

植物学报 ›› 2024, Vol. 59 ›› Issue (1): 22-33.DOI: 10.11983/CBB23025 cstr: 32102.14.CBB23025
仲昭暄1, 张冬瑞1, 李璐1, 苏颖2, 王黛宁1, 王泽冉1, 刘洋1, 常缨1,*( )
)
收稿日期:2023-02-27
接受日期:2023-08-29
出版日期:2024-01-10
发布日期:2024-01-10
通讯作者:
*E-mail: 基金资助:
Zhaoxuan Zhong1, Dongrui Zhang1, Lu Li1, Ying Su2, Daining Wang1, Zeran Wang1, Yang Liu1, Ying Chang1,*( )
)
Received:2023-02-27
Accepted:2023-08-29
Online:2024-01-10
Published:2024-01-10
Contact:
*E-mail: 摘要: 为深入探究miRNA参与调控香鳞毛蕨(Dryopteris fragrans)生长发育的分子机理, 依据实验室前期建立的miRNA数据库, 筛选出与非生物胁迫相关的差异表达dfr-miR160a前体(dfr-pri-mir160a), 预测其靶基因为DfARF10, 并通过本氏烟草(Nicotiana benthamiana)瞬时共转化GUS以及双荧光素酶(LUC)系统验证dfr-miR160a和DfARF10的靶向关系。结果显示, 共注射dfr-pri-mir160a和DfARF10的本氏烟草叶片中GUS活性和LUC活性明显降低; qRT-PCR分析显示, dfr-miR160a及其靶基因DfARF10在香鳞毛蕨的根、配子体、叶柄、叶片和孢子囊中均有表达, 在叶片中表达量最高, 在根中表达量最低; 通过qRT-PCR分析干旱、盐(NaCl)、高温和低温胁迫对dfr-miR160a及其靶基因DfARF10表达的影响。结果表明, 在干旱和高温处理下, dfr-miR160a的表达均上调, 但在NaCl处理下, dfr-miR160a的表达下调; 低温处理下, dfr-miR160a的表达在0-1小时下调, 在3-48小时上调。在NaCl、高温以及低温处理下DfARF10表达均上调; 但在干旱处理下, DfARF10表达下调, 与dfr-miR160a呈现相反的表达趋势。综上, dfr-miR160a靶向DfARF10基因且二者均能响应非生物胁迫。研究结果为从分子层面揭示香鳞毛蕨非生物胁迫抗性机制提供了新的科学依据。
仲昭暄, 张冬瑞, 李璐, 苏颖, 王黛宁, 王泽冉, 刘洋, 常缨. 香鳞毛蕨dfr-miR160a和靶基因DfARF10的生物信息学及表达模式分析. 植物学报, 2024, 59(1): 22-33.
Zhaoxuan Zhong, Dongrui Zhang, Lu Li, Ying Su, Daining Wang, Zeran Wang, Yang Liu, Ying Chang. Bioinformatic and Expression Pattern Analysis of dfr-miR160a and Target Gene DfARF10 in Dryopteris fragrans. Chinese Bulletin of Botany, 2024, 59(1): 22-33.
| Primer name | Sequence (5′-3′) | Purpose |
|---|---|---|
| DfARF10F | ATGCCCGGCCCTTTATCAAC | Gene clone |
| DfARF10R | TCACCTTGTAATGTTTTCACCG | Gene clone |
| dfr-pri-mir160aF | AAAATCACTCTGCCTGGCTC | Gene clone |
| dfr-pri-mir160aR | AGCGAGAAACTCTGCGTGG | Gene clone |
| DfARF10F-EGFP | CTCGGTACCCGGGGATCCATGCCCGGCCCTTTATCAAC | Gene clone |
| DfARF10R-EGFP | GGTGTCGACTCTAGAGGATCCCCTTGTAATGTTTTCACCG | Gene clone |
| pCAMBIA2301-dfr-pri-mir160aF | GGGCATCGATACGGGATCCATAAAATCACTCTGCCTGGCTC | Gene clone |
| pCAMBIA2301-dfr-pri-mir160aR | TCGAGCTCGATGGATCCCGTAAGCGAGAAACTCTGCGTGG | Gene clone |
| pCAMBIA2301-DfARF10F-GUS | GGGCATCGATACGGGATCCATATGCCCGGCCCTTTATCAAC | Gene clone |
| pCAMBIA2301-DfARF10R-GUS | TCGAGCTCGATGGATCCCGTACCTTGTAATGTTTTCACCG | Gene clone |
| pGreenII-dfr-pri-mir160aF | CAGTGGTCTCACACC AAAATCACTCTGCCTGGCTC | Gene clone |
| pGreenII-dfr-pri-mir160aR | CAGTGGTCTCAAGCGAGCGAGAAACTCTGCGTGG | Gene clone |
| pGreenII-LUC-DfARF10F | CAGTGGTCTCAGATCTCCATGGCAAGTGGAGCTA | Gene clone |
| pGreenII-LUC-DfARF10R | CAGTGGTCTCAAATTGTTCACAAGGCTACCCATGTTA | Gene clone |
| Df18sRNAF | GCTTTCGCAGTAGTTCGTCTTTC | qRT-PCR |
| Df18sRNAR | TGGTCCTATTATGTTGGTCTTCGG | qRT-PCR |
| DfARF10F | GCAATGCGGCGGGAGATCTT | qRT-PCR |
| DfARF10R | CAGAGCTCGAGCGCAAAGCC | qRT-PCR |
| dfr-miR160aF | TGCCTGGCTCCCTGTATGCCA | qRT-PCR |
| dfr-miR160aR | mRQ 3′ Primer | qRT-PCR |
表1 引物序列
Table1 Primer sequences
| Primer name | Sequence (5′-3′) | Purpose |
|---|---|---|
| DfARF10F | ATGCCCGGCCCTTTATCAAC | Gene clone |
| DfARF10R | TCACCTTGTAATGTTTTCACCG | Gene clone |
| dfr-pri-mir160aF | AAAATCACTCTGCCTGGCTC | Gene clone |
| dfr-pri-mir160aR | AGCGAGAAACTCTGCGTGG | Gene clone |
| DfARF10F-EGFP | CTCGGTACCCGGGGATCCATGCCCGGCCCTTTATCAAC | Gene clone |
| DfARF10R-EGFP | GGTGTCGACTCTAGAGGATCCCCTTGTAATGTTTTCACCG | Gene clone |
| pCAMBIA2301-dfr-pri-mir160aF | GGGCATCGATACGGGATCCATAAAATCACTCTGCCTGGCTC | Gene clone |
| pCAMBIA2301-dfr-pri-mir160aR | TCGAGCTCGATGGATCCCGTAAGCGAGAAACTCTGCGTGG | Gene clone |
| pCAMBIA2301-DfARF10F-GUS | GGGCATCGATACGGGATCCATATGCCCGGCCCTTTATCAAC | Gene clone |
| pCAMBIA2301-DfARF10R-GUS | TCGAGCTCGATGGATCCCGTACCTTGTAATGTTTTCACCG | Gene clone |
| pGreenII-dfr-pri-mir160aF | CAGTGGTCTCACACC AAAATCACTCTGCCTGGCTC | Gene clone |
| pGreenII-dfr-pri-mir160aR | CAGTGGTCTCAAGCGAGCGAGAAACTCTGCGTGG | Gene clone |
| pGreenII-LUC-DfARF10F | CAGTGGTCTCAGATCTCCATGGCAAGTGGAGCTA | Gene clone |
| pGreenII-LUC-DfARF10R | CAGTGGTCTCAAATTGTTCACAAGGCTACCCATGTTA | Gene clone |
| Df18sRNAF | GCTTTCGCAGTAGTTCGTCTTTC | qRT-PCR |
| Df18sRNAR | TGGTCCTATTATGTTGGTCTTCGG | qRT-PCR |
| DfARF10F | GCAATGCGGCGGGAGATCTT | qRT-PCR |
| DfARF10R | CAGAGCTCGAGCGCAAAGCC | qRT-PCR |
| dfr-miR160aF | TGCCTGGCTCCCTGTATGCCA | qRT-PCR |
| dfr-miR160aR | mRQ 3′ Primer | qRT-PCR |
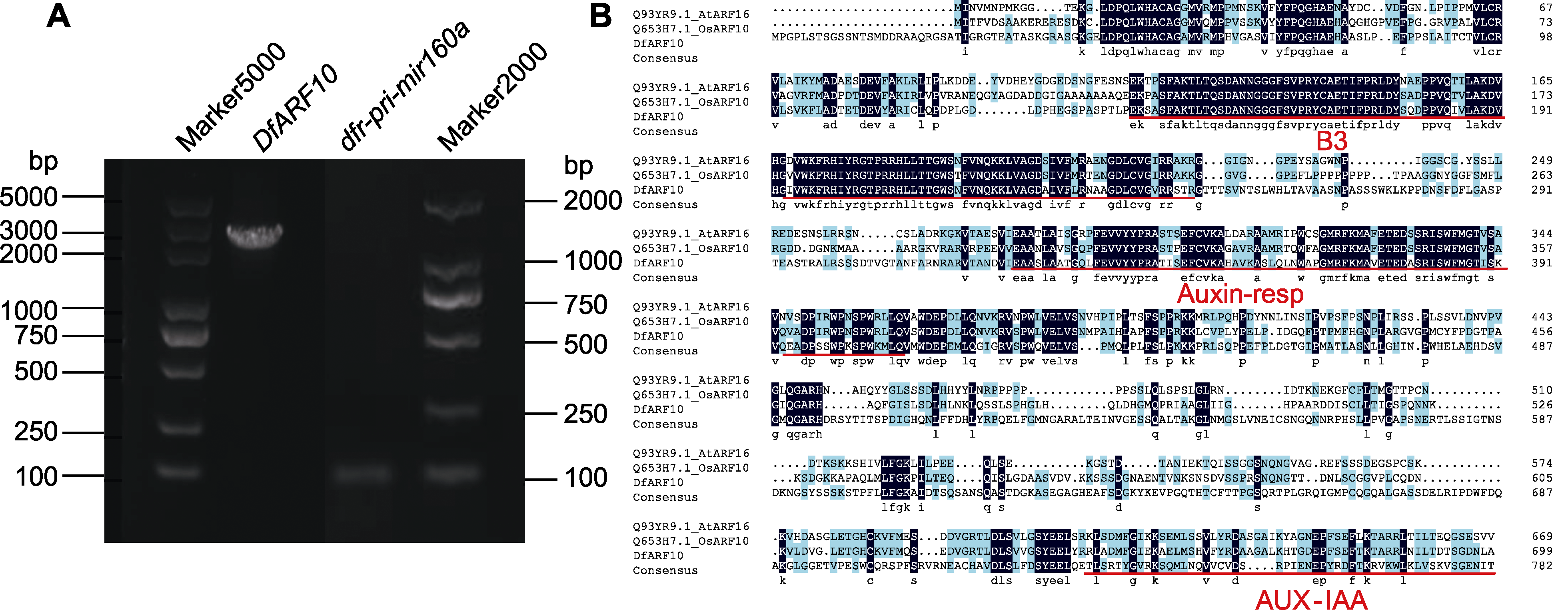
图2 dfr-pri-mir160a和DfARF10基因的RT-PCR扩增(A)以及DfARF10蛋白多序列比对(B) Marker: DNA分子标准品
Figure 2 RT-PCR of dfr-pri-mir160a and DfARF10 gene (A) and multiple sequence alignment of DfARF10 proteins (B) Marker: DNA marker
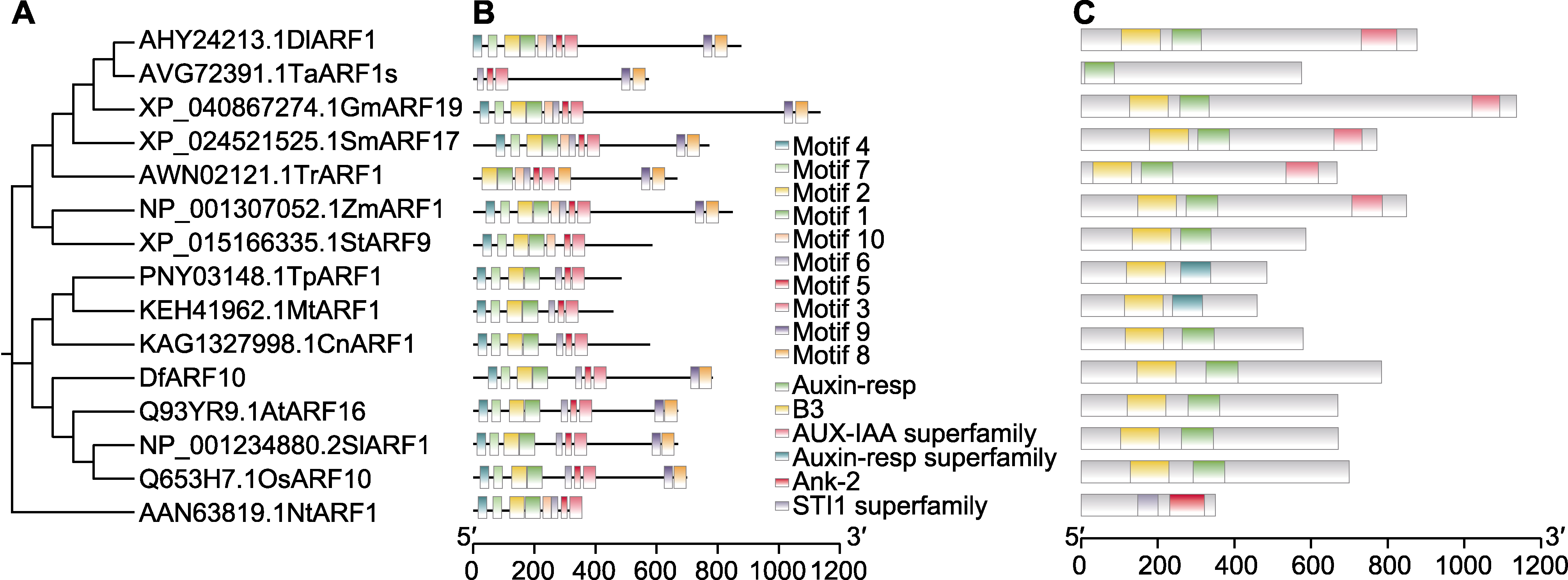
图3 香鳞毛蕨DfARF10蛋白质保守基序分析 (A) DfARF10系统进化树; (B) DfARF10基序分析; (C) DfARF10保守结构域分析
Figure 3 Conservative motif analysis of DfARF10 protein in Dryopteris fragrans (A) Phylogenetic tree of DfARF10 protein; (B) Motif analysis of DfARF10; (C) Conservative domains analysis of DfARF10
| Motif | Sequence | PFAM analysis |
|---|---|---|
| Motif1 | WKFRHIYRGQPRRHLLTTGWSVFVNQKKLVAGDSVVFLRNENGELRVGIR | B3 |
| Motif2 | SFCKTLTASDTNNGGGFSVPRRCAETIFPPLDYSQDPPVQELVAKDVHG | Auxin-resp |
| Motif3 | DPVRWPNSKWRMLQVGWDEPEALZRPKRVSPWZIEPVSAPP | - |
| Motif4 | LWHACAGPLVSIPPVGSKVYYFPQGHAEQ | - |
| Motif5 | GMRFKMAFETEESSRRRYFG | - |
| Motif6 | FEVVYYPRASPSEFVVPAKKV | - |
| Motif7 | PKILCRVLNVKLLADPETDEVYAKITLQP | - |
| Motif8 | WQVVYVDAEGDILLVGDDPWSEFVKTVRRIKILSPEEVQKM | AUX-IAA |
| Motif9 | VGRSLDLSKFSSYEELREELARMFGIEG | - |
| Motif10 | MPSSVISSHSMHIGVLAAAAHAVATNTM | - |
表2 DfARF10基序的序列
Table 2 Motif sequences of DfARF10
| Motif | Sequence | PFAM analysis |
|---|---|---|
| Motif1 | WKFRHIYRGQPRRHLLTTGWSVFVNQKKLVAGDSVVFLRNENGELRVGIR | B3 |
| Motif2 | SFCKTLTASDTNNGGGFSVPRRCAETIFPPLDYSQDPPVQELVAKDVHG | Auxin-resp |
| Motif3 | DPVRWPNSKWRMLQVGWDEPEALZRPKRVSPWZIEPVSAPP | - |
| Motif4 | LWHACAGPLVSIPPVGSKVYYFPQGHAEQ | - |
| Motif5 | GMRFKMAFETEESSRRRYFG | - |
| Motif6 | FEVVYYPRASPSEFVVPAKKV | - |
| Motif7 | PKILCRVLNVKLLADPETDEVYAKITLQP | - |
| Motif8 | WQVVYVDAEGDILLVGDDPWSEFVKTVRRIKILSPEEVQKM | AUX-IAA |
| Motif9 | VGRSLDLSKFSSYEELREELARMFGIEG | - |
| Motif10 | MPSSVISSHSMHIGVLAAAAHAVATNTM | - |
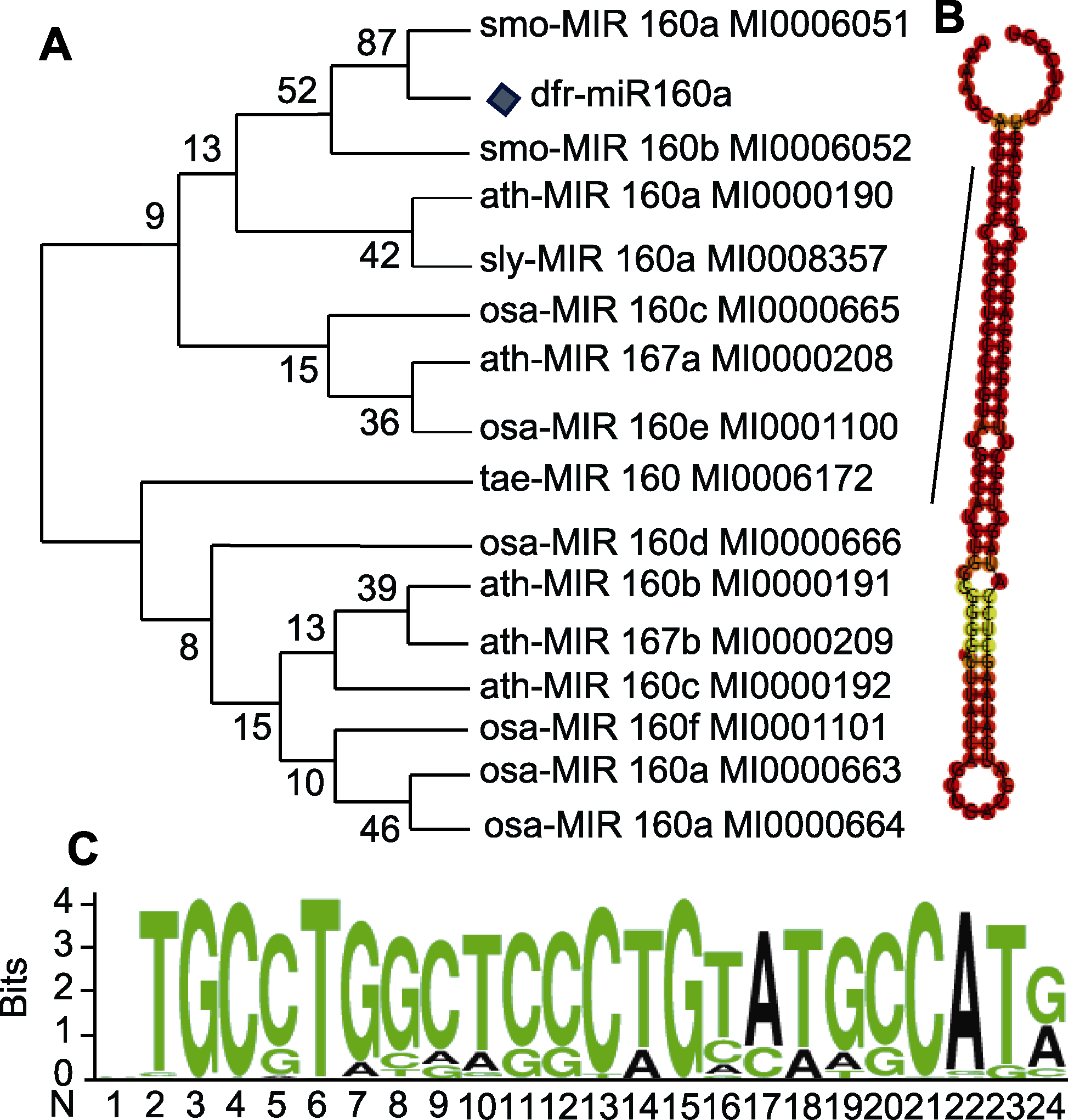
图4 香鳞毛蕨dfr-miR160a保守基序分析 (A) dfr-miR160a系统进化树; (B) dfr-miR160a二级茎环结构 (黑线代表成熟miR160a序列); (C) dfr-miR160a成熟序列碱基保守性分析
Figure 4 Conservative motif analysis of dfr-miR160a in Dryopteris fragrans (A) Phylogenetic tree of dfr-miR160a; (B) Stem-loop structure of dfr-miR160a (black line represents the mature miR160a sequence); (C) Conservative base analysis of dfr-miR160a mature sequence
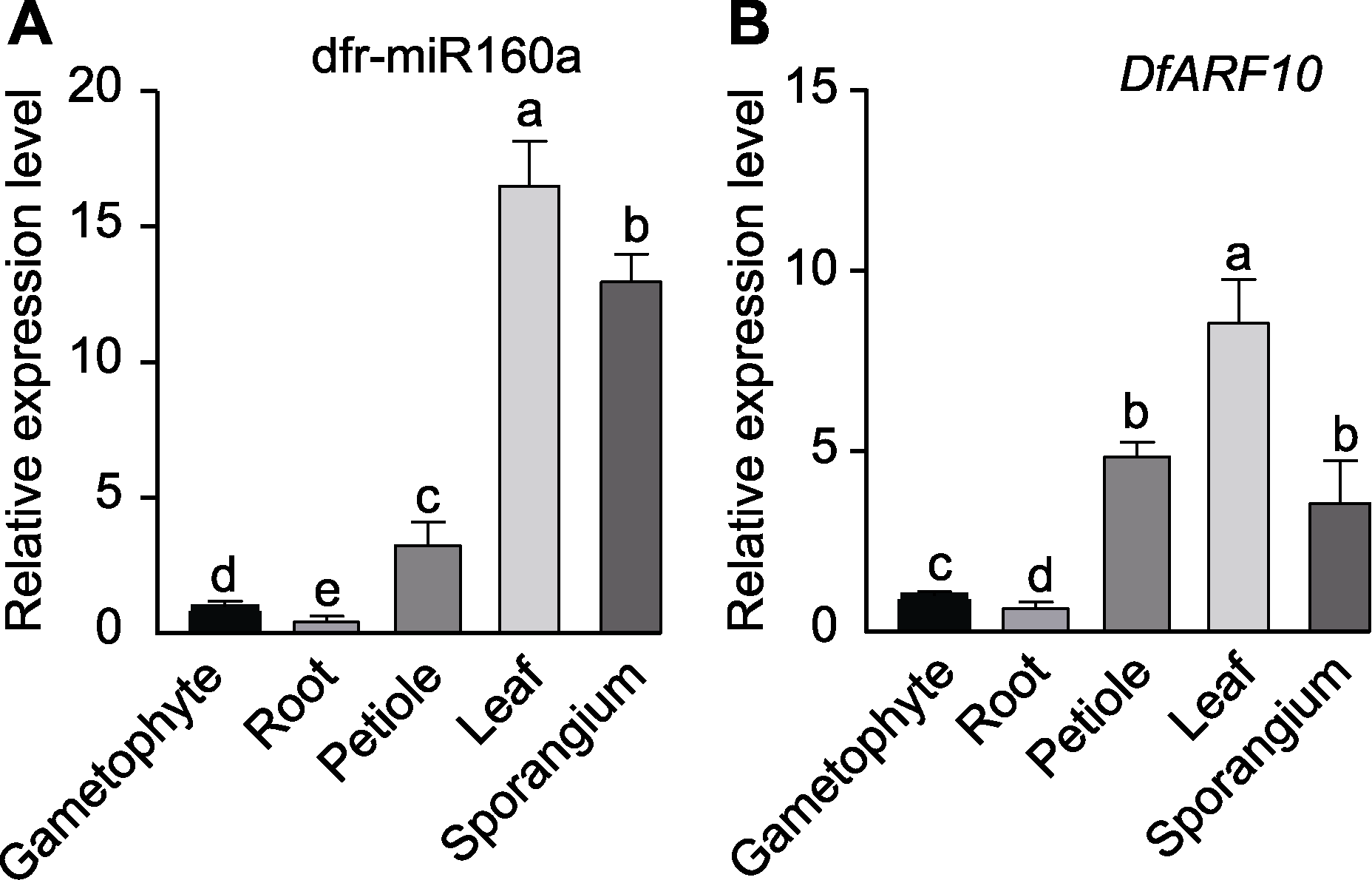
图5 香鳞毛蕨不同组织中dfr-miR160a (A)及其靶基因DfARF10 (B)的相对表达量 P值用One-way ANOVA计算, 不同小写字母表示不同组织间差异显著(P<0.05)。
Figure 5 Relative expression of dfr-miR160a (A) and target gene DfARF10 (B) in different tissues of Dryopteris fragrans P value is calculated with One-way ANOVA, different lowercase letters indicate significant differences among different tissues (P<0.05).
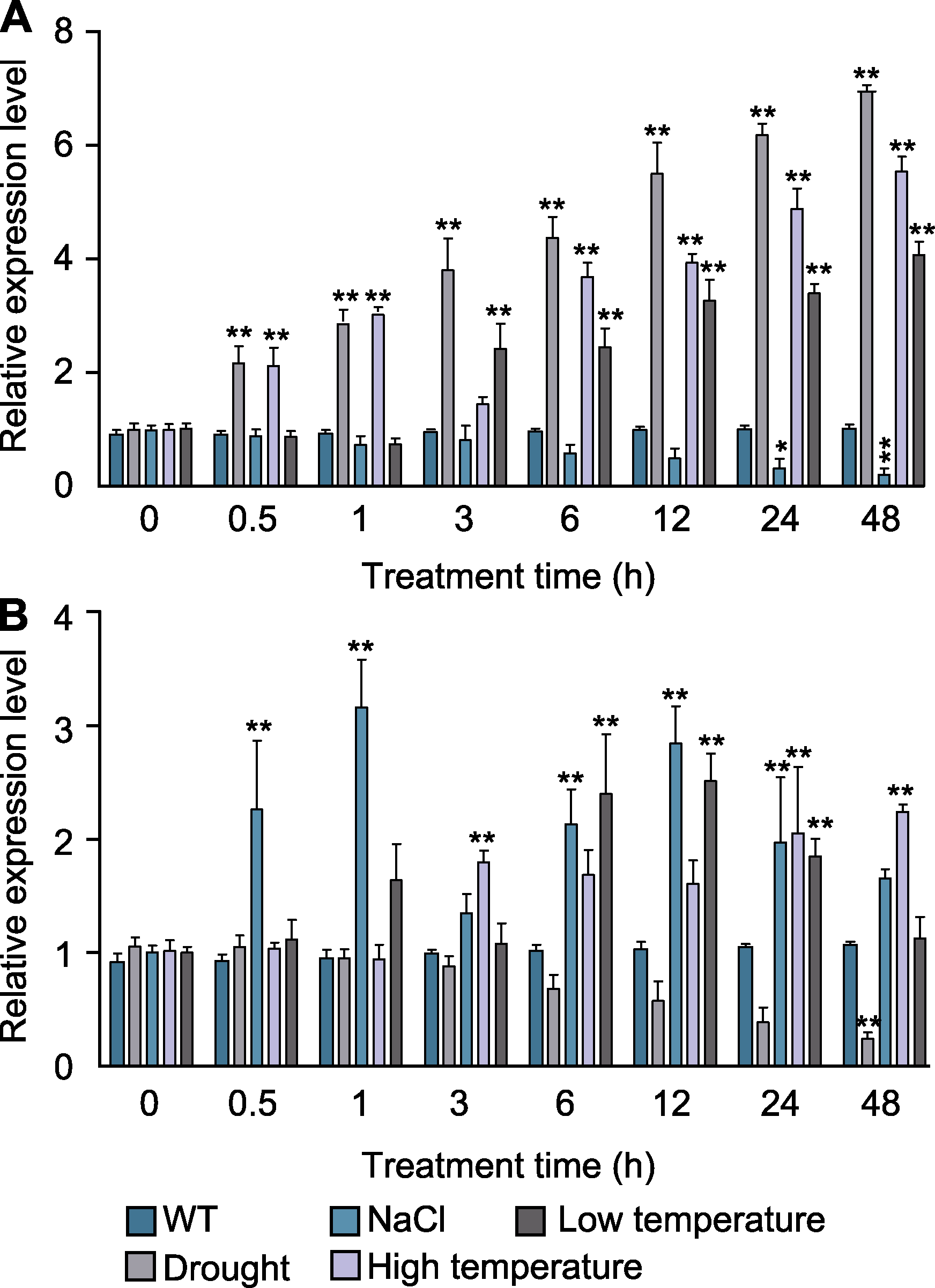
图6 不同处理时间下非生物胁迫对香鳞毛蕨叶片dfr-miR160a (A)及其靶基因DfARF10 (B)相对表达量的影响 在不同处理时间下, 将4种胁迫下检测的表达量分别与对照(WT)相比较。P值(以野生型为对照)用One-way ANOVA计算; * P<0.05; ** P<0.01
Figure 6 Effects of abiotic stress on the relative expression level of dfr-miR160a (A) and target gene DfARF10 (B) in the leaves of Dryopteris fragrans under different treatment times The expression level under four kinds of stresses were compared with control (WT) at different treatment times. P value (wild type as control) is calculated with One-way ANOVA; * P<0.05; ** P<0.01
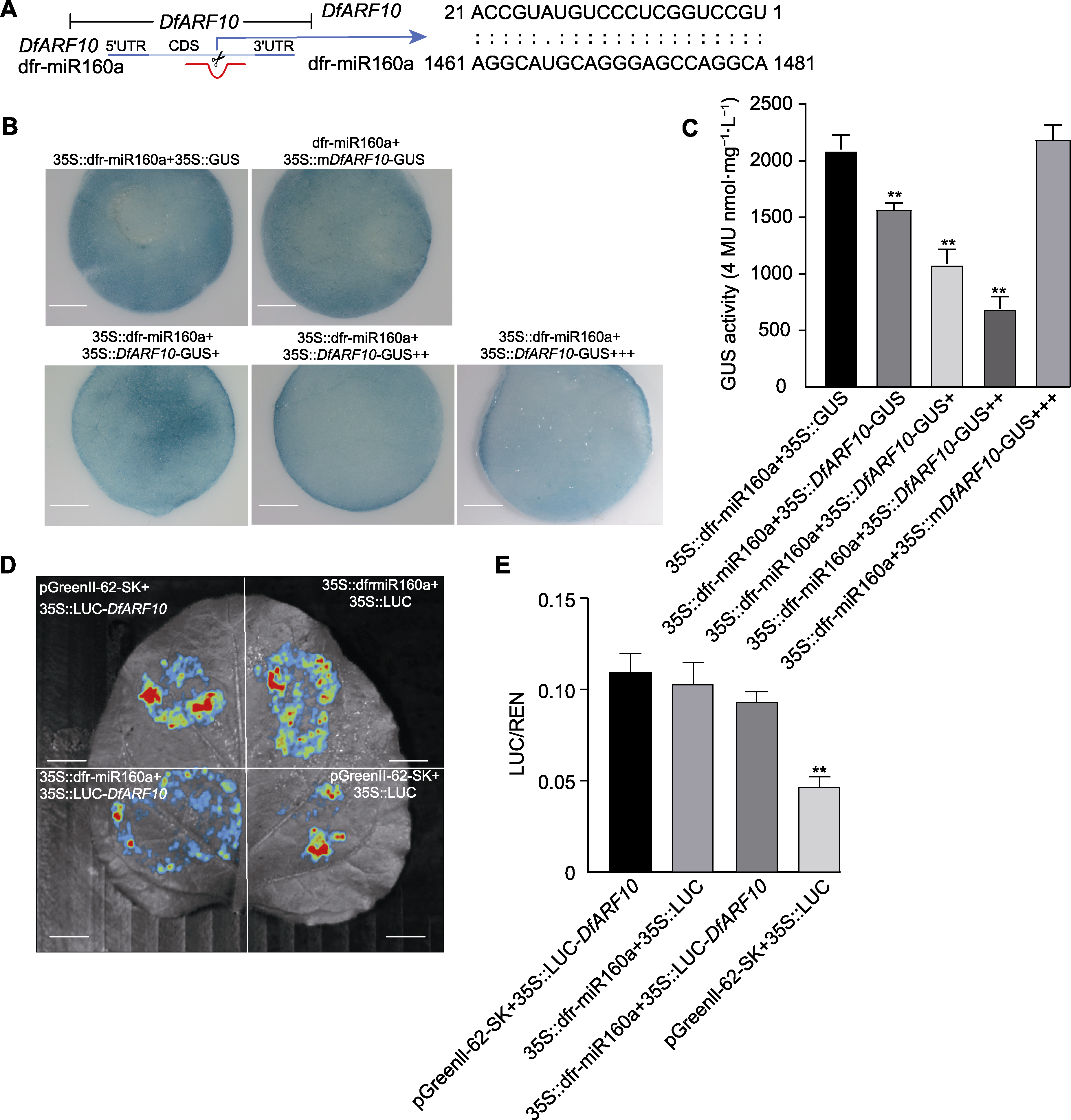
图7 农杆菌介导瞬时共转本氏烟草GUS以及双荧光素酶(LUC)活性验证dfr-miR160a靶向切割DfARF10 (A) DfARF10基因结构中dfr-miR160a的切割位点; (B) 35S::dfr-pri-mir160a和35S::DfARF10-GUS瞬时共转本氏烟草组织化学染色分析(+、++、+++分别代表不同浓度的35S::DfARF10-GUS (bars=5 mm)); (C) 35S::dfr-pri-mir160a和35S::DfARF10-GUS瞬时共转本氏烟草的GUS活性(将GUS活性标准化为本氏烟草的表达水平, +、++、+++分别代表不同浓度的35S::DfARF10-GUS); (D) 双荧光素酶验证系统通过LUC活性可视化dfr-miR160a的靶向降解能力(bars=3 cm); (E) 用LUC法定量计算荧光发光强度。
Figure 7 Agrobacterium mediated transient co-transformation of tobacco and double luciferase (LUC) activity to verify that dfr-miR160a targeted cleavage of DfARF10 (A) Cutting site of dfr-miR160a in DfARF10 gene structure; (B) β-glucuronidase (GUS) phenotype observed by histochemical staining analysis of 35S::dfr-pri-mir160a and 35S::DfARF10-GUS (+, ++, and +++ represent different concentrations of 35S::DfARF10-GUS, respectively (bars=5 mm)); (C) Co-expression of the constructs containing 35S::DfARF10-GUS and 35S::dfr-pri-mir160a in tobacco leaves (GUS activity were normalized to the expression levels of tobacco, +, ++, and +++ represent different concentrations of 35S::DfARF10-GUS, respectively); (D) The dual luciferase validation system visualizes the targeted degradation ability of dfr-miR160a through LUC activity (bars=3 cm); (E) Quantitative calculate intensity of fluorescence by LUC method.
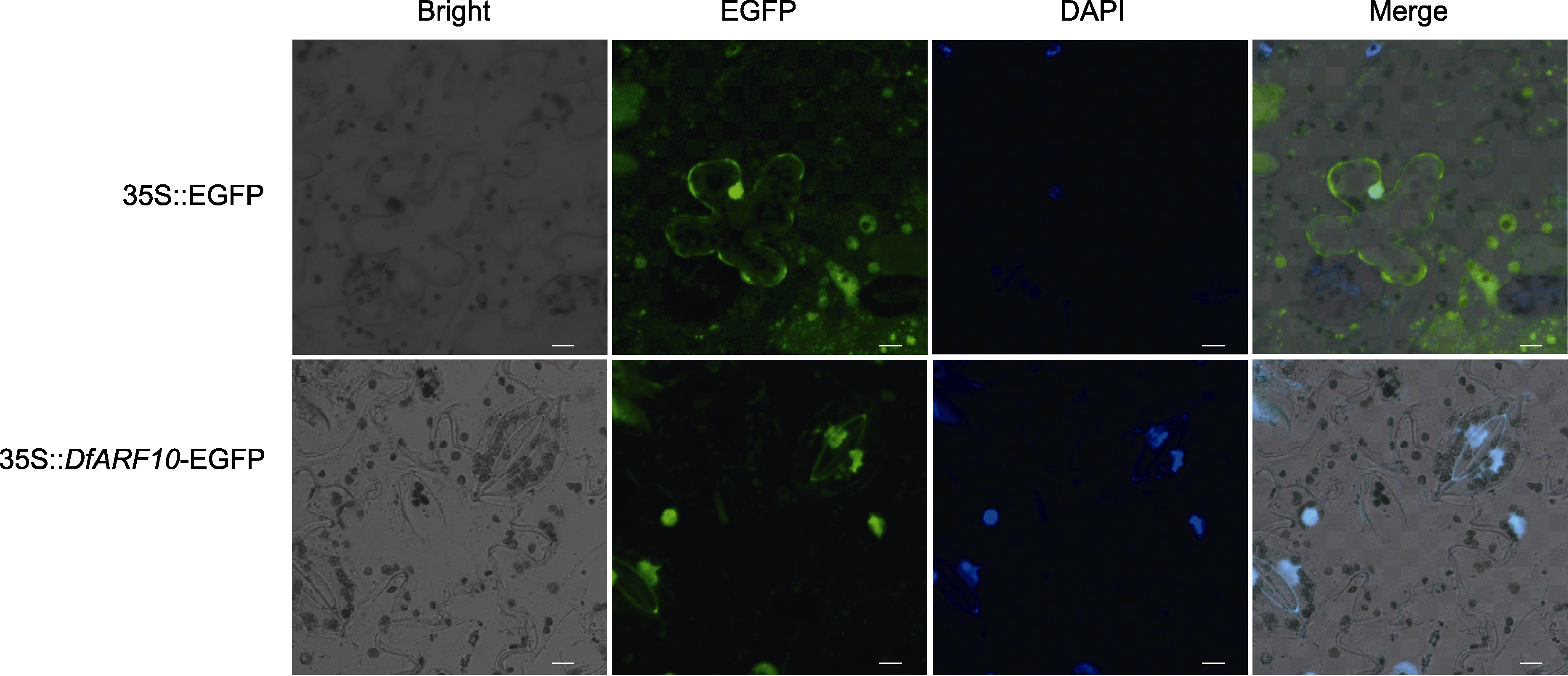
图8 香鳞毛蕨DfARF10蛋白质在本氏烟草叶片中的亚细胞定位 DAPI的蓝色荧光指示细胞核(bars=10 µm)。
Figure 8 Subcellular localization of DfARF10 proteins of Dryopteris fragrans in tobacco leaf Blue fluorescence of DAPI indicate cell nucleus (bars=10 µm).
| [1] |
曹丽茹, 张前进, 郭子宁, 鲁晓民, 张新, 魏昕, 皇甫柏树, 王振华 (2021). 玉米生长素响应因子基因家族全基因组鉴定及表达分析. 核农学报 35, 2016-2026.
DOI |
| [2] | 陈丽 (2018). 甘蓝型油菜株型及角果长度相关miRNA和靶基因的挖掘. 博士论文. 武汉: 华中农业大学. pp. 1-74. |
| [3] | 陈文浩, 宋国强, 贾小舟, 唐春萍, 冯淡开, 沈志滨 (2017). 香鳞毛蕨中1对间苯三酚类同分异构体的分离与抗真菌活性研究. 中草药 48, 433-436. |
| [4] |
官亚琳, 汤珣, 张冬瑞, 夏德鑫, 李杰, 刘守银, 宋春华, 常缨 (2020). 香鳞毛蕨DfTCP的生物信息学及其表达模式分析. 华北农学报 35(5), 39-46.
DOI |
| [5] | 邱晓杰, 汤珣, 官亚琳, 张冬瑞, 苏颖, 常缨 (2021). 香鳞毛蕨DfGNOM基因的克隆及其表达分析. 西北植物学报 41, 1279-1286. |
| [6] | 苏佳萌, 袁强, 张冬瑞, 汤珣, 常缨 (2022). 香鳞毛蕨1-脱氧- D-木酮糖-5-磷酸合酶基因的克隆及表达分析. 西北植物学报 42, 1441-1449. |
| [7] | 孙仙泽, 王政委, 娄贵诚, 王多佳, 齐越, 于晶, 苍晶 (2022). 外源脱落酸和茉莉酸甲酯调控低温胁迫下冬小麦miR444a及其靶基因TaMADS57表达. 植物生理学报 58, 708-722. |
| [8] | 汤珣, 官亚琳, 陈玲玲, 夏德鑫, 宋春华, 刘丽艳, 常缨 (2020). 香鳞毛蕨DfDREB基因克隆与表达模式分析. 西北植物学报 40, 1105-1113. |
| [9] | 吴书昌 (2016). MiR160在棉花胚珠发育中功能分析. 硕士论文. 武汉: 华中农业大学. pp. 1-39. |
| [10] | 张冬瑞, 卜志刚, 陈玲玲, 常缨 (2020). 香鳞毛蕨的组织培养和快速繁殖体系构建. 植物学报 55, 760-767. |
| [11] | 朱冲冲, 彭冰, 曾祖平, 韩旭阳, 王宏, 王天园, 何薇 (2017). 香鳞毛蕨的化学成分及药理作用研究进展. 中国药房 28, 1418-1423. |
| [12] |
Bustos-Sanmamed P, Mao GH, Deng Y, Elouet M, Khan GA, Bazin JRM, Turner M, Subramanian S, Yu O, Crespi M, Lelandais-Brière C (2013). Overexpression of miR160 affects root growth and nitrogen-fixing nodule number in Medicago truncatula. Funct Plant Biol 40, 1208-1220.
DOI PMID |
| [13] |
Cui J, Li XY, Li JL, Wang CY, Cheng DY, Dai CH (2020). Genome-wide sequence identification and expression analysis of ARF family in sugar beet (Beta vulgaris L.) under salinity stresses. PeerJ 8, e9131.
DOI URL |
| [14] |
Damodharan S, Zhao DZ, Arazi T (2016). A common miRNA160-based mechanism regulates ovary patterning, floral organ abscission and lamina outgrowth in tomato. Plant J 86, 458-471.
DOI URL |
| [15] |
Ferdous J, Hussain SS, Shi BJ (2015). Role of microRNAs in plant drought tolerance. Plant Biotechnol J 13, 293-305.
DOI PMID |
| [16] |
Guo ZY, Hao K, Lv ZY, Yu LY, Bu QT, Ren JZ, Zhang HN, Chen RB, Zhang L (2023). Profiling of phytohormone- specific microRNAs and characterization of the miR160- ARF1 module involved in glandular trichome development and artemisinin biosynthesis in Artemisia annua. Plant Biotechnol J 21, 591-605.
DOI URL |
| [17] |
Gutierrez L, Bussell JD, Pacurar DI, Schwambach J, Pacurar M, Bellini C (2009). Phenotypic plasticity of adventitious rooting in Arabidopsis is controlled by complex regulation of AUXIN RESPONSE FACTOR transcripts and microRNA abundance. Plant Cell 21, 3119-3132.
DOI URL |
| [18] |
Hao K, Wang Y, Zhu ZP, Wu Y, Chen RB, Zhang L (2022). miR160: an indispensable regulator in plant. Front Plant Sci 13, 833322.
DOI URL |
| [19] |
Huang J, Zhao L, Malik S, Gentile BR, Xiong V, Arazi T, Owen HA, Friml J, Zhao DZ (2022). Specification of female germline by microRNA orchestrated auxin signaling in Arabidopsis. Nat Commun 13, 6960.
DOI PMID |
| [20] |
Kumar R, Dhanda SK (2020). Bird eye view of protein subcellular localization prediction. Life (Basel) 10, 347.
DOI URL |
| [21] |
Liu XD, Zhang H, Zhao Y, Feng ZY, Li Q, Yang HQ, Luan S, Li JM, He ZH (2013). Auxin controls seed dormancy through stimulation of abscisic acid signaling by inducing ARF-mediated ABI3 activation in Arabidopsis. Proc Natl Acad Sci USA 110, 15485-15490.
DOI URL |
| [22] |
Luo J, Zhou JJ, Zhang JZ (2018). Aux/IAA gene family in plants: molecular structure, regulation, and function. Int J Mol Sci 19, 259.
DOI URL |
| [23] |
Peng Y, Fang T, Zhang YY, Zhang MY, Zeng LH (2020). Genome-wide identification and expression analysis of auxin response factor (ARF) gene family in Longan (Dimocarpus longan L.). Plants (Basel) 9, 221.
DOI URL |
| [24] |
Shen XX, He JQ, Ping YK, Guo JX, Hou N, Cao FG, Li XW, Geng DL, Wang SC, Chen PX, Qin GG, Ma FW, Guan QM (2022). The positive feedback regulatory loop of miR160-auxin response factor 17-HYPONASTIC LEAVES 1 mediates drought tolerance in apple trees. Plant Physiol 188, 1686-1708.
DOI URL |
| [25] |
Song CH, Fan Q, Tang YQ, Sun YN, Wang L, Wei MC, Chang Y (2022a). Overexpression of DfRaf from fragrant woodfern (Dryopteris fragrans) enhances high-temperature tolerance in tobacco (Nicotiana tabacum). Genes (Basel) 13, 1212.
DOI URL |
| [26] |
Song CH, Guan YL, Zhang DR, Tang X, Chang Y (2022b). Integrated mRNA and miRNA transcriptome analysis suggests a regulatory network for UV-B-controlled terpenoid synthesis in fragrant woodfern (Dryopteris fragrans). Int J Mol Sci 23, 5708.
DOI URL |
| [27] |
Su LY, Xu M, Zhang JD, Wang YH, Lei YS, Li Q (2021). Genome-wide identification of auxin response factor (ARF) family in kiwifruit (Actinidia chinensis) and analysis of their inducible involvements in abiotic stresses. Physiol Mol Biol Plants 27, 1261-1276.
DOI |
| [28] |
Tang YY, Du GN, Xiang J, Hu CL, Li XT, Wang WH, Zhu H, Qiao LX, Zhao CM, Wang JS, Yu SL, Sui JM (2022). Genome-wide identification of auxin response factor (ARF) gene family and the miR160-ARF18-mediated response to salt stress in peanut (Arachis hypogaea L.). Genomics 114, 171-184.
DOI URL |
| [29] |
Wang M, Wu HJ, Fang J, Chu CC, Wang XJ (2017). A long noncoding RNA involved in rice reproductive development by negatively regulating osa-miR160. Sci Bull 62, 470-475.
DOI PMID |
| [30] |
Wang YJ, Deng DX, Shi YT, Miao N, Bian YL, Yin ZT (2012). Diversification, phylogeny and evolution of auxin response factor (ARF) family: insights gained from analyzing maize ARF genes. Mol Biol Rep 39, 2401-2415.
DOI URL |
| [31] |
Wójcik AM, Nodine MD, Gaj MD (2017). miR160 and miR166/165 contribute to the LEC2-mediated auxin response involved in the somatic embryogenesis induction in Arabidopsis. Front Plant Sci 8, 2024.
DOI PMID |
| [32] | Yang TX, Wang YY, Teotia S, Wang ZH, Shi CN, Sun HW, Gu YY, Zhang ZH, Tang GL (2019). The interaction between miR160 and miR165/166 in the control of leaf development and drought tolerance in Arabidopsis. Sci Rep 9, 2832. |
| [33] |
Zhang YQ, Zeng ZH, Chen CJ, Li CQ, Xia R, Li JG (2019). Genome-wide characterization of the auxin response factor (ARF) gene family of litchi (Litchi chinensis Sonn.): phylogenetic analysis, miRNA regulation and expression changes during fruit abscission. PeerJ 7, e6677.
DOI URL |
| [1] | 熊良林, 梁国鲁, 郭启高, 景丹龙. 基因可变剪接调控植物响应非生物胁迫研究进展[J]. 植物学报, 2025, 60(3): 435-448. |
| [2] | 杜庆国, 李文学. lncRNA调控玉米生长发育和非生物胁迫研究进展[J]. 植物学报, 2024, 59(6): 950-962. |
| [3] | 周文杰, 张文瀚, 贾玮, 许自成, 黄五星. 植物miRNA响应非生物胁迫研究进展[J]. 植物学报, 2024, 59(5): 810-833. |
| [4] | 张悦婧, 桑鹤天, 王涵琦, 石珍珍, 李丽, 王馨, 孙坤, 张继, 冯汉青. 植物对非生物胁迫系统性反应中信号传递的研究进展[J]. 植物学报, 2024, 59(1): 122-133. |
| [5] | 许亚楠, 闫家榕, 孙鑫, 王晓梅, 刘玉凤, 孙周平, 齐明芳, 李天来, 王峰. 红光和远红光在调控植物生长发育及应答非生物胁迫中的作用[J]. 植物学报, 2023, 58(4): 622-637. |
| [6] | 张嘉, 李启东, 李翠, 王庆海, 侯新村, 赵春桥, 李树和, 郭强. 植物MATE转运蛋白研究进展[J]. 植物学报, 2023, 58(3): 461-474. |
| [7] | 任晓童, 张冉冉, 魏绍巍, 罗晓峰, 徐佳慧, 舒凯. 种子际微生物研究展望[J]. 植物学报, 2023, 58(3): 499-509. |
| [8] | 吴霖升, 张永光, 章钊颖, 张小康, 吴云飞. 日光诱导叶绿素荧光遥感及其在陆地生态系统监测中的应用[J]. 植物生态学报, 2022, 46(10): 1167-1199. |
| [9] | 谢玲玲, 王金龙, 伍国强. 植物CBL-CIPK信号系统响应非生物胁迫的调控机制[J]. 植物学报, 2021, 56(5): 614-626. |
| [10] | 赵菲, 党刘毅, 魏敏惠, 刘春莹, 冷伟, 尚琛晶. 黄瓜苋科凝集素基因的表达分析与逆境调控[J]. 植物学报, 2021, 56(2): 183-190. |
| [11] | 张冬瑞, 卜志刚, 陈玲玲, 常缨. 香鳞毛蕨的组织培养和快速繁殖体系构建[J]. 植物学报, 2020, 55(6): 760-767. |
| [12] | 肖银燕, 袁伟娜, 刘静, 孟建, 盛奇明, 谭烨欢, 徐春香. 木葡聚糖及其在植物抗逆过程中的功能研究进展[J]. 植物学报, 2020, 55(6): 777-787. |
| [13] | 洪林,杨蕾,杨海健,王武. AP2/ERF转录因子调控植物非生物胁迫响应研究进展[J]. 植物学报, 2020, 55(4): 481-496. |
| [14] | 王梦龙,彭小群,陈竹锋,唐晓艳. 植物凝集素类受体蛋白激酶研究进展[J]. 植物学报, 2020, 55(1): 96-105. |
| [15] | 张洵,喻娟娟,王思竹,李莹,戴绍军. 植物DREPP基因家族研究进展[J]. 植物学报, 2019, 54(5): 582-595. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||