

Chinese Bulletin of Botany ›› 2019, Vol. 54 ›› Issue (3): 378-384.DOI: 10.11983/CBB18181 cstr: 32102.14.CBB18181
• TECHNIQUES AND METHODS • Previous Articles Next Articles
Fengluan Tang,Jian Zhao,Zhiguo Zhao,Ke Xia,Shuo Qiu( )
)
Received:2018-08-22
Accepted:2018-12-29
Online:2019-05-01
Published:2019-11-24
Contact:
Shuo Qiu
Fengluan Tang,Jian Zhao,Zhiguo Zhao,Ke Xia,Shuo Qiu. Tissue Culture and Rapid Propagation of Ardisia gigantifolia[J]. Chinese Bulletin of Botany, 2019, 54(3): 378-384.
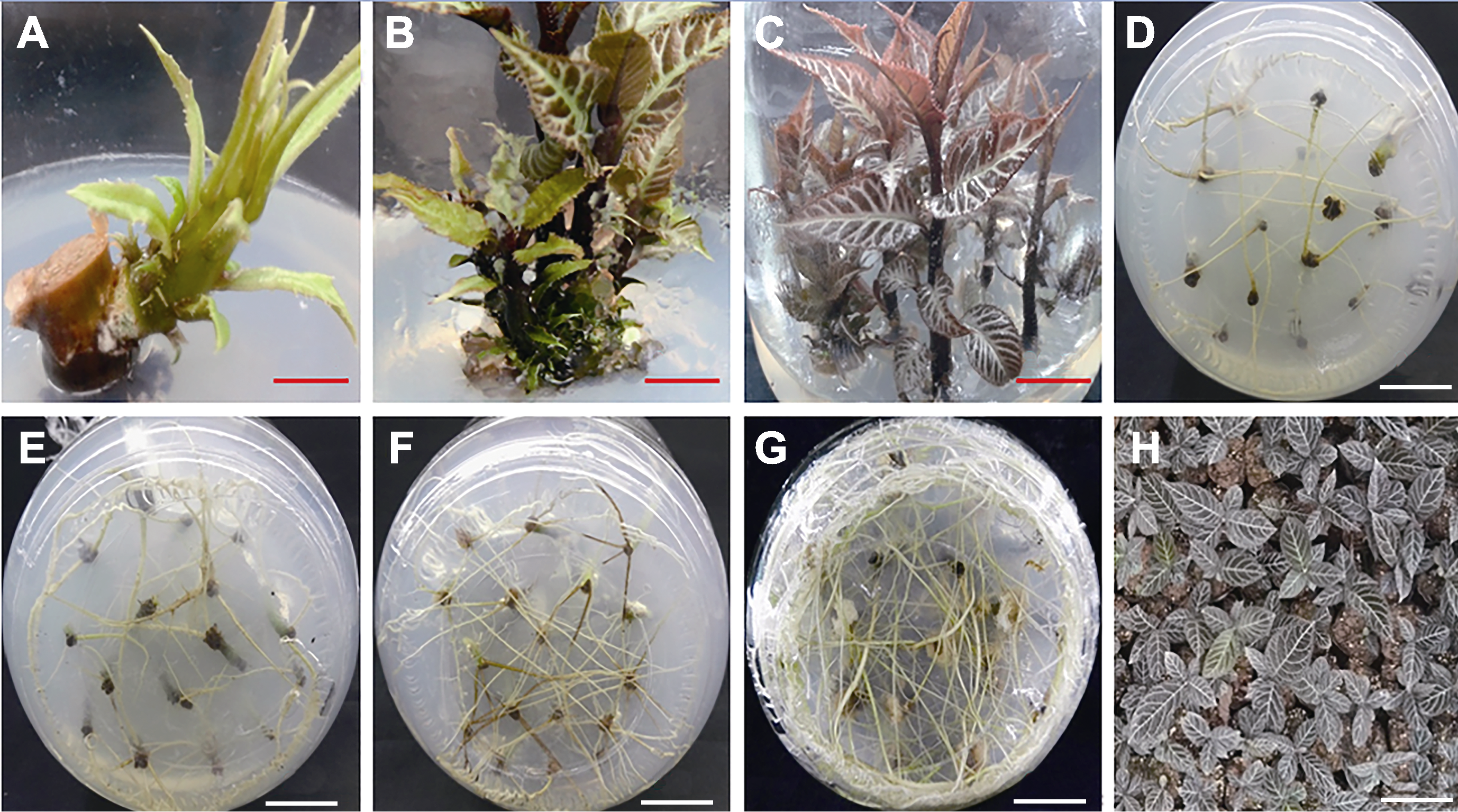
Figure 1 Axillary bud culture, root induction and transplantation of Ardisia gigantifolia(A) The induction of axillary buds; (B) The subculture of axillary buds; (C) The proliferation of buds; (D)-(G) The rooting of plantlets; (H) The transplantation of rooting seedlings. (A) Bar=1 cm, (B)-(H) Bars=2 cm
| No. | Plant growth regulator (mg·L-1) | Average height (cm) | Average propagation coefficient | State of growth | ||
|---|---|---|---|---|---|---|
| 6-BA | ZT | NAA | ||||
| 1 | 0.1 | 0.1 | 0 | 5.5 | 2.5 | Buds grew generally; leaves were medium, dark red |
| 2 | 0.1 | 0.2 | 0.1 | 8.0 | 3.5 | Buds grew well; leaves were big, dark red |
| 3 | 0.1 | 0.4 | 0.5 | 8.3 | 3.5 | Buds grew well; leaves were big, dark red |
| 4 | 0.1 | 0.8 | 1.0 | 6.5 | 3.0 | Buds grew generally; leaves were big, dark red |
| 5 | 0.5 | 0.1 | 0.1 | 8.6 | 4.3 | Buds grew well; leaves were big, dark red |
| 6 | 0.5 | 0.2 | 0 | 7.2 | 3.5 | Buds grew well; leaves were medium, dark red |
| 7 | 0.5 | 0.4 | 1.0 | 7.5 | 4.0 | Buds grew well; leaves were big, dark red |
| 8 | 0.5 | 0.8 | 0.5 | 6.5 | 3.2 | Buds grew well; leaves were big, dark red |
| 9 | 1.0 | 0.1 | 0.5 | 5.5 | 4.0 | Buds grew generally; leaves were medium; there are two or four lateral buds |
| 10 | 1.0 | 0.2 | 1.0 | 6.5 | 4.5 | Buds grew well; leaves were medium; there are two or four lateral buds |
| 11 | 1.0 | 0.4 | 0 | 4.8 | 3.5 | Buds grew generally; leaves were small; there are one or three lateral buds |
| 12 | 1.0 | 0.8 | 0.1 | 3.5 | 3.0 | Buds grew generally; leaves were small; there are one or two lateral buds |
| 13 | 2.0 | 0.1 | 1.0 | 3.0 | 2.5 | Buds grew poorly; leaves were small and yellow; base of seedlings had many calli |
| 14 | 2.0 | 0.2 | 0.5 | 3.0 | 1.8 | Seedlings had variation; leaves were small and yellow; base of seedlings had many calli |
| 15 | 2.0 | 0.4 | 0.1 | 2.0 | 1.5 | Buds grew poorly; leaves were very small and some of them had fell off; base of seedlings had many calli |
| 16 | 2.0 | 0.8 | 0 | 1.8 | 0.9 | Buds grew poorly and died partially |
Table 1 Design and result of orthogonal test on axillary buds propagation of Ardisia gigantifolia
| No. | Plant growth regulator (mg·L-1) | Average height (cm) | Average propagation coefficient | State of growth | ||
|---|---|---|---|---|---|---|
| 6-BA | ZT | NAA | ||||
| 1 | 0.1 | 0.1 | 0 | 5.5 | 2.5 | Buds grew generally; leaves were medium, dark red |
| 2 | 0.1 | 0.2 | 0.1 | 8.0 | 3.5 | Buds grew well; leaves were big, dark red |
| 3 | 0.1 | 0.4 | 0.5 | 8.3 | 3.5 | Buds grew well; leaves were big, dark red |
| 4 | 0.1 | 0.8 | 1.0 | 6.5 | 3.0 | Buds grew generally; leaves were big, dark red |
| 5 | 0.5 | 0.1 | 0.1 | 8.6 | 4.3 | Buds grew well; leaves were big, dark red |
| 6 | 0.5 | 0.2 | 0 | 7.2 | 3.5 | Buds grew well; leaves were medium, dark red |
| 7 | 0.5 | 0.4 | 1.0 | 7.5 | 4.0 | Buds grew well; leaves were big, dark red |
| 8 | 0.5 | 0.8 | 0.5 | 6.5 | 3.2 | Buds grew well; leaves were big, dark red |
| 9 | 1.0 | 0.1 | 0.5 | 5.5 | 4.0 | Buds grew generally; leaves were medium; there are two or four lateral buds |
| 10 | 1.0 | 0.2 | 1.0 | 6.5 | 4.5 | Buds grew well; leaves were medium; there are two or four lateral buds |
| 11 | 1.0 | 0.4 | 0 | 4.8 | 3.5 | Buds grew generally; leaves were small; there are one or three lateral buds |
| 12 | 1.0 | 0.8 | 0.1 | 3.5 | 3.0 | Buds grew generally; leaves were small; there are one or two lateral buds |
| 13 | 2.0 | 0.1 | 1.0 | 3.0 | 2.5 | Buds grew poorly; leaves were small and yellow; base of seedlings had many calli |
| 14 | 2.0 | 0.2 | 0.5 | 3.0 | 1.8 | Seedlings had variation; leaves were small and yellow; base of seedlings had many calli |
| 15 | 2.0 | 0.4 | 0.1 | 2.0 | 1.5 | Buds grew poorly; leaves were very small and some of them had fell off; base of seedlings had many calli |
| 16 | 2.0 | 0.8 | 0 | 1.8 | 0.9 | Buds grew poorly and died partially |
| Average height | Average propagation coefficient | |||||
|---|---|---|---|---|---|---|
| 6-BA | ZT | NAA | 6-BA | ZT | NAA | |
| Range analysis and multiple comparisons | ||||||
| K1 | 7.1 a | 5.7 | 4.8 | 3.1 a | 3.3 | 2.6 |
| K2 | 7.4 a | 6.2 | 5.5 | 3.8 a | 3.3 | 3.1 |
| K3 | 5.1 b | 5.7 | 5.8 | 3.8 a | 3.1 | 3.1 |
| K4 | 2.5 c | 4.8 | 5.9 | 1.7 b | 2.5 | 3.5 |
| Range | 5.0 | 1.6 | 1.1 | 2.1 | 0.8 | 0.9 |
| Variance analysis | ||||||
| Degrees of freedom | 3 | 3 | 3 | 3 | 3 | 3 |
| DEVSQ | 63.1 | 5.4 | 2.8 | 11.5 | 1.7 | 1.7 |
| F value | 4.1* | 0.4 | 0.2 | 3.7* | 0.6 | 0.5 |
Table 2 Analysis and comparison of average height and propagation coefficient of buds of Ardisia gigantifolia
| Average height | Average propagation coefficient | |||||
|---|---|---|---|---|---|---|
| 6-BA | ZT | NAA | 6-BA | ZT | NAA | |
| Range analysis and multiple comparisons | ||||||
| K1 | 7.1 a | 5.7 | 4.8 | 3.1 a | 3.3 | 2.6 |
| K2 | 7.4 a | 6.2 | 5.5 | 3.8 a | 3.3 | 3.1 |
| K3 | 5.1 b | 5.7 | 5.8 | 3.8 a | 3.1 | 3.1 |
| K4 | 2.5 c | 4.8 | 5.9 | 1.7 b | 2.5 | 3.5 |
| Range | 5.0 | 1.6 | 1.1 | 2.1 | 0.8 | 0.9 |
| Variance analysis | ||||||
| Degrees of freedom | 3 | 3 | 3 | 3 | 3 | 3 |
| DEVSQ | 63.1 | 5.4 | 2.8 | 11.5 | 1.7 | 1.7 |
| F value | 4.1* | 0.4 | 0.2 | 3.7* | 0.6 | 0.5 |
| [1] |
戴卫波, 董鹏鹏, 梅全喜, 何鉴洪, 张远军 ( 2018). 走马胎石油醚提取物抗类风湿性关节炎的作用机制. 中药材 41, 459-463.
DOI URL |
| [2] | 邓小梅, 戴小英, 万小婷 ( 2003). 紫金牛的组织培养. 江西林业科技( 1), 1, 24. |
| [3] | 丁爱萍, 史正军 ( 2010). 6-BA对红掌组织培养中红叶变异的影响. 植物生理学通讯 46, 571-573. |
| [4] |
符运柳, 徐立, 李志英, 黄碧兰, 李克烈 ( 2017). 走马胎离体培养及植株再生. 北方园艺 ( 4), 98-101.
DOI URL |
| [5] | 刘拥海, 俞乐, 丁君辉, 王若仲, 黄志刚, 萧浪涛 ( 2012). 植物激素对分枝发育的协同调控作用研究进展. 植物生理学报 48, 941-948. |
| [6] |
毛世忠, 唐文秀, 骆文华, 邓涛, 丁莉, 隗红燕 ( 2010). 广西紫金牛属药用植物资源及可持续利用初探. 福建林业科技 37, 119-126.
DOI URL |
| [7] |
穆丽华, 张静, 刘屏 ( 2018). 走马胎三萜皂苷衍生物的生物转化制备及其抗肿瘤活性研究. 中草药 49, 1266-1271.
DOI URL |
| [8] |
穆丽华, 赵海霞, 龚强强, 周小江, 刘屏 ( 2011). 走马胎中的三萜皂苷类成分及其体外抗肿瘤活性研究. 解放军药学学报 27, 1-6.
DOI URL |
| [9] |
孙英坤, 胡绍庆, 庞基良, 高凯, 刘华红, 陈焕伟, 姚涛, 陈林敬, 沈柏春 ( 2017). 珍稀濒危物种堇叶紫金牛高效快繁体系的建立. 植物学报 52, 764-773.
DOI URL |
| [10] | 王清, 王蒂, 戴朝曦, 王玉萍 ( 1997). 萘乙酸、2,4-D对马铃薯愈伤组织细胞染色体倍性的影响. 甘肃农业大学学报 32, 304-307. |
| [11] |
王燕, 汪一婷, 吕永平, 牟豪杰, 李海营, 陈剑平 ( 2015). 组培增殖方式对网纹草嵌合性状稳定性的影响. 植物学报 50, 372-377.
DOI URL |
| [12] | 张宝红, 李秀兰, 李凤莲, 李付广 ( 1996). 棉花组织培养中异常苗的发生与转化. 植物学报 38, 845-852. |
| [13] |
Barbier F, Péron T, Lecerf M, Perez-Garcia MD, Barrière Q, Rolčík J, Boutet-Mercey S, Citerne S, Lemoine R, Porcheron B, Roman H, Leduc N, Le Gourrierec J, Bertheloot J, Sakr S ( 2015). Sucrose is an early modulator of the key hormonal mechanisms controlling bud outgrowth in Rosa hybrida. J Exp Bot 66, 2569-2582.
DOI URL PMID |
| [14] |
Faiss M, Zalubìlová J, Strnad M, Schmülling T ( 1997). Conditional transgenic expression of the ipt gene indicates a function for cytokinins in paracrine signaling in whole tobacco plants. Plant J 12, 401-415.
DOI URL |
| [15] |
Janssen BJ, Drummond RSM, Snowden KC ( 2014). Regulation of axillary shoot development. Curr Opin Plant Biol 17, 28-35.
DOI URL PMID |
| [16] |
Mason MG, Ross JJ, Babst BA, Wienclaw BN, Beveridge CA ( 2014). Sugar demand, not auxin, is the initial regulator of apical dominance. Proc Natl Acad Sci USA 111, 6092-6097.
DOI URL |
| [17] |
Müller D, Leyser O ( 2011). Auxin, cytokinin and the control of shoot branching. Ann Bot 107, 1203-1212.
DOI URL PMID |
| [18] |
Müller D, Waldie T, Miyawaki K, To JPC, Melnyk CW, Kieber JJ, Kakimoto T, Leyser O ( 2015). Cytokinin is required for escape but not release from auxin mediated apical dominance. Plant J 82, 874-886.
DOI URL PMID |
| [1] |
Tong Li, Churan Li, Zhiyu Zhang, Xiaoman Fu, Yun Liu, Yingjun Zhang, Liying Yang, Ping Zhao.
A Preliminary Study on Tissue Culture and Rapid Propagation Technology of Phyllanthus acidus [J]. Chinese Bulletin of Botany, 2025, 60(4): 1-0. |
| [2] | Wen Feng, Yuguo Wang. Establishment of an In Vitro Regeneration System for Stem Segments of Cultivated Dioscorea polystachya [J]. Chinese Bulletin of Botany, 2024, 59(5): 792-799. |
| [3] | Yuze Liu, Yifei Wang, Weizhen Ren, Hao Li, Bin Lu, Bingshe Lu, Xiaoyue Yu. Establishment of Immature Embryo Rescue and Regeneration System for Pyrus calleryana cv. ‘Cleveland’ [J]. Chinese Bulletin of Botany, 2024, 59(5): 800-809. |
| [4] | Hao Zeng, Peifang Li, Zhihui Guo, Chunlin Liu, Ying Ruan. Establishment of a Regeneration System for Lunaria annua [J]. Chinese Bulletin of Botany, 2024, 59(3): 433-440. |
| [5] | Shangwen Zhang, Shiyu Huang, Tianwei Yang, Ting Li, Xiangjun Zhang, Manrong Gao. Establishment of a Tissue Culture and Rapid Propagation System for Erythropalum scandens Based on Orthogonal Test [J]. Chinese Bulletin of Botany, 2024, 59(1): 99-109. |
| [6] | Chungang Xie, Zhe Liu, Shusheng Zhang, Haitao Hu. Establishment of In Vitro Regeneration System of Citrus australasica [J]. Chinese Bulletin of Botany, 2023, 58(6): 926-934. |
| [7] | Yefei Liu, Haixia Zhao, Xiping Jiang, Rui Qiu, Xinyue Zhou, Yan Zhao, Chunxiang Fu. Establishment of Highly Efficient Tissue Culture and Agrobacterium-mediated Callus Infection Systems for Hordeum brevisubulatum [J]. Chinese Bulletin of Botany, 2023, 58(3): 440-448. |
| [8] | Jinchun Lu, Lina Cao, Guanjie Tong, Xinying Wang, Liying Zhang, Xin Yu, Huifang Li, Yanhui Li. Establishment of Callus Induction and Regeneration System of Anemone silvestris [J]. Chinese Bulletin of Botany, 2022, 57(2): 217-226. |
| [9] | Mengyue Li, Liu Liu, Yan Liu, Xiaoman Zhang. Establishment of Tissue Culture System for Axillary Bud Regeneration of Primula × pubescens [J]. Chinese Bulletin of Botany, 2021, 56(6): 732-739. |
| [10] | Qian Luo, Yansha Zhang, Jing Ou. Callus Induction and Plant Regeneration of Cerasus serrulata var. lannesiana cv. ‘Grandiflora’ [J]. Chinese Bulletin of Botany, 2021, 56(4): 451-461. |
| [11] | Sha Deng, Yanni Wu, Kunlin Wu, Lin Fang, Lin Li, Songjun Zeng. Breeding characteristics and artificial propagation of 14 species of Wild Plant with Extremely Small Populations (WPESP) in China [J]. Biodiv Sci, 2020, 28(3): 385-400. |
| [12] | Hong Luo, Xiaohui Wen, Yuanyuan Zhou, Silan Dai. Establishment of In Vitro Regeneration System of Helenium aromaticum [J]. Chinese Bulletin of Botany, 2020, 55(3): 318-328. |
| [13] | Wenting Zhang,Yanhong He,Ning Shu,Jingjing Xing,Baojun Liu,Manzhu Bao,Guofeng Liu. Plant Regeneration and Rapid Propagation System of Lilium bakerianum var. aureum [J]. Chinese Bulletin of Botany, 2019, 54(6): 773-778. |
| [14] | Yang Xian,Xin Dong,Xiaoman Xie,Dan Wu,Biao Han,Yan Wang. Effect of Conservation Conditions on Restricting Conservation of Acer rubrum cv. ‘Somerset’ [J]. Chinese Bulletin of Botany, 2019, 54(1): 64-71. |
| [15] | Zheng Yunfeng, Zhang Xiaoman, Liu Xiao. Plant Regeneration by Inducing Axillary Buds of Sterile Seedlings of Primula denticulata [J]. Chinese Bulletin of Botany, 2018, 53(5): 686-692. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||