

植物学报 ›› 2021, Vol. 56 ›› Issue (2): 147-150.DOI: 10.11983/CBB21048 cstr: 32102.14.CBB21048
收稿日期:2021-03-15
接受日期:2021-03-24
出版日期:2021-03-01
发布日期:2021-04-09
通讯作者:
孙蒙祥
作者简介:*E-mail: mxsun@whu.edu.cn基金资助:Received:2021-03-15
Accepted:2021-03-24
Online:2021-03-01
Published:2021-04-09
Contact:
Mengxiang Sun
摘要: 植物有性生殖过程中, 花粉-柱头间的识别作用是确保亲和花粉萌发、完成受精并保持后代遗传稳定性的重要环节, 也是农业生产上杂交育种的一个障碍。相关研究历来备受关注。然而, 经过几十年的研究, 亲和花粉如何被识别仍是未解之谜。最近, 华东师范大学李超团队的研究成果, 揭示花粉外被B类小肽PCP-Bs与柱头分泌的RALF23/33小肽竞争性地与柱头乳突细胞质膜上的ANJ-FER受体激酶复合体直接结合, 通过下游RAC/ROP-NADPH氧化酶信号途径调节柱头活性氧水平, 从而调节亲和花粉水合作用的机制。这一发现是认识花粉与柱头识别机制的重要突破。
王伟, 孙蒙祥. 花粉外被蛋白B类小肽, 花粉打开柱头大门的一把钥匙. 植物学报, 2021, 56(2): 147-150.
Wei Wang, Mengxiang Sun. POLLENCOAT PROTEIN B-class Peptides (PCP-Bs), a Key of Compatible Pollen to Open the Gate of Stigma. Chinese Bulletin of Botany, 2021, 56(2): 147-150.
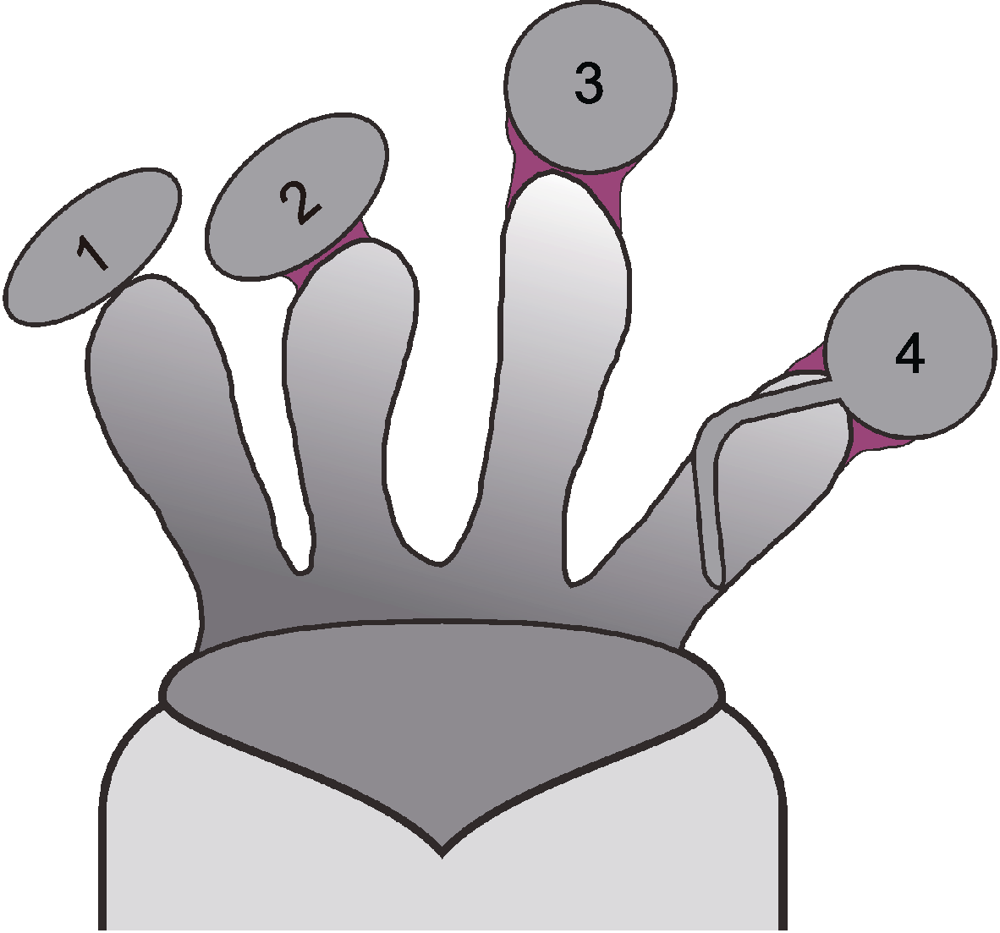
图1 拟南芥花粉与柱头的相互作用 1: 花粉落到柱头上; 2, 3: 花粉黏附与水合; 4: 花粉萌发。花粉黏附到柱头上之后, 形成花粉足(红色部分), 花粉足是花粉与柱头之间信号交流的通道。目前已知在花粉中参与水合的因子包括花粉外壁脂质以及相关的脂质酶、脂质结合蛋白和PCP-Bs小肽等。柱头中涉及水合的因子包括钙离子和Exo 70A1 (囊泡运输)等。
Figure 1 Pollen-stigma interaction in Arabidopsis 1: Pollen landing on the stigma; 2, 3: Pollen adhesion and hydration; 4: Pollen germination. After pollen adhesion a foot structure (red) established, which bridges pollen and papillar cells for the signaling between them. Currently, the known pollen factors involved in its hydration include pollen exine lipid and relevant lipidase, lipid binding proteins, and PCP-Bs, etc. The stigma factors involved in the hydration include cal-cium ion and Exo70A1 (vesicle trafficking), etc.
| [1] | Cabrillac D, Cock JM, Dumas C, Gaude T (2001). The S-locus receptor kinase is inhibited by thioredoxins and activated by pollen coat proteins. Nature 410,220-223. |
| [2] | Chapman LA, Goring DR (2010). Pollen-pistil interactions regulating successful fertilization in the Brassicaceae. J Exp Bot 61, 1987-1999. |
| [3] | Doucet J, Lee HK, Goring DR (2016). Pollen acceptance or rejection: a tale of two pathways. Trends Plant Sci 21,1058-1067. |
| [4] | Heslop-Harrison J (1975). Incompatibility and the pollen- stigma interaction. Annu Rev Plant Physiol 26,403-425. |
| [5] | Heslop-Harrison Y (1977). The pollen-stigma interaction: pollen-tube penetration in Crocus. Ann Bot 41,913-922. |
| [6] | Lin Z, Eaves DJ, Sanchez-Moran E, Franklin FCH, Frank-alin-Tong VE (2015). The Papaver rhoeas S determinants confer self-incompatibility to Arabidopsis thaliana in plan- ta. Science 350,684-687. |
| [7] | Liu C, Shen L, Xiao Y, Vyshedsky D, Peng C, Sun X, Liu Z, Cheng L, Zhang H, Han Z, Chai J, Wu HM, Cheung AY, Li C (2021). PCP-B peptides unlock a stigma 1 pep-tide-receptor kinase gated mechanism for pollination. Science doi: 10.1126/science.abc6107 |
| [8] | Mattsson O, Knox RB, Heslop-Harrison J, Heslop-Harri-son Y (1974). Protein pellicle of stigmatic papillae as a probable recognition site in incompatibility reactions. Nature 247,298-300. |
| [9] | Nasrallah ME, Liu P, Nasrallah JB (2002). Generation of self-incompatible Arabidopsis thaliana by transfer of two S locus genes from A. lyrata. Science 297,247-249. |
| [10] | Schopfer CR, Nasrallah ME, Nasrallah JB (1999). The male determinant of self-incompatibility in Brassica. Science 286, 1697-1700. |
| [11] | Takayama S, Shiba H, Iwano M, Shimosato H, Che FS, Kai N, Watanabe M, Suzuki G, Hinata K, Isogai A (2000). The pollen determinant of self-incompatibility in Brassica campestris. Proc Natl Acad Sci USA 97, 1920- 1925. |
| [12] | Wang LD, Clarke LA, Eason RJ, Parker CC, Qi BX, Scott RJ, Doughty J (2017). PCP-B class pollen coat proteins are key regulators of the hydration checkpoint in Arabidopsis thaliana pollen-stigma interactions. New Phytol 213,764-777. |
| [13] | Wheeler MJ, de Graaf BHJ, Hadjiosif N, Perry RM, Poulter NS, Osman K, Vatovec S, Harper A, Franklin FCH, Franklin-Tong VE (2009). Identification of the pollen self-incompatibility determinant in Papaver rhoeas. Nature 459,992-995. |
| [1] | 张悦婧, 桑鹤天, 王涵琦, 石珍珍, 李丽, 王馨, 孙坤, 张继, 冯汉青. 植物对非生物胁迫系统性反应中信号传递的研究进展[J]. 植物学报, 2024, 59(1): 122-133. |
| [2] | 周玉萍, 颜嘉豪, 田长恩. 保卫细胞中ABA信号调控机制研究进展[J]. 植物学报, 2022, 57(5): 684-696. |
| [3] | 刘晓龙, 季平, 杨洪涛, 丁永电, 付佳玲, 梁江霞, 余聪聪. 脱落酸对水稻抽穗开花期高温胁迫的诱抗效应[J]. 植物学报, 2022, 57(5): 596-610. |
| [4] | 胡海涛, 钱婷婷, 杨玲. 基于H2DCFDA荧光探针的植物活性氧检测方法[J]. 植物学报, 2022, 57(3): 320-326. |
| [5] | 王伟, 唐定中. 两类免疫受体强强联手筑牢植物免疫防线[J]. 植物学报, 2021, 56(2): 142-146. |
| [6] | 姚玉婷,马家琦,冯晓莉,潘建伟,王超. 磷酸肌醇激酶FAB1调控拟南芥根毛伸长[J]. 植物学报, 2020, 55(2): 126-136. |
| [7] | 代宇佳,罗晓峰,周文冠,陈锋,帅海威,杨文钰,舒凯. 生物和非生物逆境胁迫下的植物系统信号[J]. 植物学报, 2019, 54(2): 255-264. |
| [8] | 马丹颖,季东超,徐勇,陈彤,田世平. 活性氧调控植物细胞自噬的研究进展[J]. 植物学报, 2019, 54(1): 81-92. |
| [9] | 何光明, 邓兴旺. 死亡信号传递: 叶绿体与线粒体间信号交流调控植物程序性细胞死亡[J]. 植物学报, 2018, 53(4): 441-444. |
| [10] | 赵曦娟, 钱礼超, 刘玉乐. 中国科学家在植物程序性细胞死亡领域取得重要成果[J]. 植物学报, 2018, 53(4): 447-450. |
| [11] | 张宪省. 我国科学家在程序性细胞死亡机制研究领域取得重大突破[J]. 植物学报, 2018, 53(4): 445-446. |
| [12] | 王曦,胡红玲,胡庭兴,张城浩,王鑫,刘丹. 干旱胁迫对桢楠幼树渗透调节与活性氧代谢的影响及施氮的缓解效应[J]. 植物生态学报, 2018, 42(2): 240-251. |
| [13] | 许馨露, 李丹丹, 马元丹, 翟建云, 孙建飞, 高岩, 张汝民. 四季桂抗氧化防御系统对干旱、高温及协同胁迫的响应[J]. 植物学报, 2018, 53(1): 72-81. |
| [14] | 张盛春, 李清明, 阳成伟. 拟南芥金属蛋白酶FtSH4通过生长素与活性氧调控叶片衰老[J]. 植物学报, 2017, 52(4): 453-464. |
| [15] | 孟德云, 侯林琳, 杨莎, 孟静静, 郭峰, 李新国, 万书波. 外源多胺对盆栽花生盐胁迫的缓解作用[J]. 植物生态学报, 2015, 39(12): 1209-1215. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
