

植物学报 ›› 2020, Vol. 55 ›› Issue (5): 596-604.DOI: 10.11983/CBB20016 cstr: 32102.14.CBB20016
收稿日期:2020-02-05
接受日期:2020-06-05
出版日期:2020-09-01
发布日期:2020-09-03
通讯作者:
蒋苏
作者简介:E-mail: pepinojs@163.com基金资助:
Long Ma, Guilin Li, Shipeng Li, Su Jiang*( )
)
Received:2020-02-05
Accepted:2020-06-05
Online:2020-09-01
Published:2020-09-03
Contact:
Su Jiang
摘要: 整体透明观察技术是植物形态发育研究的基础手段之一, 是无需制作切片直接观察植物体内部形态结构的有效方法。该技术采用高折射率介质降低光在样品中的散射, 提高光通量, 增加视野深度, 从而实现组织样品透明观察。然而透明剂能改变透明液的渗透势和pH值, 从而对细胞形态保持产生负面影响。目前, 针对植物叶片和胚珠已建立了相对成熟的整体透明观察体系, 但根尖由于细胞壁较薄, 现有的整体透明方法常导致细胞形态改变, 不确定性增加(如根尖整体形态改变和细胞发生严重的质壁分离)。该研究以拟南芥(Arabidopsis thaliana)幼苗为实验材料, 通过检测根尖形态、细胞质壁分离情况和细胞清晰度, 对常用的透明液组分、pH值和透明时间进行优化, 旨在建立一种适用于根尖等较脆弱组织材料的整体透明方法。
马龙, 李桂林, 李师鹏, 蒋苏. 根尖整体透明技术改良. 植物学报, 2020, 55(5): 596-604.
Long Ma, Guilin Li, Shipeng Li, Su Jiang. An Improved Protocol for Whole Mount Clearing of Plant Root Tip. Chinese Bulletin of Botany, 2020, 55(5): 596-604.
| No. | HCG-1 | ||
|---|---|---|---|
| Composition | pH | ||
| 1 | H2O | 9 mL | 1.2 |
| Chloral hydrate | 24 g | ||
| Glycerol | 3 mL | ||
| 2 | H2O | 9 mL | 5.8 |
| Chloral hydrate | 24 g | ||
| Glycerol | 3 mL | ||
| 3 | H2O | 9 mL | 7.2 |
| Chloral hydrate | 24 g | ||
| Glycerol | 3 mL | ||
| HCG-2 | |||
| Composition | pH | ||
| 4 | H2O | 9 mL | 1.8 |
| Chloral hydrate | 12 g | ||
| Glycerol | 3 mL | ||
| 5 | H2O | 9 mL | 5.8 |
| Chloral hydrate | 12 g | ||
| Glycerol | 3 mL | ||
| 6 | H2O | 9 mL | 7.2 |
| Chloral hydrate | 12 g | ||
| Glycerol | 3 mL | ||
表1 原始pH、拟南芥培养基常用pH、中性pH透明液HCG-1和HCG-2
Table 1 Solution HCG-1 and HCG-2 in original pH, the pH used for Arabidopsis thaliana culture and neutral pH
| No. | HCG-1 | ||
|---|---|---|---|
| Composition | pH | ||
| 1 | H2O | 9 mL | 1.2 |
| Chloral hydrate | 24 g | ||
| Glycerol | 3 mL | ||
| 2 | H2O | 9 mL | 5.8 |
| Chloral hydrate | 24 g | ||
| Glycerol | 3 mL | ||
| 3 | H2O | 9 mL | 7.2 |
| Chloral hydrate | 24 g | ||
| Glycerol | 3 mL | ||
| HCG-2 | |||
| Composition | pH | ||
| 4 | H2O | 9 mL | 1.8 |
| Chloral hydrate | 12 g | ||
| Glycerol | 3 mL | ||
| 5 | H2O | 9 mL | 5.8 |
| Chloral hydrate | 12 g | ||
| Glycerol | 3 mL | ||
| 6 | H2O | 9 mL | 7.2 |
| Chloral hydrate | 12 g | ||
| Glycerol | 3 mL | ||
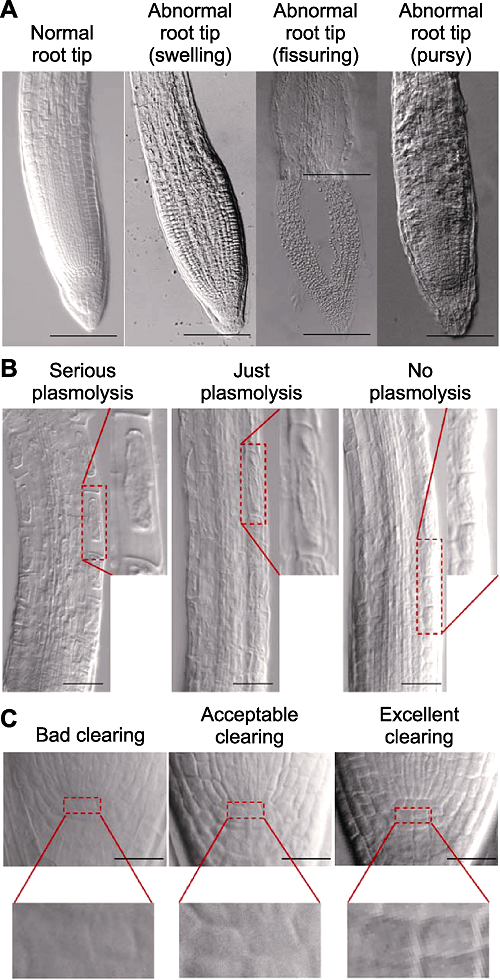
图1 拟南芥根尖透明效果界定标准图 拟南芥Col-0幼苗根尖形态(A)、伸长区表皮细胞质壁分离程度(B)和静止中心(QC)细胞清晰度(C)的透明效果((A) Bars= 100 μm; (B) Bars=50 μm; (C) Bars=20 μm)。
Figure 1 Criteria of Arabidopsis thaliana root tip clearing Root tips morphology (A), elongation zone epidermal cells plasmolysis (B) and quiescent center (QC) cells clarity (C) of cleared Arabidopsis thaliana Col-0 seedlings ((A) Bars=100 μm; (B) Bars=50 μm; (C) Bars=20 μm).
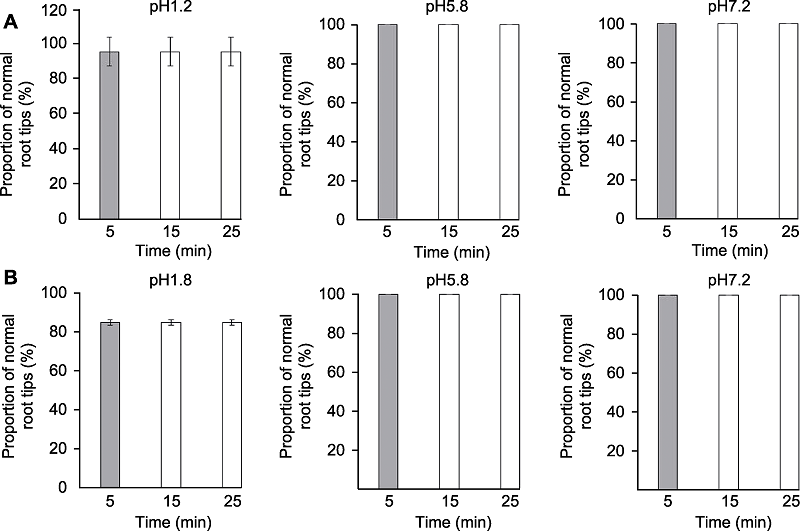
图2 透明时间对拟南芥根尖形态的影响 分别采用透明液HCG-1 (A)和HCG-2 (B)对拟南芥幼苗透明5、15和25分钟后形态正常根尖所占比例。
Figure 2 The effects of clearing times on Arabidopsis thaliana root tip morphology Proportion of normal root tips of Arabidopsis thaliana seedlings cleared by HCG-1 (A) and HCG-2 (B) for 5, 15, 25 min, respectively.
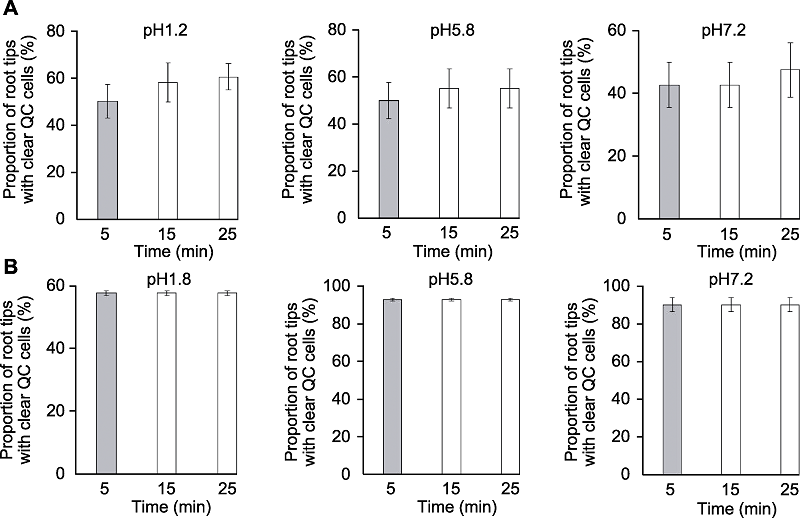
图3 透明时间对拟南芥根尖静止中心(QC)细胞清晰度的影响 分别采用透明液HCG-1 (A)和HCG-2 (B)对拟南芥幼苗透明5、15和25分钟后QC细胞清晰的根尖所占比例。
Figure 3 The effects of clearing times on clarity of quiescent center (QC) cells of Arabidopsis thaliana root tips Proportion of root tips with clear QC cells of Arabidopsis thaliana seedlings cleared by HCG-1 (A) and HCG-2 (B) for 5, 15, 25 min, respectively.
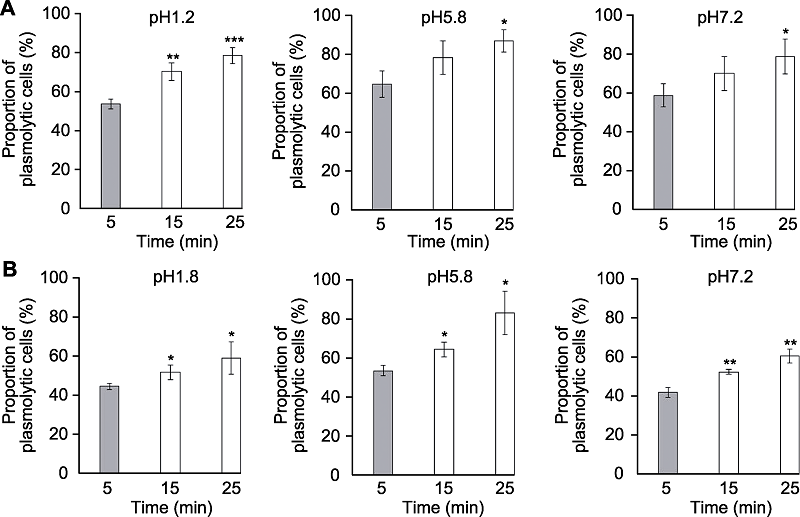
图4 透明时间对拟南芥根尖细胞质壁分离的影响 分别采用透明液HCG-1 (A)和HCG-2 (B)对拟南芥幼苗透明5、15和25分钟后根尖伸长区表皮细胞质壁分离比例(* P<0.05, ** P<0.01, *** P<0.001)。
Figure 4 The effects of clearing times on plasmolysis of Arabidopsis thaliana root tip cells Proportion of plasmolytic cells of Arabidopsis thaliana seedlings cleared by HCG-1 (A) and HCG-2 (B) for 5, 15, 25 min, respectively (* P<0.05, ** P<0.01, *** P<0.001).
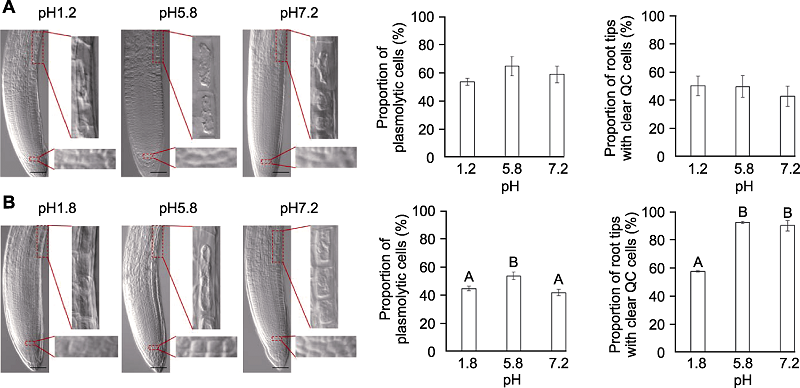
图5 透明液pH值对拟南芥根尖透明的影响 分别采用透明液HCG-1 (A)和HCG-2 (B)在pH1.2/1.8、pH5.8和pH7.2条件下, 对拟南芥幼苗透明5分钟后, 根尖伸长区表皮细胞质壁分离比例和静止中心(QC)细胞清晰的根尖所占比例。不同大写字母表示差异极显著(P<0.01)。Bars=50 μm
Figure 5 The effects of pH values on Arabidopsis thaliana root tip clearing Proportion of plasmolytic cells and proportion of root tips with clear quiescent center (QC) cells of Arabidopsis thaliana seedlings cleared by HCG-1 (A) and HCG-2 (B) for 5 min under pH1.2/1.8, pH5.8, pH7.2, respectively. Different uppercase letters indicate extremely significant differences (P<0.01). Bars=50 μm
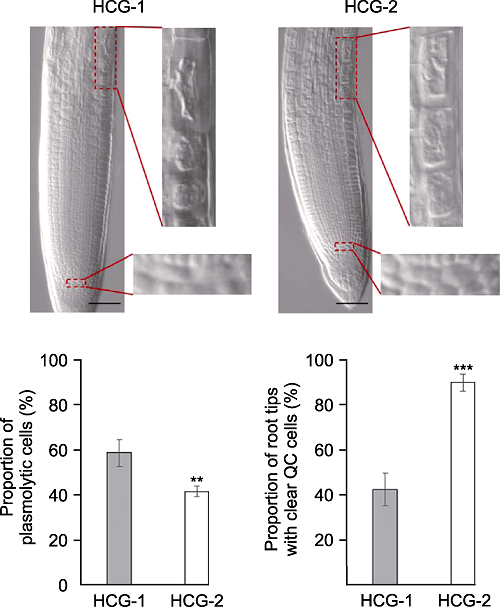
图6 透明液成分配比对拟南芥根尖透明的影响 分别采用透明液HCG-1和HCG-2在pH7.2条件下, 对拟南芥幼苗透明5分钟后, 根尖伸长区表皮细胞质壁分离比例和QC细胞清晰的根尖所占比例(** P<0.01, *** P<0.001)。Bars=50 μm
Figure 6 The comparison of solution HCG-1 with HCG-2 on Arabidopsis thaliana root tip clearing Proportion of plasmolytic cells and proportion of root tips with clear QC cells of Arabidopsis thaliana seedlings cleared by HCG-1 and HCG-2 for 5 min under pH7.2 (** P<0.01, *** P< 0.001). Bars=50 μm
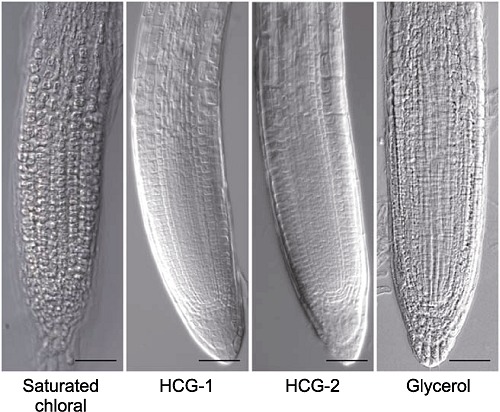
图7 三氯乙醛梯度浓度的根尖透明效果对比 分别采用饱和三氯乙醛溶液、透明液HCG-1、透明液HCG-2和25%甘油溶液对拟南芥幼苗透明5分钟后的根尖形态。Bars= 50 μm
Figure 7 The comparison of root tips cleared by gradient concentrations of chloral solutions Morphology of root tips of Arabidopsis thaliana seedlings cleared by saturated chloral, HCG-1, HCG-2 and 25% glycerol solutions for 5 min, respectively. Bars=50 μm
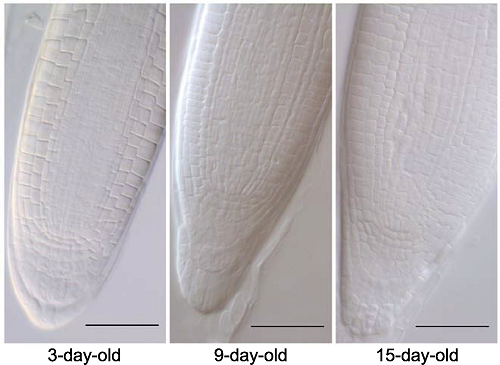
图8 用不同苗龄拟南芥验证优选透明方案 采用透明液HCG-2 (pH7.2)对拟南芥3、9和15日龄幼苗根尖透明5分钟。Bars=50 μm
Figure 8 Arabidopsis thaliana seedlings in different growth stages were used to identify the optimized protocol 3-day-old, 9-day-old and 15-day-old seedlings of Arabidopsis thaliana were cleared by HCG-2 (pH7.2) for 5 min. Bars=50 μm
| [1] | 程蔚玲, 丁兰, 李金平, 刘国安, 杨宁 ( 2017). Leukamenin E调节拟南芥幼苗生长发育的模式及其机制. 生态学杂志 36, 676-686. |
| [2] | 郝建华, 强胜 ( 2007). 整体透明技术在植物生物学中的应用实例及其剖析. 植物学通报 24, 490-497. |
| [3] | 李芳芳, 杨娜, 钱猛, 甘立军 ( 2018). 生长素参与三十烷醇诱导的拟南芥侧根发育. 南京农业大学学报 41, 473-480. |
| [4] | 李彦坤, 臧巩固, 赵立宁, 李育君, 唐蜻, 程超华 ( 2011). 整体透明技术观察悬铃叶苎麻胚胎发育的方法研究. 中国麻业科学 33, 142-146. |
| [5] | 任媛媛, 朱炎 ( 2017). INO80参与拟南芥气孔数量调控的分子机制. 复旦学报(自然科学版) 56, 653-661. |
| [6] | 王培新, 张丹, 尚爱加, 侯冰 ( 2016). 组织透明技术. 神经解剖学杂志 32, 124-128. |
| [7] | 王文婧, 刘婷, 郭磊, 刘春明 ( 2011). SLC/AGO1基因控制拟南芥细胞分裂与定向伸长. 植物学报 46, 370-378. |
| [8] | 杨弘远 ( 1986). 用整体染色与透明技术观察胚囊、胚、胚乳和胚状体. 植物学报 28, 575-581. |
| [9] | 杨弘远 ( 1988). 植物胚胎学中的整体透明技术. 植物学通报 5(2), 114-116. |
| [10] | 于明明, 李兴国, 张宪省 ( 2009). APETALA1启动子驱动AtIPT4在转基因拟南芥中表达导致花和花器官发育异常. 植物学报 44, 59-68. |
| [11] | 赵林姝, 刘录祥, 古佳玉, 郭会君, 李军辉, 谢永盾, 赵世荣 ( 2014). 一种小麦叶片气孔保卫细胞观察样品的制备方法. 植物学报 49, 120-126. |
| [12] |
Beemster GTS, Baskin TI ( 1998). Analysis of cell division and elongation underlying the developmental acceleration of root growth in Arabidopsis thaliana. Plant Physiol 116, 1515-1526.
DOI URL PMID |
| [13] |
Bougourd S, Marrison J, Haseloff J ( 2000). An aniline blue staining procedure for confocal microscopy and 3D imaging of normal and perturbed cellular phenotypes in mature Arabidopsis embryos. Plant J 24, 543-550.
URL PMID |
| [14] |
Bruzzese E, Hasan S ( 1983). A whole leaf clearing and staining technique for host specificity studies of rust fungi. Plant Pathol 32, 335-338.
DOI URL |
| [15] | Crane CF ( 1978). Apomixis and Crossing Incompatibilities in Some Zephyrantheae. Ph.D. thesis. Austin: University of Texas. |
| [16] |
Derbyshire P, Findlay K, McCann MC, Roberts K ( 2007). Cell elongation in Arabidopsis hypocotyls involves dynamic changes in cell wall thickness. J Exp Bot 58, 2079-2089.
DOI URL PMID |
| [17] |
Hager A ( 2003). Role of the plasma membrane H+-ATPase in auxin-induced elongation growth: historical and new aspects. J Plant Res 116, 483-505.
DOI URL PMID |
| [18] |
Haseloff J ( 2003). Old botanical techniques for new microscopes. BioTechniques 34, 1174-1182.
DOI URL PMID |
| [19] |
Herr JMJ ( 1971). A new clearing-squash technique for the study of ovule development in angiosperms. Am J Bot 58, 785-790.
DOI URL |
| [20] | Herr JMJ (1993). Clearing techniques for the study of vascular plant tissues in whole structures and thick sections. In: Tested Studies for Laboratory Teaching. Proceedings of the Fifth Workshop/Conference of the Association for Biology Laboratory Education (ABLE). Toronto: ABLE. pp. 63-84. |
| [21] | Hoyer H ( 1882). Beiträge zur histologischen Technik. 3. Einschlussflüssigkeiten. Biologisches Zentralblatt 2, 23-24. |
| [22] | Itoh K, Nakamura Y, Kawata H, Yamada T, Ohta E, Sakata M ( 1987). Effect of osmotic stress on turgor pressure in mung bean root cells. Plant Cell Physiol 28, 987-994. |
| [23] |
Ivanov VB, Dubrovsky JG ( 2013). Longitudinal zonation pattern in plant roots: conflicts and solutions. Trends Plant Sci 18, 237-243.
DOI URL PMID |
| [24] | Janicka-Russak M (2011). Plant plasma membrane H+- ATPase in adaptation of plants to abiotic stresses. In: Shanker A, ed. Abiotic Stress Response in Plants—Physiological, Biochemical and Genetic Perspectives. Rijeka: Intech Open Press. pp. 197-218. |
| [25] |
Kim YX, Stumpf B, Sung J, Lee SJ ( 2018). The relationship between turgor pressure change and cell hydraulics of midrib parenchyma cells in the leaves of Zea mays. Cells 7, 180.
DOI URL |
| [26] |
Kurihara D, Mizuta Y, Sato Y, Higashiyama T ( 2015). ClearSee: a rapid optical clearing reagent for whole-plant fluorescence imaging. Development 142, 4168-4179.
DOI URL PMID |
| [27] |
Lang I, Sassmann S, Schmidt B, Komis G ( 2014). Plasmolysis: loss of turgor and beyond. Plants (Basel) 3, 583-593.
DOI URL PMID |
| [28] |
Lersten NR ( 1986). Modified clearing method to show sieve tubes in minor veins of leaves. Stain Technol 61, 231-234.
DOI URL PMID |
| [29] |
Li SP, Chen M, Yu DL, Ren SC, Sun SF, Liu LD, Ketelaar T, Emons AMC, Liu CM ( 2013). EXO70A1-mediated vesicle trafficking is critical for tracheary element development in Arabidopsis. Plant Cell 25, 1774-1786.
URL PMID |
| [30] |
Li SP, van Os GMA, Ren SC, Yu DL, Ketelaar T, Emons AMC, Liu CM ( 2010). Expression and functional analyses of EXO70 genes in Arabidopsis implicate their roles in regulating cell type-specific exocytosis. Plant Physiol 154, 1819-1830.
DOI URL PMID |
| [31] |
Liberato JR, Barreto RW, Shivas RG ( 2005). Leaf-clearing and staining techniques for the observation of conidiophores in the Phyllactinioideae (Erysiphaceae). Australas Plant Pathol 34, 401-404.
DOI URL |
| [32] |
Pavelescu I, Vilarrasa-Blasi J, Planas-Riverola A, González-García MP, Caño-Delgado AI, Ibañes M ( 2018). A Sizer model for cell differentiation in Arabidopsis thaliana root growth. Mol Syst Biol 14, e7687.
DOI URL PMID |
| [33] |
Richmond PA, Métraux JP, Taiz L ( 1980). Cell expansion patterns and directionality of wall mechanical properties in Nitella. Plant Physiol 65, 211-217.
DOI URL PMID |
| [34] |
Shabala S, Babourina O, Newman I ( 2000). Ion-specific mechanisms of osmoregulation in bean mesophyll cells. J Exp Bot 51, 1243-1253.
URL PMID |
| [35] |
Shabala SN, Lew RR ( 2002). Turgor regulation in osmotically stressed Arabidopsis epidermal root cells. Direct support for the role of inorganic ion uptake as revealed by concurrent flux and cell turgor measurements. Plant Physiol 129, 290-299.
DOI URL PMID |
| [36] |
Takatsuka H, Umeda M ( 2014). Hormonal control of cell division and elongation along differentiation trajectories in roots. J Exp Bot 65, 2633-2643.
DOI URL PMID |
| [37] |
Ursache R, Andersen TG, Marhavý P, Geldner N ( 2018). A protocol for combining fluorescent proteins with histological stains for diverse cell wall components. Plant J 93, 399-412.
URL PMID |
| [38] |
Verbelen JP, de Cnodder T, Le J, Vissenberg K, Baluška F ( 2006). The root apex of Arabidopsis thaliana consists of four distinct zones of growth activities. Plant Signal Behav 1, 296-304.
DOI URL PMID |
| [39] |
Villani TS, Koroch AR, Simon JE ( 2013). An improved clearing and mounting solution to replace chloral hydrate in microscopic applications. Appl Plant Sci 1, apps. 1300016.
DOI URL |
| [1] | 刘雨函, 曹启江, 张诗晗, 李益慧, 王菁, 谭晓萌, 刘筱儒, 王显玲. 拟南芥AtFTCD-L参与根系响应土壤紧实度的机制研究[J]. 植物学报, 2025, 60(4): 1-0. |
| [2] | 景艳军, 林荣呈. 蓝光受体CRY2化身“暗黑舞者”[J]. 植物学报, 2024, 59(6): 878-882. |
| [3] | 罗燕, 刘奇源, 吕元兵, 吴越, 田耀宇, 安田, 李振华. 拟南芥光敏色素突变体种子萌发的光温敏感性[J]. 植物学报, 2024, 59(5): 752-762. |
| [4] | 陈艳晓, 李亚萍, 周晋军, 解丽霞, 彭永彬, 孙伟, 和亚男, 蒋聪慧, 王增兰, 郑崇珂, 谢先芝. 拟南芥光敏色素B氨基酸位点突变对其结构与功能的影响[J]. 植物学报, 2024, 59(3): 481-494. |
| [5] | 杨继轩, 王雪霏, 顾红雅. 西藏野生拟南芥开花时间变异的遗传基础[J]. 植物学报, 2024, 59(3): 373-382. |
| [6] | 王钢, 王二涛. “卫青不败由天幸”——WeiTsing的广谱抗根肿病机理被揭示[J]. 植物学报, 2023, 58(3): 356-358. |
| [7] | 杨永青, 郭岩. 植物细胞质外体pH感受机制的解析[J]. 植物学报, 2022, 57(4): 409-411. |
| [8] | 支添添, 周舟, 韩成云, 任春梅. PAD4突变加速拟南芥酪氨酸降解缺陷突变体sscd1的程序性细胞死亡[J]. 植物学报, 2022, 57(3): 288-298. |
| [9] | 马灵玉, 祁晓红, 胡子建, 沈微微, 王广超, 张柏林, 张曦, 林金星. 光学透明技术在植物多尺度成像中的应用[J]. 植物学报, 2022, 57(1): 98-110. |
| [10] | 李艳艳, 齐艳华. 植物Aux/IAA基因家族生物学功能研究进展[J]. 植物学报, 2022, 57(1): 30-41. |
| [11] | 李秋信, 迟伟, 季代丽. CURT1调控类囊体膜弯曲的研究进展[J]. 植物学报, 2021, 56(4): 462-469. |
| [12] | 车永梅, 孙艳君, 卢松冲, 侯丽霞, 范欣欣, 刘新. AtMYB77促进NO合成参与调控干旱胁迫下拟南芥侧根发育[J]. 植物学报, 2021, 56(4): 404-413. |
| [13] | 王婷, 羊欢欢, 赵弘巍, JosefVoglmeir, 刘丽. 蛋白质N-糖基化在拟南芥生长周期中的变化规律及去糖基化对根发育的影响[J]. 植物学报, 2021, 56(3): 262-274. |
| [14] | 林雨晴, 齐艳华. 生长素输出载体PIN家族研究进展[J]. 植物学报, 2021, 56(2): 151-165. |
| [15] | 杜斐, 焦雨铃. WUSCHEL介导的固有免疫: 植物干细胞抵御病毒侵害的新机制[J]. 植物学报, 2020, 55(5): 537-540. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||