

植物学报 ›› 2020, Vol. 55 ›› Issue (4): 421-429.DOI: 10.11983/CBB19244 cstr: 32102.14.CBB19244
张楠1,刘自广2,孙世臣3,刘圣怡4,林建辉1,彭疑芳5,张晓旭1,杨贺1,岑曦1,吴娟1,*( )
)
收稿日期:2019-12-18
接受日期:2020-04-15
出版日期:2020-07-01
发布日期:2020-05-21
通讯作者:
吴娟
基金资助:
Nan Zhang1,Ziguang Liu2,Shichen Sun3,Shengyi Liu4,Jianhui Lin1,Yifang Peng5,Xiaoxu Zhang1,He Yang1,Xi Cen1,Juan Wu1,*( )
)
Received:2019-12-18
Accepted:2020-04-15
Online:2020-07-01
Published:2020-05-21
Contact:
Juan Wu
摘要: 长链非编码RNA (lncRNA)是一类长度大于200个核苷酸且不编码蛋白质的非编码RNA, 主要由RNA聚合酶II转录生成, 大量存在于生物体内并具有多种生物学功能。AtR8 lncRNA是拟南芥(Arabidopsis thaliana)中RNA聚合酶III转录的长链非编码RNA。前期研究发现, 水杨酸(SA)处理诱导萌发种子中AtR8 lncRNA的表达, AtR8 lncRNA缺失抑制SA胁迫下的种子萌发。进一步研究发现, AtR8 lncRNA转录区域内存在保守的盐胁迫响应元件(TCTTCTTCTTTA); NaCl处理抑制萌发种子中AtR8 lncRNA的表达; 与野生型相比, 高浓度NaCl处理明显抑制了atr8 (AtR8 lncRNA部分缺失型拟南芥)种子萌发。研究结果表明, AtR8 lncRNA在拟南芥种子萌发期的盐胁迫中起重要作用。
张楠,刘自广,孙世臣,刘圣怡,林建辉,彭疑芳,张晓旭,杨贺,岑曦,吴娟. 拟南芥AtR8 lncRNA对盐胁迫响应及其对种子萌发的调节作用. 植物学报, 2020, 55(4): 421-429.
Nan Zhang,Ziguang Liu,Shichen Sun,Shengyi Liu,Jianhui Lin,Yifang Peng,Xiaoxu Zhang,He Yang,Xi Cen,Juan Wu. Response of AtR8 lncRNA to Salt Stress and Its Regulation on Seed Germination in Arabidopsis. Chinese Bulletin of Botany, 2020, 55(4): 421-429.
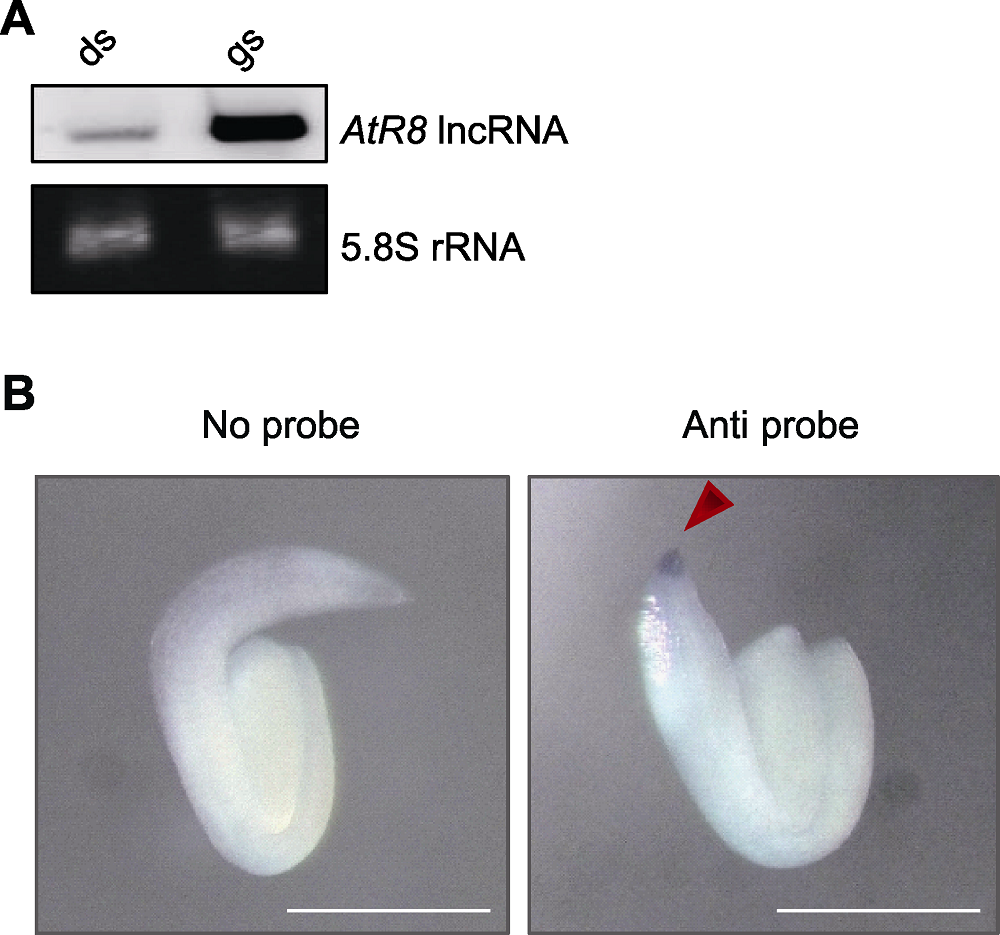
图1 拟南芥种子萌发过程中AtR8 lncRNA的表达特性分析 (A) 拟南芥种子萌发过程中AtR8 lncRNA表达特性的Northern分析(以5.8S rRNA作为上样对照); (B) 拟南芥种子萌发过程中AtR8 lncRNA组织表达特性的整体原位杂交(箭头指示AtR8 lncRNA信号) (Bars=200 μm)。
Figure 1 Analysis of AtR8 lncRNA expression during seed germination in Arabidopsis thaliana (A) Northern blotting analysis of AtR8 lncRNA expression during seed germination (expression of 5.8S rRNA serves as loading controls); (B) In situ hybridization of AtR8 lncRNA expression during seed germination (arrow indicates AtR8 lncRNA signal) (Bars=200 μm).
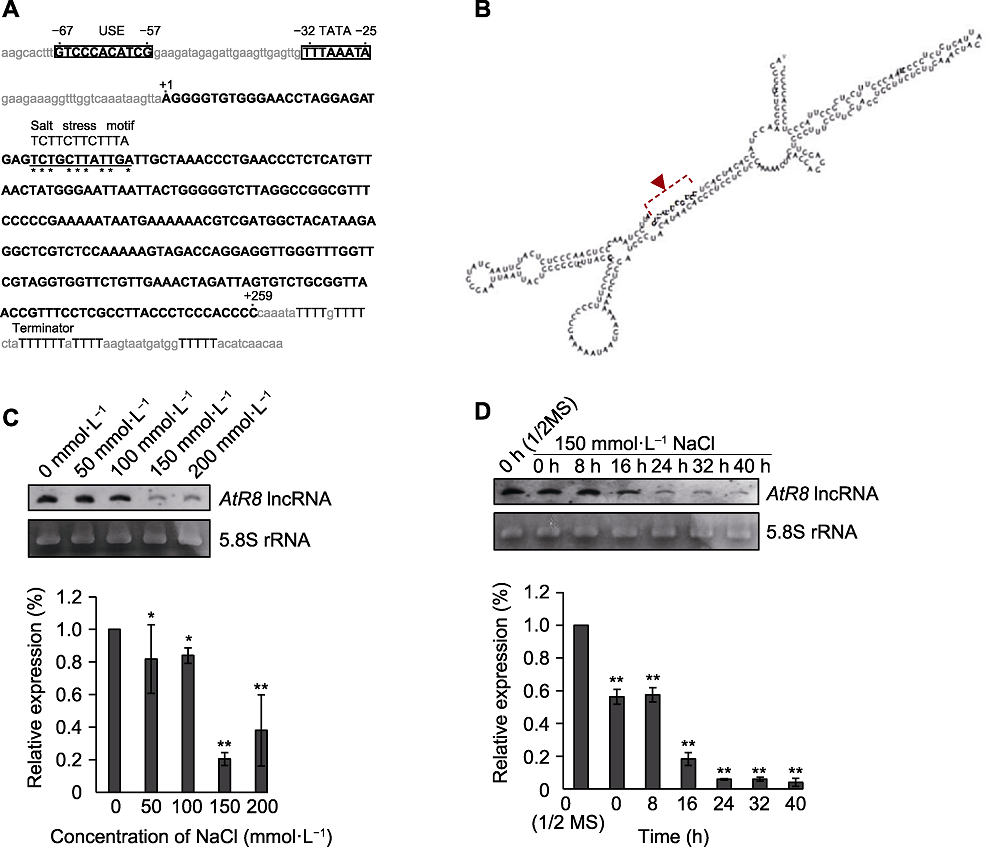
图2 拟南芥种子萌发过程中AtR8 lncRNA表达响应NaCl逆境胁迫 (A) AtR8 lncRNA与UCC盐胁迫响应元件的序列比较分析(USE、TATA启动子序列为大写、加粗并加框, AtR8 lncRNA转录区域为大写并加粗, 保守的盐胁迫响应元件用星号标注)。(B) AtR8 lncRNA二级结构中盐胁迫响应元件存在位置的RNAlogo预测(盐胁迫响应元件为大写、加粗并用箭头指出)。(C) 拟南芥种子萌发过程中不同浓度NaCl处理下AtR8 lncRNA表达特性的Northern分析, 以5.8S rRNA作为上样对照。下方为Northern半定量分析, 2个独立的实验给出了相似的结果, 并显示了1个代表性的例子。值为平均值±标准误(t-检验, *P<0.05, **P<0.01)。(D) 拟南芥种子萌发过程中150 mmol·L-1 NaCl处理不同时间AtR8 lncRNA表达特性的Northern分析, 以5.8S rRNA作为上样对照。下方为Northern半定量分析, 2个独立的实验给出了相似的结果, 并显示了1个代表性的例子。值为平均值±标准误(t-检验, **P<0.01)。
Figure 2 AtR8 lncRNA expression during seed germination of Arabidopsis thaliana after NaCl treatment (A) Sequence comparison between AtR8 lncRNA and UCC salt stress-responsive element (the USE and TATA promoter sequences are capitalized, bloded and framed; the AtR8 lncRNA transcriptional region is capitalized and bolded; and the conserved salt stress-responsive element is marked with asterisk). (B) The location of the salt stress-responsive element in the secondary structure of AtR8 lncRNA predicted by RNAlogo (the salt stress-responsive motif is capitalized, bloded and indicated with an arrow). (C) Northern blotting analysis of AtR8 lncRNA expression in germinating seeds under different NaCl treatments, 5.8S rRNA was used as a loading control. The lower panel shows semi-quantitative analysis of the Northern blotting signals. Two independent experiments gave similar results, and a representative example is shown. Values are means ± SE (t-test, *P<0.05, **P<0.01). (D) Northern blotting analysis of AtR8 lncRNA in germinating seeds under different periods of 150 mmol·L-1 NaCl treatment, 5.8S rRNA was used as a loading control. The lower panel shows semi-quantitative analysis of the Northern blotting signals. Two independent experiments gave similar results, and a representative example is shown. Values are means ± SE (t-test, **P<0.01).
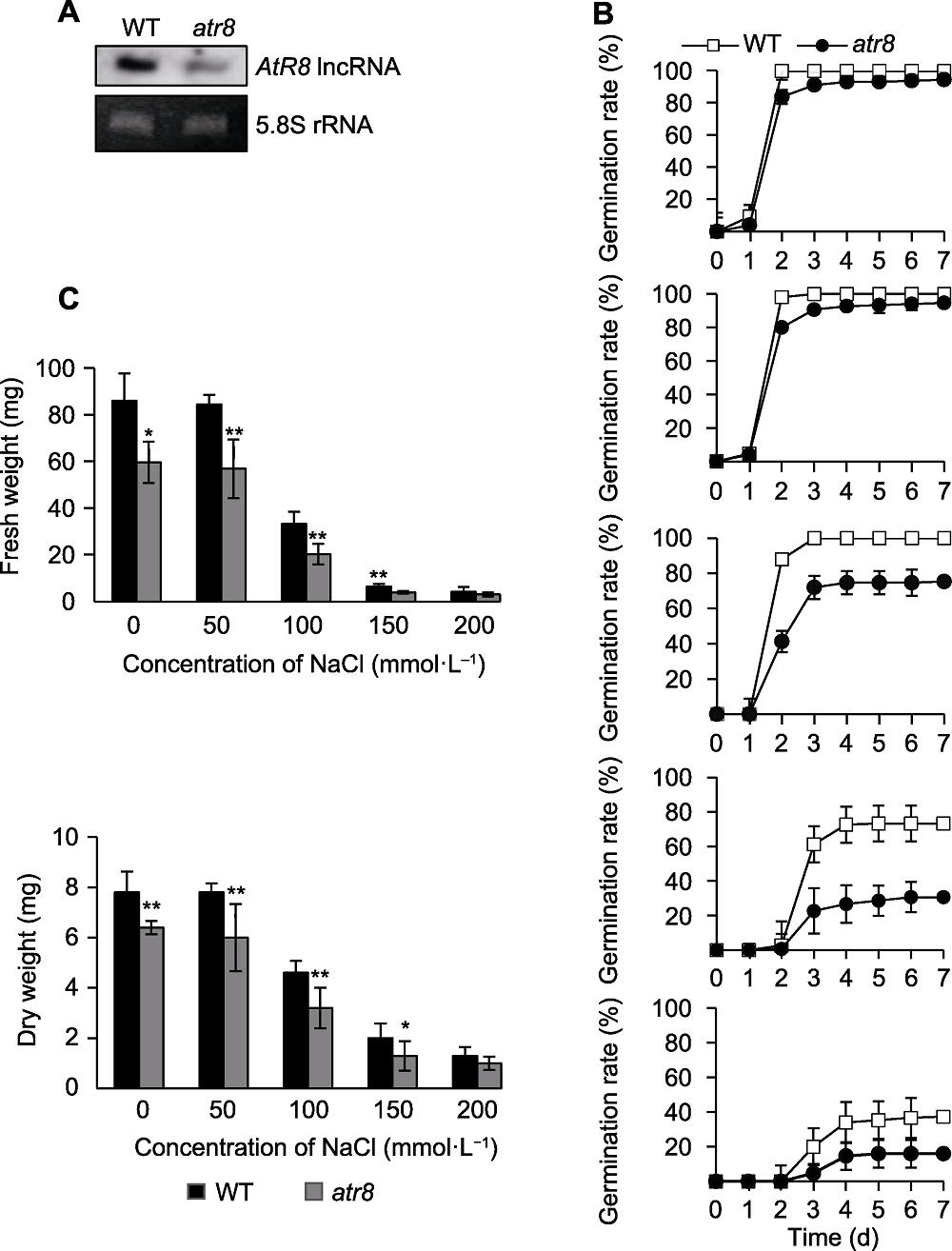
图3 盐胁迫下AtR8 lncRNA缺失抑制拟南芥种子萌发 (A) Northern分析鉴定AtR8 lncRNA缺失突变体atr8 (以5.8S rRNA作为上样对照); (B) 不同浓度NaCl处理下, 野生型和atr8种子生长状况及萌发率统计分析(数据为3次独立试验的平均值, 误差为标准误); (C) 不同浓度NaCl处理下, 野生型和atr8萌发7天种子的鲜重及干重(数值为3次独立试验的平均值, 误差为标准误, 星号表示atr8与野生型的显著性差异(t-检验, *P<0.05, **P<0.01))。WT: 野生型
Figure 3 Loss of AtR8 lncRNA inhibits Arabidopsis thaliana seed germination under salt stress (A) Northern blotting analysis of the AtR8 lncRNA loss-of-function mutant atr8 (5.8S rRNA was used as the loading control); (B) Statistical analysis of growth and germination rate of the wild-type and atr8 seeds under different concentrations of NaCl (data are average of three independent experiments, and bars indicate standard error); (C) Fresh and dry weight of the wild-type and atr8 seeds after 7 d of germination under NaCl treatment (the values are average of three independent experiments, and bars indicate standard error (the asterisk indicate significant differences between atr8 and wild type (t-test, * P < 0.05, ** P < 0.01)). WT: Wild type
| [1] | 陈洁, 林栖凤 (2003). 植物耐盐生理及耐盐机理研究进展. 海南大学学报(自然科学版) 21(2), 177-182. |
| [2] | 窦伟 (2010). 硫化氢对盐和铝胁迫下小麦种子萌发及氧化损伤的缓解效应. 硕士论文. 合肥: 合肥工业大学. pp. 28. |
| [3] | 韩志平, 张海霞, 周凤 (2015). 盐胁迫对植物的影响及植物对盐胁迫的适应性. 山西大同大学学报(自然科学版) 31(3), 59-62. |
| [4] | 郝雪峰, 高惠仙, 燕平梅, 李晓春, 李珊珊 (2013). 盐胁迫对大豆种子萌发及生理的影响. 湖北农业科学 52, 1263-1266. |
| [5] | 黄小庆, 李丹丹, 吴娟 (2015). 植物长链非编码RNA研究进展. 遗传 37, 344-359. |
| [6] | 刘春晓, 黄小庆, 刘自广, 彭疑芳, 李丹丹, 张晓旭, 李爽, 汤川泰, 吴娟 (2019). 十字花科植物种子低分子RNA提取方法比较. 基因组学与应用生物学 38, 1236-1241. |
| [7] | 陆玉建, 高春明, 郑香峰, 钮松召 (2012). 盐胁迫对拟南芥种子萌发的影响. 湖北农业科学 51, 5099-5104. |
| [8] | 乔慧萍, 李建设, 雍立华, 艾凤舞 (2007). 植物盐胁迫生理及其适应性调控机制的研究进展. 宁夏农林科技 (3), 34-36, 24. |
| [9] | 苏永全, 吕迎春 (2007). 盐分胁迫对植物的影响研究简述. 甘肃农业科技 (3), 23-27. |
| [10] | 孙兰菊, 岳国峰, 王金霞, 周百成 (2001). 植物耐盐机制的研究进展. 海洋科学 25(4), 28-31. |
| [11] | 王泳超 (2016). γ-氨基丁酸(GABA)调控盐胁迫下玉米种子萌发和幼苗生长的机制. 博士论文. 哈尔滨: 东北农业大学. pp. 73. |
| [12] | 张新宇, 赵兰杰, 李艳军, 孙杰, 刘永昌 (2014). 盐胁迫对拟南芥AtPUB18基因的诱导表达及其启动子分析. 西北植物学报 34, 54-59. |
| [13] |
Bergler J, Hoth S (2011). Plant U-box armadillo repeat proteins AtPUB18 and AtPUB19 are involved in salt inhibition of germination in Arabidopsis. Plant Biol 13, 725-730.
DOI URL PMID |
| [14] |
Di C, Yuan JP, Wu Y, Li JR, Lin HX, Hu L, Zhang T, Qi YJ, Gerstein MB, Guo Y, Lu ZJ (2014). Characterization of stress-responsive lncRNAs in Arabidopsis thaliana by integrating expression, epigenetic and structural features. Plant J 80, 848-861.
DOI URL PMID |
| [15] |
Ding JH, Lu Q, Ouyang YD, Mao HL, Zhang PB, Yao JL, Xu CG, Li XH, Xiao JH, Zhang QF (2012). A long noncoding RNA regulates photoperiod-sensitive male sterility, an essential component of hybrid rice. Proc Natl Acad Sci USA 109, 2654-2659.
DOI URL PMID |
| [16] |
Ding ZJ, Yan JY, Li GX, Wu ZC, Zhang SQ, Zheng SJ (2014). WRKY41 controls Arabidopsis seed dormancy via direct regulation of ABI3 transcript levels not downstream of ABA. Plant J 79, 810-823.
URL PMID |
| [17] |
Franco-Zorrilla JM, Valli A, Todesco M, Mateos I, Puga MI, Rubio-Somoza I, Leyva A, Weigel D, García JA, Paz-Ares J (2007). Target mimicry provides a new me- chanism for regulation of microRNA activity. Nat Genet 39, 1033-1037.
DOI URL PMID |
| [18] |
Guo GH, Liu XY, Sun FL, Cao J, Huo N, Wuda B, Xin MM, Hu ZR, Du JK, Xia R, Rossi V, Peng HR, Ni ZF, Sun QX, Yao YY (2018). Wheat miR9678 affects seed germination by generating phased siRNAs and modulating abscisic acid/gibberellin signaling. Plant Cell 30, 796-814.
DOI URL PMID |
| [19] |
Heo JB, Sung S (2011). Vernalization-mediated epigenetic silencing by a long intronic noncoding RNA. Science 331, 76-79.
URL PMID |
| [20] |
Huang Y, Feng CZ, Ye Q, Wu WH, Chen YF (2016). Arabidopsis WRKY6 transcription factor acts as a positive regulator of abscisic acid signaling during seed germination and early seedling development. PLoS Genet 12, e1005833.
DOI URL PMID |
| [21] |
Jiang WB, Yu DQ (2009). Arabidopsis WRKY2 transcription factor mediates seed germination and postgermination arrest of development by abscisic acid. BMC Plant Biol 9, 96.
URL PMID |
| [22] |
Kim DH, Sung S (2012). Environmentally coordinated epigenetic silencing of FLC by protein and long noncoding RNA components. Curr Opin Plant Biol 15, 51-56.
DOI URL PMID |
| [23] | Li DD, Huang XQ, Liu ZG, Li S, Okada T, Yukawa Y, Wu J (2016). Effect of AtR8 lncRNA partial deletion on Arabidopsis seed germination. Mol Soil Biol 7, 1-7. |
| [24] |
Liu F, Xu YR, Chang KX, Li SN, Liu ZG, Qi SD, Jia JB, Zhang M, Crawford NM, Wang Y (2019). The long noncoding RNA T5120 regulates nitrate response and assimilation in Arabidopsis. New Phytol 224, 117-131.
DOI URL PMID |
| [25] | Martin R, Liu PP, Nonogaki H (2005). Simple purification of small RNAs from seeds and efficient detection of multiple microRNAs expressed in Arabidopsis thaliana and tomato(Lycopersicon esculentum) seeds. Seed Sci Res 15, 319-328. |
| [26] | Martin RC, Asahina M, Liu PP, Kristof JR, Coppersmith JL, Pluskota WE, Bassel GW, Goloviznina NA, Nguyen TT, Martínez-Andújar C, Arun Kumar MB, Pupel P, Nonogaki H (2010a). The regulation of post-germinative transition from the cotyledon- to vegetative-leaf stages by microRNA-targeted SQUAMOSA PROMOTER-BINDING PROTEIN LIKE13 in Arabidopsis. Seed Sci Res 20, 89-96. |
| [27] | Martin RC, Asahina M, Liu PP, Kristof JR, Coppersmith JL, Pluskota WE, Bassel GW, Goloviznina NA, Nguyen TT, Martínez-Andújar C, Arun Kumar MB, Pupel P, Nonogaki H (2010b). The microRNA156 and microRNA172 gene regulation cascades at post-germinative stages in Arabidopsis. Seed Sci Res 20, 79-87. |
| [28] |
Qin T, Zhao HY, Cui P, Albesher N, Xiong LM (2017). A nucleus-localized long non-coding RNA enhances drought and salt stress tolerance. Plant Physiol 175, 1321-1336.
DOI URL PMID |
| [29] |
Reyes JL, Chua NH (2007). ABA induction of miR159 controls transcript levels of two MYB factors during Arabidopsis seed germination. Plant J 49, 592-606.
DOI URL PMID |
| [30] |
Rhoades MW, Reinhart BJ, Lim LP, Burge CB, Bartel B, Bartel DP (2002). Prediction of plant microRNA targets. Cell 110, 513-520.
DOI URL PMID |
| [31] |
Shkolnik D, Finkler A, Pasmanik-Chor M, Fromm H (2019). CALMODULIN-BINDING TRANSCRIPTION ACTIVATOR 6: a key regulator of Na+ homeostasis during germination. Plant Physiol 180, 1101-1118.
DOI URL PMID |
| [32] |
Swiezewski S, Liu FQ, Magusin A, Dean C (2009). Cold-induced silencing by long antisense transcripts of an Arabidopsis polycomb target. Nature 462, 799-802.
DOI URL PMID |
| [33] |
Wang AH, Hu JH, Gao CB, Chen GL, Wang BC, Lin CF, Song LP, Ding Y, Zhou GL (2019). Genome-wide analysis of long non-coding RNAs unveils the regulatory roles in the heat tolerance of Chinese cabbage (Brassica rapa ssp. chinensis). Sci Rep 9, 5002.
DOI URL PMID |
| [34] |
Wu J, Liu CX, Liu ZG, Li S, Li DD, Liu SY, Huang XQ, Liu SK, Yukawa Y (2019a). Pol III-dependent cabbage BoNR8 long ncRNA affects seed germination and growth in Arabidopsis. Plant Cell Physiol 60, 421-435.
DOI URL PMID |
| [35] |
Wu J, Okada T, Fukushima T, Tsudzuki T, Sugiura M, Yukawa Y (2012). A novel hypoxic stress-responsive long non-coding RNA transcribed by RNA polymerase III in Arabidopsis. RNA Biol 9, 302-313.
DOI URL |
| [36] |
Wu XX, Shi T, Iqbal S, Zhang Y, Liu L, Gao ZH (2019b). Genome-wide discovery and characterization of flower development related long non-coding RNAs in Prunus mume. BMC Plant Biol 19, 64.
DOI URL PMID |
| [37] |
Xu W, Yang TQ, Wang B, Han B, Zhou HK, Wang Y, Li DZ, Liu AZ (2018). Differential expression networks and inheritance patterns of long non-coding RNAs in castor bean seeds. Plant J 95, 324-340.
DOI URL PMID |
| [38] |
Yang BC, Song ZH, Li CN, Jiang JH, Zhou YY, Wang RP, Wang Q, Ni C, Liang Q, Chen HD, Fan LM (2018). RSM1, an Arabidopsis MYB protein, interacts with HY5/ HYH to modulate seed germination and seedling development in response to abscisic acid and salinity. PLoS Genet 14, e1007839.
DOI URL |
| [39] |
Yao WJ, Zhao K, Cheng ZH, Li XY, Zhou BR, Jiang TB (2018). Transcriptome analysis of poplar under salt stress and over-expression of transcription factor NAC57 gene confers salt tolerance in transgenic Arabidopsis. Front Plant Sci 9, 1121.
DOI URL PMID |
| [40] |
Yin DD, Li SS, Shu QY, Gu ZY, Wu Q, Feng CY, Xu WZ, Wang LS (2018). Identification of microRNAs and long non-coding RNAs involved in fatty acid biosynthesis in tree peony seeds. Gene 666, 72-82.
DOI URL PMID |
| [41] |
Zhang GY, Chen DG, Zhang T, Duan AG, Zhang JG, He CY (2018). Transcriptomic and functional analyses unveil the role of long non-coding RNAs in anthocyanin biosynthesis during sea buckthorn fruit ripening. DNA Res 25, 465-476.
URL PMID |
| [42] |
Zhang XP, Dong J, Deng FN, Wang W, Cheng YY, Song LR, Hu MJ, Shen J, Xu QJ, Shen FF (2019). The long non-coding RNA lncRNA 973 is involved in cotton response to salt stress. BMC Plant Biol 19, 459.
DOI URL PMID |
| [43] |
Zhao XY, Li JR, Lian B, Gu HQ, Li Y, Qi YJ (2018). Global identification of Arabidopsis lncRNAs reveals the regulation of MAF4 by a natural antisense RNA. Nat Commun 9, 5056.
DOI URL PMID |
| [44] | Zhu M, Zhang M, Xing LJ, Li WZ, Jiang HY, Wang L, Xu MY (2017). Transcriptomic analysis of long non-coding RNAs and coding genes uncovers a complex regulatory network that is involved in maize seed development. Genes (Basel) 8, 274. |
| [1] | 周鑫宇, 刘会良, 高贝, 卢妤婷, 陶玲庆, 文晓虎, 张岚, 张元明. 新疆特有濒危植物雪白睡莲繁殖生物学研究[J]. , 2025, 49(濒危植物的保护与恢复): 0-. |
| [2] | 刘雨函, 曹启江, 张诗晗, 李益慧, 王菁, 谭晓萌, 刘筱儒, 王显玲. 拟南芥AtFTCD-L参与根系响应土壤紧实度的机制研究[J]. 植物学报, 2025, 60(4): 1-0. |
| [3] | 徐田甜, 杨培建, 周晓茜, 曹怡, 陈艳红, 刘国元, 张健, 魏辉. 紫薇GolS家族基因的理化特性与表达特征[J]. 植物学报, 2025, 60(3): 393-406. |
| [4] | 杜庆国, 李文学. lncRNA调控玉米生长发育和非生物胁迫研究进展[J]. 植物学报, 2024, 59(6): 950-962. |
| [5] | 孙龙, 李文博, 娄虎, 于澄, 韩宇, 胡同欣. 火干扰对兴安落叶松种子萌发的影响[J]. 植物生态学报, 2024, 48(6): 770-779. |
| [6] | 景艳军, 林荣呈. 蓝光受体CRY2化身“暗黑舞者”[J]. 植物学报, 2024, 59(6): 878-882. |
| [7] | 罗燕, 刘奇源, 吕元兵, 吴越, 田耀宇, 安田, 李振华. 拟南芥光敏色素突变体种子萌发的光温敏感性[J]. 植物学报, 2024, 59(5): 752-762. |
| [8] | 袁涵, 钟爱文, 刘送平, 彭焱松, 徐磊. 水毛花种子萌发特性的差异及休眠解除方法[J]. 植物生态学报, 2024, 48(5): 638-650. |
| [9] | 路笃贤, 张严妍, 刘艳, 李岩竣, 左新秀, 林金星, 崔亚宁. 非编码RNA在植物生长发育及逆境响应中的研究进展[J]. 植物学报, 2024, 59(5): 709-725. |
| [10] | 朱晓博, 董张, 祝梦瑾, 胡晋, 林程, 陈敏, 关亚静. 重要的种子储存物质长寿命mRNA[J]. 植物学报, 2024, 59(3): 355-372. |
| [11] | 杨继轩, 王雪霏, 顾红雅. 西藏野生拟南芥开花时间变异的遗传基础[J]. 植物学报, 2024, 59(3): 373-382. |
| [12] | 陈艳晓, 李亚萍, 周晋军, 解丽霞, 彭永彬, 孙伟, 和亚男, 蒋聪慧, 王增兰, 郑崇珂, 谢先芝. 拟南芥光敏色素B氨基酸位点突变对其结构与功能的影响[J]. 植物学报, 2024, 59(3): 481-494. |
| [13] | 杜锦瑜, 孙震, 苏彦龙, 王贺萍, 刘亚玲, 吴振映, 何峰, 赵彦, 付春祥. 蒙古冰草咖啡酸氧甲基转移酶基因AmCOMT1的鉴定及功能分析[J]. 植物学报, 2024, 59(3): 383-396. |
| [14] | 蔡淑钰, 刘建新, 王国夫, 吴丽元, 宋江平. 褪黑素促进镉胁迫下番茄种子萌发的调控机理[J]. 植物学报, 2023, 58(5): 720-732. |
| [15] | 王钢, 王二涛. “卫青不败由天幸”——WeiTsing的广谱抗根肿病机理被揭示[J]. 植物学报, 2023, 58(3): 356-358. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||