

植物学报 ›› 2017, Vol. 52 ›› Issue (4): 453-464.DOI: 10.11983/CBB17044 cstr: 32102.14.CBB17044
收稿日期:2017-03-09
接受日期:2017-05-06
出版日期:2017-07-01
发布日期:2017-05-05
通讯作者:
阳成伟
作者简介:# 共同第一作者
基金资助:Shengchun Zhang, Qingming Li, Chengwei Yang*
Received:2017-03-09
Accepted:2017-05-06
Online:2017-07-01
Published:2017-05-05
Contact:
Yang Chengwei
About author:# Co-first authors
摘要: 植物金属蛋白酶FtSH基因家族在拟南芥(Arabidopsis thaliana)中有12个成员, 目前各基因的功能还不清楚。该文利用细胞生物学和遗传学方法初步分析了拟南芥FtSH4在叶片衰老中的功能。ftsh4-4突变体叶片中H2O2含量及细胞死亡率增加, 叶绿素含量降低; 此外, 突变体中过氧化物酶基因表达上调, 过氧化物酶活性增加, 出现早衰表型。外源抗氧化剂AsA、内源和外源生长素能够通过降低ftsh4-4体内H2O2含量、过氧化物酶基因的表达及过氧化物酶活性, 恢复ftsh4-4叶片的衰老表型。ftsh4-4突变体中生长素响应因子基因ARF2和ARF7上调表达, 外源生长素和抗氧化剂能够降低ARF2和ARF7的表达, 并且ARF2突变能够降低ftsh4-4的H2O2含量并恢复其早衰表型。以上结果表明, FtSH4基因通过生长素与活性氧在调控植物叶片衰老中起重要作用。
张盛春, 李清明, 阳成伟. 拟南芥金属蛋白酶FtSH4通过生长素与活性氧调控叶片衰老. 植物学报, 2017, 52(4): 453-464.
Shengchun Zhang, Qingming Li, Chengwei Yang. Arabidopsis Metalloprotease FtSH4 Regulates Leaf Senescence Through Auxin and Reactive Oxygen Species. Chinese Bulletin of Botany, 2017, 52(4): 453-464.
| Primer name | Primer sequence (5ʹ-3ʹ) |
|---|---|
| PER33F | TCTTCTCCATCACTTCTTCTTA |
| PER33R | ATCCTCCAACACATATTCTCTA |
| PER37F | CGCCAACACTCTTTGACAACAAG |
| PER37R | ACTCATCCTTATCATTGCCTTCGC |
| ARF2F | AATATAGCACCTTCATCTCCT |
| ARF2R | ATCACACTCTACACTCTCAG |
| ARF7F | GCTAATGCTAATAACAGTCCTT |
| ARF7R | TCCACATTCTTCAGTCTCAA |
| SAG12F | ATGATGAGCAAGCACTGATGAAGG |
| SAG12R | TCCGTTAGTAGATTCGCCGTATCC |
| SAG13F | GCGACAACATAAGGACGAACTCTG |
| SAG13R | GAAGACAAAGAAATGCCACAAGCG |
| SAG101F | GGGATGAGAGACGATGTGAGAGAG |
| SAG101R | CGGGTGTTCATAAACTCGGTCAAG |
| SEN1F | GGACATCCGACTAGAGCCATCAAC |
| SEN1R | ATCGCCGTGAAGCCAGCAG |
| SEN4F | AACCGCCAATTTCCACACTTACTC |
| SEN4R | CTCTTGTTGCCCAATCGTCTGC |
| UBQ10F | CCGACTACAACATTCAGAAG |
| UBQ10R | TATCAATGGTGTCAGAACTCT |
表1 引物序列
Table 1 Primers used in this study
| Primer name | Primer sequence (5ʹ-3ʹ) |
|---|---|
| PER33F | TCTTCTCCATCACTTCTTCTTA |
| PER33R | ATCCTCCAACACATATTCTCTA |
| PER37F | CGCCAACACTCTTTGACAACAAG |
| PER37R | ACTCATCCTTATCATTGCCTTCGC |
| ARF2F | AATATAGCACCTTCATCTCCT |
| ARF2R | ATCACACTCTACACTCTCAG |
| ARF7F | GCTAATGCTAATAACAGTCCTT |
| ARF7R | TCCACATTCTTCAGTCTCAA |
| SAG12F | ATGATGAGCAAGCACTGATGAAGG |
| SAG12R | TCCGTTAGTAGATTCGCCGTATCC |
| SAG13F | GCGACAACATAAGGACGAACTCTG |
| SAG13R | GAAGACAAAGAAATGCCACAAGCG |
| SAG101F | GGGATGAGAGACGATGTGAGAGAG |
| SAG101R | CGGGTGTTCATAAACTCGGTCAAG |
| SEN1F | GGACATCCGACTAGAGCCATCAAC |
| SEN1R | ATCGCCGTGAAGCCAGCAG |
| SEN4F | AACCGCCAATTTCCACACTTACTC |
| SEN4R | CTCTTGTTGCCCAATCGTCTGC |
| UBQ10F | CCGACTACAACATTCAGAAG |
| UBQ10R | TATCAATGGTGTCAGAACTCT |
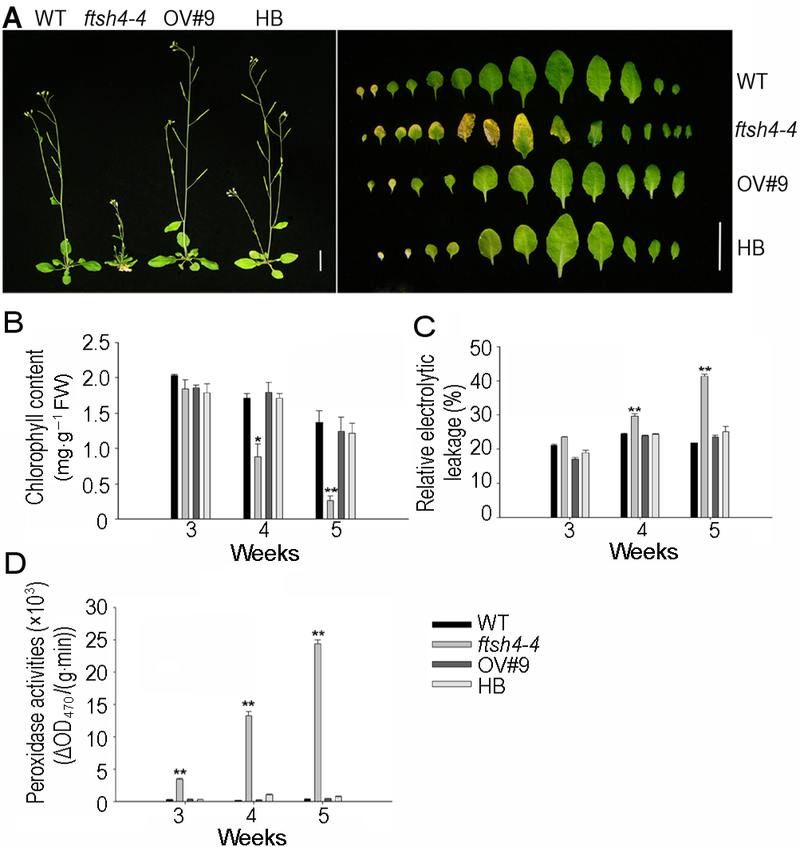
图1 FtSH4突变导致拟南芥叶片早衰^(A) ftsh4-4突变体表现出衰老表型(Bar=1 cm); (B) ftsh4-4突变体叶绿素含量降低; (C) ftsh4-4突变体相对电导率增加; (D) ftsh4-4突变体过氧化物酶活性增加。* 表示差异显著(P<0.05); ** 表示差异极显著(P<0.01) (Student’s t-test)。
Figure 1 FtSH4 mutation causes leaf senescence of Arabi- dopsis^(A) The phenotype of premature senescence observed in the ftsh4-4 mutant (Bar=1 cm); (B) The chlorophyll content decreased in ftsh4-4 mutant; (C) The relative electrolytic leakage increased in ftsh4-4 mutant; (D) The peroxidase activities increased in ftsh4-4 mutant. * indicates significant difference at P<0.05; ** indicates significant difference at P<0.01 (Student’s t-test).
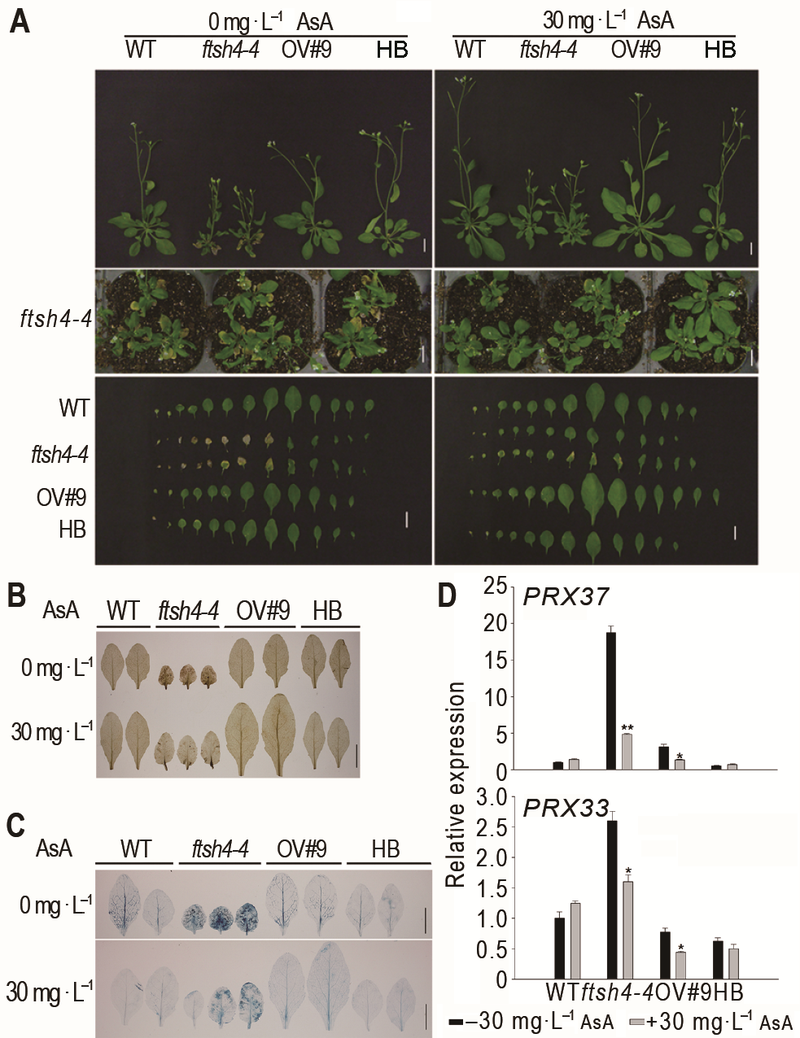
图2 外源抗氧化剂AsA能恢复拟南芥ftsh4-4突变体叶片衰老表型^(A) 外源AsA处理能恢复ftsh4-4突变体早衰表型(Bar=1 cm); (B) 外源AsA处理能降低ftsh4-4突变体中H2O2含量(Bar=1 cm); (C) 外源AsA处理能减少ftsh4-4突变体中细胞死亡数目(Bar=1 cm); (D) 外源AsA处理能降低ftsh4-4突变体中过氧化物酶基因的表达。* 表示差异显著(P<0.05); ** 表示差异极显著(P<0.01) (Student’s t-test)。
Figure 2 Exogenous AsA rescued the leaf senescence phenotype of Arabidopsis ftsh4-4 mutant^(A) Exogenous AsA rescued the leaf senescence phenotype of ftsh4-4 mutant (Bar=1 cm); (B) Exogenous AsA reduced the H2O2 level of ftsh4-4 mutant (Bar=1 cm); (C) Exogenous AsA reduced the cell death of ftsh4-4 mutant (Bar=1 cm); (D) Exogenous AsA reduced the expression of peroxidase genes in ftsh4-4 mutant. * indicates significant difference at P<0.05; ** indicates significant difference at P<0.01 (Student’s t-test).
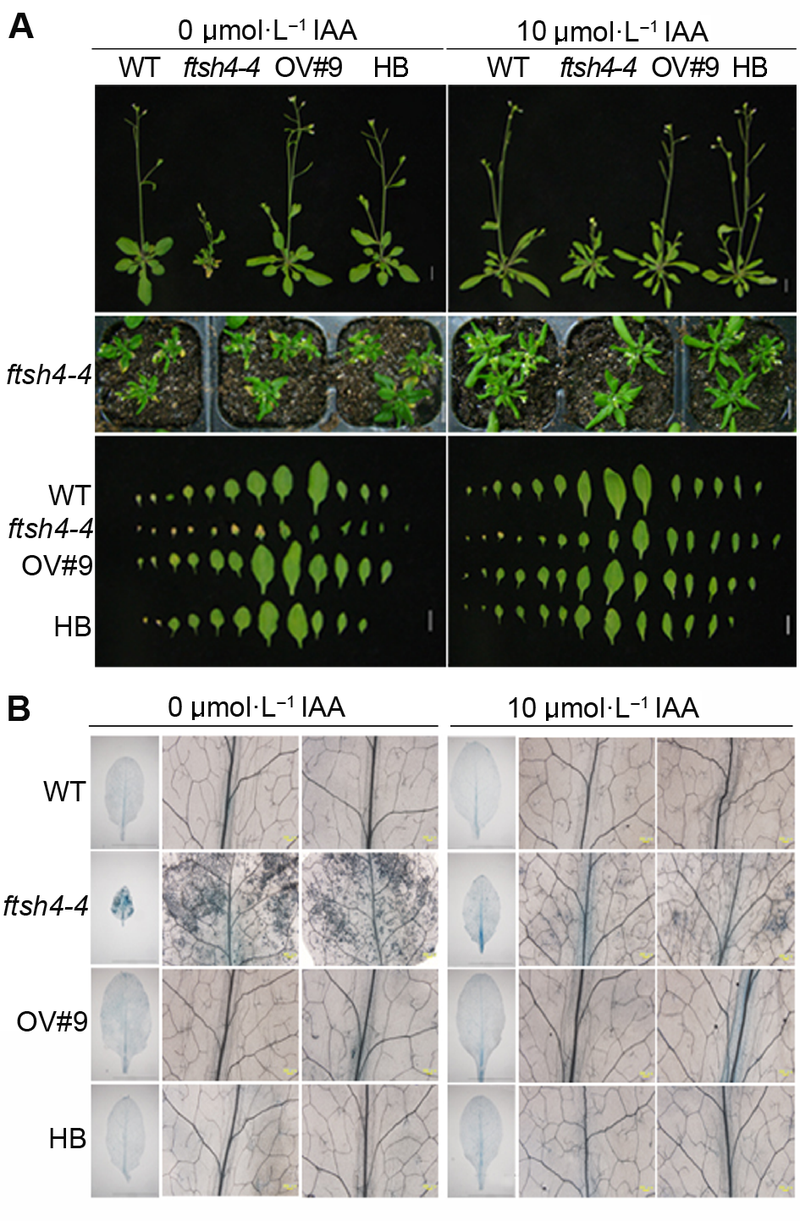
图3 外源生长素(IAA)能恢复拟南芥ftsh4-4突变体早衰表型^(A) 外源IAA处理能恢复ftsh4-4突变体早衰表型(Bar=1 cm); (B) 外源IAA处理能减少ftsh4-4突变体中细胞死亡数目
Figure 3 Exogenous IAA restored the leaf senescence phenotype of Arabidopsis ftsh4-4 mutant^(A) Exogenous IAA restored the leaf senescence phenotype of ftsh4-4 mutant (Bar=1 cm); (B) Exogenous IAA reduced the cell death of ftsh4-4 mutant
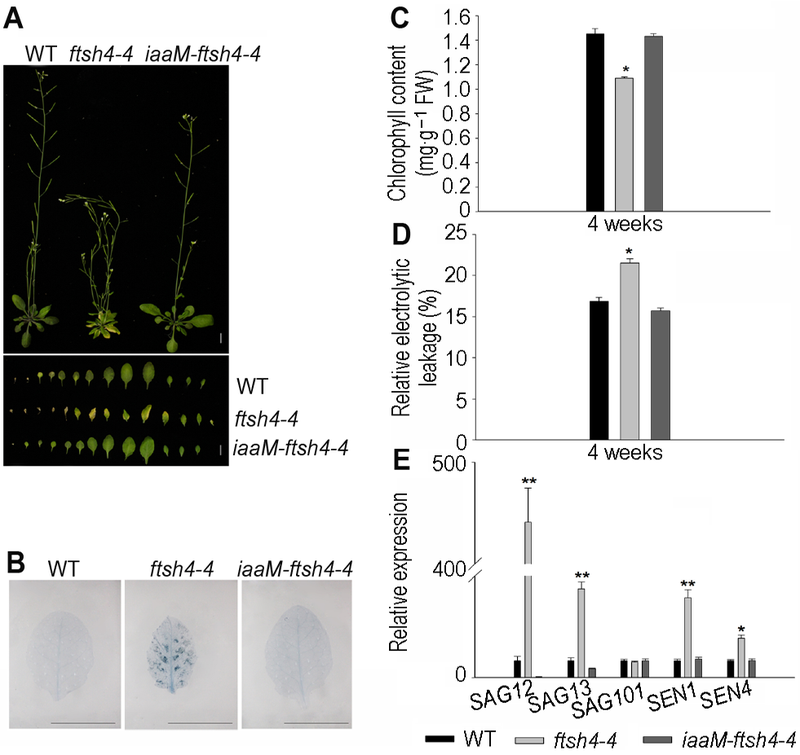
图4 增加内源生长素含量能恢复拟南芥ftsh4-4突变体早衰表型^(A) 增加内源IAA含量能恢复ftsh4-4突变体早衰表型(Bar=1 cm); (B) iaaM-ftsh4-4中细胞死亡数目减少(Bar=1 cm); (C) iaaM-ftsh4-4中叶绿素含量恢复到WT水平; (D) iaaM-ftsh4-4中相对电导率恢复到WT水平; (E) 内源激素降低ftsh4-4突变体中衰老基因SAG12、SAG13、SAG101、SEN1及SEN4的表达。* 表示差异显著(P<0.05); ** 表示差异极显著(P<0.01) (Student’s t-test)。
Figure 4 Increasing endogenous IAA restored the leaf senescence phenotype of Arabidopsis ftsh4-4 mutant^(A) Increasing endogenous IAA restored the leaf senescence phenotype of ftsh4-4 mutant (Bar=1 cm); (B) Cell death decreased in iaaM-ftsh4-4 (Bar=1 cm); (C) The chlorophyll content of iaaM-ftsh4-4 transgeneic line restored to the wild type level; (D) The relative electrolytic leakage of iaaM- ftsh4-4 transgeneic line restored to the wild type level; (E) Endogenous IAA decreased the expression of senescence-associated genes SAG12, SAG13, SAG101, SEN1 and SEN4 in ftsh4-4 mutant. * indicates significant difference at P<0.05; ** indicates significant difference at P<0.01 (Student’s t-test).
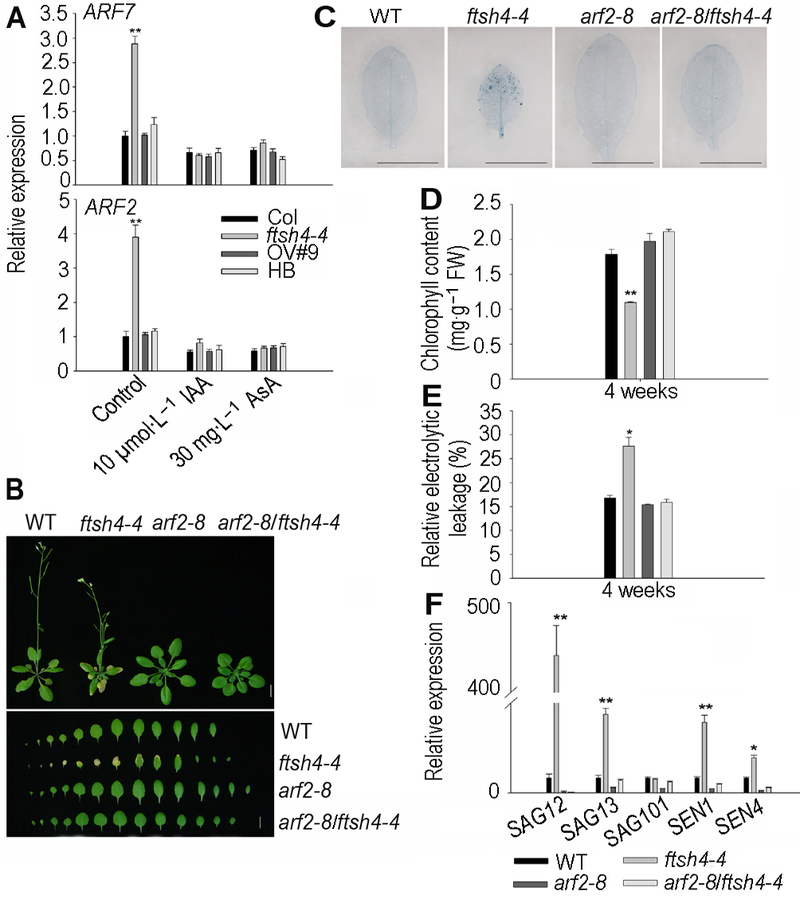
图5 ARF2参与FtSH4介导的拟南芥叶片衰老过程^(A) 生长素响应因子基因ARF2和ARF7在ftsh4-4中上调表达并受外源IAA和AsA抑制; (B) ARF2突变抑制了ftsh4-4的叶片衰老(Bar=1 cm); (C) ARF2突变使ftsh4-4中细胞死亡数目减少(Bar=1 cm); (D) ARF2突变使ftsh4-4中叶绿素含量增加; (E) ARF2突变使ftsh4-4中相对电导率降低; (F) ARF2突变使ftsh4-4中衰老相关标记基因SAG12、SAG13、SAG101、SEN1及SEN4下调表达。* 表示差异显著(P<0.05); ** 表示差异极显著(P<0.01) (Student’s t-test)。
Figure 5 ARF2 is involved in FtSH4-mediated leaf senescence of Arabidopsis^(A) The expression levels of ARF2 and ARF7 increased in the ftsh4-4 and were inhibited by the exogenous IAA and AsA; (B) ARF2 mutation rescued the leaf senescence of ftsh4-4 mutant (Bar=1 cm); (C) ARF2 mutation reduced the cell death of ftsh4-4 mutant (Bar=1 cm); (D) ARF2 mutation increased the chlorophyll content of ftsh4-4 mutant; (E) ARF2 mutation reduced the relative electrolytic leakage of ftsh4-4 mutant; (F) ARF2 mutation reduced the expression of senescence-associated genes SAG12, SAG13, SAG101, SEN1 and SEN4. * indicates significant difference at P<0.05; ** indicates significant difference at P<0.01 (Student’s t-test).
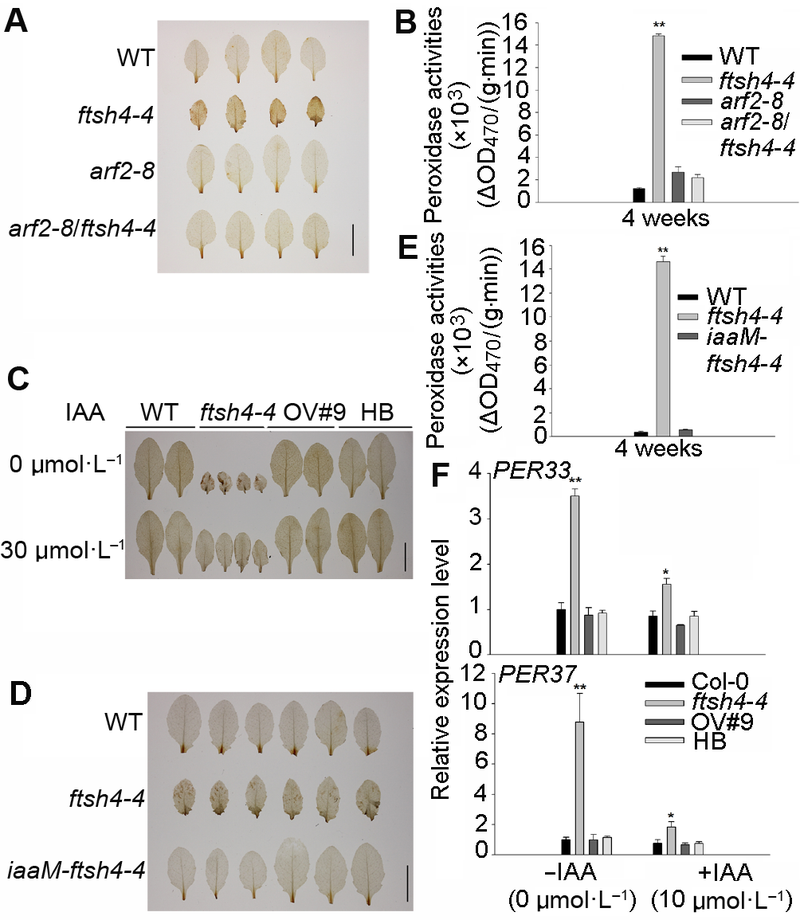
图6 生长素处理降低拟南芥ftsh4-4突变体中H2O2含量和过氧化物酶活性^(A) ARF2突变使ftsh4-4中H2O2含量减少(Bar=1 cm); (B) ARF2突变使ftsh4-4中过氧化物酶活性降低; (C)外施生长素能降低ftsh4-4突变体中H2O2含量(Bar=1 cm); (D) 增加内源生长素含量能降低ftsh4-4突变体中H2O2含量(Bar=1 cm); (E) 增加内源生长素含量能降低ftsh4-4突变体中过氧化物酶活性; (F) 外源生长素处理降低ftsh4-4突变体中过氧化物酶基因的表达。* 表示差异显著(P<0.05); ** 表示差异极显著(P<0.01) (Student’s t-test)。
Figure 6 IAA treatment reduces the H2O2 level and peroxidase activities of Arabidopsis ftsh4-4 mutant^(A) ARF2 mutation reduced the H2O2 level of ftsh4-4 mutant (Bar=1 cm); (B) ARF2 mutation reduced the peroxidase activities of ftsh4-4 mutant; (C) Exogenous IAA rescued the H2O2 level of ftsh4-4 mutant (Bar=1 cm); (D) Increasing endogenous IAA reduced the H2O2 level of ftsh4-4 mutant (Bar=1 cm); (E) Increasing endogenous IAA reduced the peroxidase activities of ftsh4-4 mutant; (F) Exogenous IAA reduced the peroxidase genes expression of ftsh4-4 mutant. * indicates significant difference at P<0.05; ** indicates significant difference at P<0.01 (Student’s t-test).
| [1] | Apel K, Hirt H (2004). Reactive oxygen species: metabolism, oxidative stress, and signal transduction.Annu Rev Plant Biol 55, 373-399. |
| [2] | Arnon DI (1949). Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol 24, 1-15. |
| [3] | Bashandy T, Guilleminot J, Vernoux T, Caparros-Ruiz D, Ljung K, Meyer Y, Reichheld JP (2010). Interplay bet- ween the NADP-linked thioredoxin and glutathione systems in Arabidopsis auxin signaling.Plant Cell 22 376-391. |
| [4] | Blomster T, Salojarvi J, Sipari N, Brosche M, Ahlfors R, Keinanen M, Overmyer K, Kangasjarvi J (2011). Apo- plastic reactive oxygen species transiently decrease auxin signaling and cause stress-induced morphogenic res- ponse in Arabidopsis.Plant Physiol 157, 1866-1883. |
| [5] | Camilleri C, Jouanin L (1991). The TR-DNA region carrying the auxin synthesis genes of the Agrobacterium rhizo- genes agropine-type plasmid pRiA4: nucleotide sequence analysis and introduction into tobacco plants. Mol Plant Microbe Interact 4, 155-162. |
| [6] | Chen GH, Liu CP, Chen SC, Wang LC (2012). Role of ARABIDOPSIS A-FIFTEEN in regulating leaf senescence involves response to reactive oxygen species and is dependent on ETHYLENE INSENSITIVE2. J Exp Bot 63, 275-292. |
| [7] | Chen JP, Burke JJ, Velten J, Xin ZU (2006). FtsH11 protease plays a critical role in Arabidopsis thermotolerance.Plant J 48, 73-84. |
| [8] | Ellis CM, Nagpal P, Young JC, Hagen G, Guilfoyle TJ, Reed JW (2005). AUXIN RESPONSE FACTOR1 and AUXIN RESPONSE FACTOR2 regulate senescence and floral organ abscission in Arabidopsis thaliana. Development 132, 4563-4574. |
| [9] | Garcia-Lorenzo M, Sjodin A, Jansson S, Funk C (2006). Protease gene families in Populus and Arabidopsis. BMC Plant Biol 6, 30. |
| [10] | Gazarian IG, Lagrimini LM, Mellon FA, Naldrett MJ, Ashby GA, Thorneley RN (1998). Identification of skatolyl hydroperoxide and its role in the peroxidase-catalysed oxidation of indol-3-yl acetic acid.Biochem J 333, 223-232. |
| [11] | Gibala M, Kicia M, Sakamoto W, Gola EM, Kubrakiewicz J, Smakowska E, Janska H (2009). The lack of mitochondrial AtFtsH4 protease alters Arabidopsis leaf morphology at the late stage of rosette development under short-day photoperiod.Plant J 59, 685-699. |
| [12] | Guo Y, Gan SS (2012). Convergence and divergence in gene expression profiles induced by leaf senescence and 27 senescence-promoting hormonal, pathological and environmental stress treatments.Plant Cell Environ 35, 644-655. |
| [13] | He J, Duan Y, Hua D, Fan G, Wang L, Liu Y, Chen Z, Han L, Qu LJ, Gong Z (2012). DEXH box RNA helicase- mediated mitochondrial reactive oxygen species production in Arabidopsis mediates crosstalk between abscisic acid and auxin signaling.Plant Cell 24, 1815-1833. |
| [14] | Hou K, Wu W, Gan SS (2013). SAUR36, a small auxin up RNA gene, is involved in the promotion of leaf senescence in Arabidopsis.Plant Physiol 161, 1002-1009. |
| [15] | Huang YC, Chang YL, Hsu JJ, Chuang HW (2008). Trans- criptome analysis of auxin-regulated genes of Arabidopsis thaliana. Gene 420, 118-124. |
| [16] | Jibran R, Hunter DA, Dijkwel PP (2013). Hormonal regulation of leaf senescence through integration of developmental and stress signals.Plant Mol Biol 82, 547-561. |
| [17] | Joo JH, Bae YS, Lee JS (2001). Role of auxin-induced reactive oxygen species in root gravitropism.Plant Physiol 126, 1055-1060. |
| [18] | Kant S, Bi YM, Zhu T, Rothstein SJ (2009). SAUR39, a small auxin-up RNA gene, acts as a negative regulator of auxin synthesis and transport in rice.Plant Physiol 151, 691-701. |
| [19] | Kato Y, Miura E, Ido K, Ifuku K, Sakamoto W (2009). The variegated mutants lacking chloroplastic FtsHs are defective in D1 degradation and accumulate reactive oxygen sp- ecies.Plant Physiol 151, 1790-1801. |
| [20] | Kim JI, Murphy AS, Baek D, Lee SW, Yun DJ, Bressan RA, Narasimhan ML (2011). YUCCA6 over-expression demonstrates auxin function in delaying leaf senescence inArabidopsis thaliana. J Exp Bot 62, 3981-3992. |
| [21] | Kolodziejczak M, Kolaczkowska A, Szczesny B, Urantowka A, Knorpp C, Kieleczawa J, Janska H (2002). A higher plant mitochondrial homologue of the yeast m-AAA protease-molecular cloning, localization, and putative func- tion.J Biol Chem 277, 43792-43798. |
| [22] | Kovtun Y, Chiu WL, Tena G, Sheen J (2000). Functional analysis of oxidative stress-activated mitogen-activated protein kinase cascade in plants.Proc Natl Acad Sci USA 97, 2940-2945. |
| [23] | Li Z, Peng J, Wen X, Guo H (2012). Gene network analysis and functional studies of senescence-associated genes reveal novel regulators of Arabidopsis leaf senescence.J Integr Plant Biol 54, 526-539. |
| [24] | Lim PO, Kim HJ, Nam HG (2007). Leaf senescence.Annu Rev Plant Biol 58, 115-136. |
| [25] | Lim PO, Lee IC, Kim J, Kim HJ, Ryu JS, Woo HR, Nam HG (2010). Auxin response factor 2 (ARF2) plays a major role in regulating auxin-mediated leaf longevity.J Exp Bot 61, 1419-1430. |
| [26] | Ljung K, Hull AK, Kowalczyk M, Marchant A, Celenza J, Cohen JD, Sandberg G (2002). Biosynthesis, conjugation, catabolism and homeostasis of indole-3-acetic acid in Arabidopsis thaliana. Plant Mol Biol 49, 249-272. |
| [27] | Malnoe A, Wang F, Girard-Bascou J, Wollman FA, de Vitry C (2014). Thylakoid FtsH protease contributes to photosystem II and cytochrome b6f remodeling in Chlamydomonas reinhardtii under stress conditions.Plant Cell 26, 373-390. |
| [28] | Meudt WJ, Gaines TP (1967). Studies on the oxidation of indole-3-acetic acid by peroxidase enzymes. I. Colorimetric determination of indole-3-acetic acid oxidation produ- cts.Plant Physiol 144, 118-128. |
| [29] | Mittler R, Vanderauwera S, Suzuki N, Miller G, Tognetti VB, Vandepoele K, Gollery M, Shulaev V, Van Breu- segem F (2011). ROS signaling: the new wave?Trends Plant Sci 16, 300-309. |
| [30] | Nakagami H, Soukupova H, Schikora A, Zarsky V, Hirt H (2006). A mitogen-activated protein kinase kinase kinase mediates reactive oxygen species homeostasis in Arabidopsis.J Biol Chem 281, 38697-38704. |
| [31] | Nolden M, Ehses S, Koppen M, Bernacchia A, Rugarli EI, Langer T (2005). The m-AAA protease defective in hereditary spastic paraplegia controls ribosome assembly in mitochondria.Cell 123, 277-289. |
| [32] | Okushima Y, Fukaki H, Onoda M, Theologis A, Tasaka M (2007). ARF7 and ARF19 regulate lateral root formation via direct activation ofLBD/ASL genes in Arabidopsis. Pla- nt Cell 19, 118-130. |
| [33] | Piechota J, Kolodziejczak M, Juszczak I, Sakamoto W, Janska H (2010). Identification and characterization of high molecular weight complexes formed by matrix AAA proteases and prohibitins in mitochondria ofArabidopsis thaliana. J Biol Chem 285, 12512-12521. |
| [34] | Potters G, Pasternak TP, Guisez Y, Palme KJ, Jansen MA (2007). Stress-induced morphogenic responses: growing out of trouble?Trends Plant Sci 12, 98-105. |
| [35] | Queval G, Issakidis-Bourguet E, Hoeberichts FA, Vandorpe M, Gakiere B, Vanacker H, Miginiac-Maslow M, Van Breusegem F, Noctor G (2007). Conditional oxidative stress responses in the Arabidopsis photorespiratory mutant cat2 demonstrate that redox state is a key modulator of daylength-dependent gene expression, and define photoperiod as a crucial factor in the regulation of H2O2- induced cell death. Plant J 52, 640-657. |
| [36] | Ren G, Zhou Q, Wu S, Zhang Y, Zhang L, Huang J, Sun Z, Kuai B (2010). Reverse genetic identification of CRN1 and its distinctive role in chlorophyll degradation in Arabidopsis.J Integr Plant Biol 52, 496-504. |
| [37] | Romano CP, Robson PR, Smith H, Estelle M, Klee H (1995). Transgene-mediated auxin overproduction in Ara- bidopsis: hypocotyl elongation phenotype and interactions with the hy6-1 hypocotyl elongation and axr1 auxin-resis- tant mutants.Plant Mol Biol 27, 1071-1083. |
| [38] | Sakamoto W, Tamura T, Hanba-Tomita Y, Murata M (2002). The VAR1 locus of Arabidopsis encodes a chloroplastic FtsH and is responsible for leaf variegation in the mutant alleles.Genes Cells 7, 769-780. |
| [39] | Salleh FM, Evans K, Goodall B, Machin H, Mowla SB, Mur LA, Runions J, Theodoulou FL, Foyer CH, Rogers HJ (2012). A novel function for a redox-related LEA protein (SAG21/AtLEA5) in root development and biotic stress re- sponses.Plant Cell Environ 35, 418-429. |
| [40] | Savitsky PA, Gazaryan IG, Tishkov VI, Lagrimini LM, Ruzgas T, Gorton L (1999). Oxidation of indole-3-acetic acid by dioxygen catalysed by plant peroxidases: specifi- city for the enzyme structure.Biochem J 340, 579-583. |
| [41] | Sierla M, Rahikainen M, Salojarvi J, Kangasjarvi J, Kangasjarvi S (2013). Apoplastic and chloroplastic redox signaling networks in plant stress responses.Antioxid Re- dox Signal 18, 2220-2239. |
| [42] | Suzuki N, Miller G, Morales J, Shulaev V, Torres MA, Mittler R (2011). Respiratory burst oxidases: the engines of ROS signaling.Curr Opin Plant Biol 14, 691-699. |
| [43] | Wagner R, Aigner H, Pruzinska A, Jankanpaa HJ, Jansson S, Funk C (2011). Fitness analyses of Arabidopsis thaliana mutants depleted of FtsH metalloproteases and characterization of three FtsH6 deletion mutants exposed to high light stress, senescence and chilling. New Phytol 191, 449-458. |
| [44] | Wang P, Song CP (2008). Guard-cell signaling for hydrogen peroxide and abscisic acid.New Phytol 178, 703-718. |
| [45] | Watanabe M, Balazadeh S, Tohge T, Erban A, Giavalisco P, Kopka J, Mueller-Roeber B, Fernie AR, Hoefgen R (2013). Comprehensive dissection of spatiotemporal meta- bolic shifts in primary, secondary, and lipid metabolism during developmental senescence in Arabidopsis.Plant Physiol 162, 1290-1310. |
| [46] | Woodward AW, Bartel B (2005). Auxin: regulation, action, and interaction.Ann Bot 95, 707-735. |
| [47] | Xu F, Meng T, Li P, Yu Y, Cui Y, Wang Y, Gong Q, Wang NN (2011). A soybean dual-specificity kinase, GmSARK, and its Arabidopsis homolog, AtSARK, regulate leaf senescence through synergistic actions of auxin and ethyle- ne.Plant Physiol 157, 2131-2153. |
| [48] | Yuan HM, Liu WC, Jin Y, Lu YT (2013). Role of ROS and auxin in plant response to metal-mediated stress.Plant Signal Behav 8, e24671. |
| [49] | Zentgraf U, Laun T, Miao Y (2010). The complex regulation of WRKY53 during leaf senescence of Arabidopsis thali- ana.Eur J Cell Biol 89, 133-137. |
| [50] | Zhang S, Wu J, Yuan D, Zhang D, Huang Z, Xiao L, Yang C (2014). Perturbation of auxin homeostasis caused by mitochondrial FtSH4 gene-mediated peroxidase accumulation regulates Arabidopsis architecture.Mol Plant 7, 856-873. |
| [51] | Zhang S, Li C, Wang R, Chen Y, Shu S, Huang R, Zhang D, Xiao S, Yao N, Li J, Yang CW (2017). The mitochondrial protease FtSH4 regulates leaf senescence via WRKY- dependent salicylic acid signal.Plant Physiol 173, 2294-2307. |
| [52] | Zimmermann P, Heinlein C, Orendi G, Zentgraf U (2006). Senescence-specific regulation of catalases in Arabidopsis thaliana(L.) Heynh. Plant Cell Environ 29, 1049-1060. |
| [1] | 刘雨函, 曹启江, 张诗晗, 李益慧, 王菁, 谭晓萌, 刘筱儒, 王显玲. 拟南芥AtFTCD-L参与根系响应土壤紧实度的机制研究[J]. 植物学报, 2025, 60(4): 1-0. |
| [2] | 杨文丽, 李钊, 刘志铭, 张志华, 杨今胜, 吕艳杰, 王永军. 不同熟期玉米叶片衰老特性及其对叶际细菌的影响[J]. 植物学报, 2024, 59(6): 1024-1040. |
| [3] | 景艳军, 林荣呈. 蓝光受体CRY2化身“暗黑舞者”[J]. 植物学报, 2024, 59(6): 878-882. |
| [4] | 罗燕, 刘奇源, 吕元兵, 吴越, 田耀宇, 安田, 李振华. 拟南芥光敏色素突变体种子萌发的光温敏感性[J]. 植物学报, 2024, 59(5): 752-762. |
| [5] | 周玉滢, 陈辉, 刘斯穆. 植物非典型Aux/IAA蛋白应答生长素研究进展[J]. 植物学报, 2024, 59(4): 651-658. |
| [6] | 杨继轩, 王雪霏, 顾红雅. 西藏野生拟南芥开花时间变异的遗传基础[J]. 植物学报, 2024, 59(3): 373-382. |
| [7] | 陈艳晓, 李亚萍, 周晋军, 解丽霞, 彭永彬, 孙伟, 和亚男, 蒋聪慧, 王增兰, 郑崇珂, 谢先芝. 拟南芥光敏色素B氨基酸位点突变对其结构与功能的影响[J]. 植物学报, 2024, 59(3): 481-494. |
| [8] | 张悦婧, 桑鹤天, 王涵琦, 石珍珍, 李丽, 王馨, 孙坤, 张继, 冯汉青. 植物对非生物胁迫系统性反应中信号传递的研究进展[J]. 植物学报, 2024, 59(1): 122-133. |
| [9] | 孔祥培, 张蒙悦, 丁兆军. 柳暗花明:胞外生长素信号感受的新突破[J]. 植物学报, 2023, 58(6): 861-865. |
| [10] | 园园, 恩和巴雅尔, 齐艳华. 植物GH3基因家族生物学功能研究进展[J]. 植物学报, 2023, 58(5): 770-782. |
| [11] | 王钢, 王二涛. “卫青不败由天幸”——WeiTsing的广谱抗根肿病机理被揭示[J]. 植物学报, 2023, 58(3): 356-358. |
| [12] | 周淑瑶, 李建明, 毛娟. AtGH3.17调控拟南芥生长素和油菜素甾醇的响应[J]. 植物学报, 2023, 58(3): 373-384. |
| [13] | 周玉萍, 颜嘉豪, 田长恩. 保卫细胞中ABA信号调控机制研究进展[J]. 植物学报, 2022, 57(5): 684-696. |
| [14] | 刘晓龙, 季平, 杨洪涛, 丁永电, 付佳玲, 梁江霞, 余聪聪. 脱落酸对水稻抽穗开花期高温胁迫的诱抗效应[J]. 植物学报, 2022, 57(5): 596-610. |
| [15] | 叶青, 闫晓燕, 陈慧泽, 冯金林, 韩榕. 氮掺杂石墨烯量子点对拟南芥主根生长方向的影响[J]. 植物学报, 2022, 57(5): 623-634. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
