

Chinese Bulletin of Botany ›› 2016, Vol. 51 ›› Issue (1): 81-88.DOI: 10.11983/CBB14196 cstr: 32102.14.CBB14196
Previous Articles Next Articles
Guiping Ren1,2, Xiaojing Wang1, Genfa Zhu2*
Received:2014-11-15
Accepted:2015-04-14
Online:2016-01-01
Published:2016-02-01
Contact:
Zhu Genfa
About author:? These authors contributed equally to this paper
Guiping Ren, Xiaojing Wang, Genfa Zhu. Effect of LED in Different Light Qualities on Growth of Phalaenopsis Plantlets[J]. Chinese Bulletin of Botany, 2016, 51(1): 81-88.
| Light treatment | Factors | Wavelength |
|---|---|---|
| RED(630) | Red | 630 nm |
| R(630)8B2 | Red:Blue=8:2 | 630 nm/450-480 nm |
| R(630)7B3 | Red:Blue=7:3 | 630 nm/450-480 nm |
| R(630)5B5 | Red:Blue=5:5 | 630 nm/450-480 nm |
| R(630)3B7 | Red:Blue=3:7 | 630 nm/450-480 nm |
| R(630)2B8 | Red:Blue=2:8 | 630 nm/450-480 nm |
| BLUE | Blue | 450-480 nm |
| RED(660) | Red | 660 nm |
| R(660)7B3 | Red:Blue=7:3 | 660 nm/450-480 nm |
| R(660)5B5 | Red:Blue=5:5 | 660 nm/450-480 nm |
| CW5R(630)5 | Cold white:Red=5:5 | 630 nm/400-780 nm |
| Warm W | Warm white LED | 400 nm-780 nm (the range of 500-780 nm accounts for 91.80%) |
| R(630)6B3FR1 | Red:Blue:Far red=6:3:1 | 630 nm/450-480 nm/730 nm |
| R(630)4B4FR2 | Red:Blue:Far red=4:4:2 | 630 nm/450-480 nm/730 nm |
| R(630)3B6FR1 | Red:Blue:Far red=3:6:1 | 630 nm/450-480 nm/730 nm |
| WFL | White fluorescent tube | 360-750 nm |
Table 1 The ratio of different light qualities of LED
| Light treatment | Factors | Wavelength |
|---|---|---|
| RED(630) | Red | 630 nm |
| R(630)8B2 | Red:Blue=8:2 | 630 nm/450-480 nm |
| R(630)7B3 | Red:Blue=7:3 | 630 nm/450-480 nm |
| R(630)5B5 | Red:Blue=5:5 | 630 nm/450-480 nm |
| R(630)3B7 | Red:Blue=3:7 | 630 nm/450-480 nm |
| R(630)2B8 | Red:Blue=2:8 | 630 nm/450-480 nm |
| BLUE | Blue | 450-480 nm |
| RED(660) | Red | 660 nm |
| R(660)7B3 | Red:Blue=7:3 | 660 nm/450-480 nm |
| R(660)5B5 | Red:Blue=5:5 | 660 nm/450-480 nm |
| CW5R(630)5 | Cold white:Red=5:5 | 630 nm/400-780 nm |
| Warm W | Warm white LED | 400 nm-780 nm (the range of 500-780 nm accounts for 91.80%) |
| R(630)6B3FR1 | Red:Blue:Far red=6:3:1 | 630 nm/450-480 nm/730 nm |
| R(630)4B4FR2 | Red:Blue:Far red=4:4:2 | 630 nm/450-480 nm/730 nm |
| R(630)3B6FR1 | Red:Blue:Far red=3:6:1 | 630 nm/450-480 nm/730 nm |
| WFL | White fluorescent tube | 360-750 nm |
| Light treatment | Green Bear | Big Chilli | ||||
|---|---|---|---|---|---|---|
| Multiplication coefficient | Ratio (%) (number of shoot≥3) | Ratio (%) (number of shoot≥4) | Multiplication coefficient | Ratio (%) (number of shoot≥3) | ||
| RED(630) | 2.85±0.16 c | 51.39 | 25.00 | 2.20±0.14 g | 34.55 | |
| R(630)8B2 | 2.26±0.13 a | 38.46 | 11.54 | 1.85±0.11 cdef | 18.52 | |
| R(630)7B3 | 2.33±0.14 a | 36.84 | 13.16 | 1.78±0.12 bcdef | 12.73 | |
| R(630)5B5 | 2.19±0.15 a | 32.43 | 9.46 | 1.63±0.09 abcde | 9.26 | |
| R(630)3B7 | 2.14±0.12 a | 25.71 | 10.00 | 1.69±0.11 abcdef | 14.55 | |
| R(630)2B8 | 2.37±0.11 a | 36.90 | 11.90 | 1.50±0.08 abc | 3.57 | |
| BLUE | 2.27±0.13 a | 33.33 | 14.67 | 1.66±0.13 abcdef | 16.00 | |
| RED(660) | 2.80±0.15 bc | 56.25 | 25.00 | 2.00±0.12 fg | 32.14 | |
| R(660)7B3 | 2.13±0.13 a | 35.94 | 9.38 | 1.86±0.10 defg | 21.57 | |
| R(660)5B5 | 2.39±0.14 ab | 40.26 | 18.18 | 1.75±0.11 bcdef | 19.23 | |
| CW5R(630)5 | 2.43±0.15 ab | 38.67 | 20.00 | 1.94±0.12 efg | 20.75 | |
| Warm W | 3.14±0.17 c | 62.16 | 36.49 | 2.02±0.12 fg | 26.98 | |
| R(630)6B3FR1 | 2.32±0.17 a | 34.78 | 14.49 | 1.49±0.09 ab | 6.38 | |
| R(630)4B4FR2 | 2.29±0.19 a | 31.82 | 13.64 | 1.56±0.09 abcd | 8.33 | |
| R(630)3B6FR1 | 2.35±0.11 a | 37.84 | 10.81 | 1.45±0.09 ab | 5.45 | |
| WFL | 2.05±0.12 a | 22.08 | 6.49 | 1.38±0.07 a | 1.82 | |
Table 2 Multiplication coefficient of Phalaenopsis shoot under different light qualities of LED
| Light treatment | Green Bear | Big Chilli | ||||
|---|---|---|---|---|---|---|
| Multiplication coefficient | Ratio (%) (number of shoot≥3) | Ratio (%) (number of shoot≥4) | Multiplication coefficient | Ratio (%) (number of shoot≥3) | ||
| RED(630) | 2.85±0.16 c | 51.39 | 25.00 | 2.20±0.14 g | 34.55 | |
| R(630)8B2 | 2.26±0.13 a | 38.46 | 11.54 | 1.85±0.11 cdef | 18.52 | |
| R(630)7B3 | 2.33±0.14 a | 36.84 | 13.16 | 1.78±0.12 bcdef | 12.73 | |
| R(630)5B5 | 2.19±0.15 a | 32.43 | 9.46 | 1.63±0.09 abcde | 9.26 | |
| R(630)3B7 | 2.14±0.12 a | 25.71 | 10.00 | 1.69±0.11 abcdef | 14.55 | |
| R(630)2B8 | 2.37±0.11 a | 36.90 | 11.90 | 1.50±0.08 abc | 3.57 | |
| BLUE | 2.27±0.13 a | 33.33 | 14.67 | 1.66±0.13 abcdef | 16.00 | |
| RED(660) | 2.80±0.15 bc | 56.25 | 25.00 | 2.00±0.12 fg | 32.14 | |
| R(660)7B3 | 2.13±0.13 a | 35.94 | 9.38 | 1.86±0.10 defg | 21.57 | |
| R(660)5B5 | 2.39±0.14 ab | 40.26 | 18.18 | 1.75±0.11 bcdef | 19.23 | |
| CW5R(630)5 | 2.43±0.15 ab | 38.67 | 20.00 | 1.94±0.12 efg | 20.75 | |
| Warm W | 3.14±0.17 c | 62.16 | 36.49 | 2.02±0.12 fg | 26.98 | |
| R(630)6B3FR1 | 2.32±0.17 a | 34.78 | 14.49 | 1.49±0.09 ab | 6.38 | |
| R(630)4B4FR2 | 2.29±0.19 a | 31.82 | 13.64 | 1.56±0.09 abcd | 8.33 | |
| R(630)3B6FR1 | 2.35±0.11 a | 37.84 | 10.81 | 1.45±0.09 ab | 5.45 | |
| WFL | 2.05±0.12 a | 22.08 | 6.49 | 1.38±0.07 a | 1.82 | |
| Light treatment | Green Bear | Big Chilli | |||||
|---|---|---|---|---|---|---|---|
| FW (g) | DW (mg) | Height (mm) | FW (g) | DW (mg) | Height (mm) | ||
| RED(630) | 0.94±0.05 bcde | 49.56±2.81 abc | 38.16±1.31 ef | 0.74±0.04 bc | 41.28±5.24 ab | 42.77±1.11 f | |
| R(630)8B2 | 0.90±0.05 bcde | 49.58±2.81 abc | 36.63±1.13 cde | 0.81±0.05 bcd | 42.01±2.45 ab | 36.61±1.03 cd | |
| R(630)7B3 | 0.84±0.05 abc | 47.30±3.33 ab | 33.15±1.00 ab | 0.75±0.05 bc | 42.82±2.59 abc | 34.21±0.99 bc | |
| R(630)5B5 | 0.79±0.05 ab | 47.86±3.34 ab | 34.59±0.88 bcd | 0.74±0.04 bcd | 41.89±2.19 ab | 34.20±0.86 bc | |
| R(630)3B7 | 0.71±0.05 a | 43.16±2.70 a | 32.94±0.77 ab | 0.73±0.04 bc | 46.04±2.37 bc | 28.98±1.09 a | |
| R(630)2B8 | 0.80±0.06 ab | 45.01±3.61 a | 30.94±0.78 a | 0.76±0.05 bcd | 43.65±2.53 bc | 32.53±0.85 b | |
| BLUE | 0.79±0.05 ab | 53.30±3.35 abcd | 39.68±0.89 f | 0.74±0.04 bc | 41.88±2.46 ab | 37.79±1.01 de | |
| RED(660) | 0.83±0.05 abc | 47.80±2.49 ab | 37.41±0.90 def | 0.89±0.05 d | 53.17±7.59 c | 42.78±1.26 f | |
| R(660)7B3 | 1.04±0.05 e | 56.86±1.77 bcde | 37.18±0.92 def | 0.68±0.04 b | 40.16±2.74 ab | 32.92±1.13 b | |
| R(660)5B5 | 0.95±0.05 bcde | 58.26±3.12 cde | 32.93±0.76 ab | 0.83±0.05 cd | 42.41±2.14 ab | 34.37±1.14 bc | |
| CW5R(630)5 | 0.99±0.05 cde | 56.80±3.06 bcde | 34.94±0.96 bcd | 0.75±0.04 bcd | 47.30±2.94 bc | 38.83±1.14 de | |
| Warm W | 1.04±0.06 e | 60.25±3.61 de | 36.37±1.08 cde | 0.81±0.05 bc | 41.76±2.27 ab | 40.70±1.19 ef | |
| R(630)6B3FR1 | 0.88±0.05 abcde | 50.44±2.82 abcd | 34.96±0.98 bcd | 0.70±0.04 bc | 43.67±2.11 bc | 32.41±0.87 b | |
| R(630)4B4FR2 | 1.04±0.08 de | 63.37±4.68 e | 34.47±1.22 bcd | 0.81±0.03 bcd | 49.82±2.56 bc | 33.02±1.16 b | |
| R(630)3B6FR1 | 0.86±0.04 abcd | 51.89±2.46 abcd | 34.69±0.91 bcd | 0.69±0.04 bc | 42.69±1.96 abc | 31.55±0.98 ab | |
| WFL | 0.87±0.06 abcde | 50.63±3.17 abcd | 33.74±0.83 abc | 0.53±0.03 a | 32.59±1.60 a | 31.32±0.98 ab | |
Table 3 Effects of different light qualities of LED on morphology of Phalaenopsis
| Light treatment | Green Bear | Big Chilli | |||||
|---|---|---|---|---|---|---|---|
| FW (g) | DW (mg) | Height (mm) | FW (g) | DW (mg) | Height (mm) | ||
| RED(630) | 0.94±0.05 bcde | 49.56±2.81 abc | 38.16±1.31 ef | 0.74±0.04 bc | 41.28±5.24 ab | 42.77±1.11 f | |
| R(630)8B2 | 0.90±0.05 bcde | 49.58±2.81 abc | 36.63±1.13 cde | 0.81±0.05 bcd | 42.01±2.45 ab | 36.61±1.03 cd | |
| R(630)7B3 | 0.84±0.05 abc | 47.30±3.33 ab | 33.15±1.00 ab | 0.75±0.05 bc | 42.82±2.59 abc | 34.21±0.99 bc | |
| R(630)5B5 | 0.79±0.05 ab | 47.86±3.34 ab | 34.59±0.88 bcd | 0.74±0.04 bcd | 41.89±2.19 ab | 34.20±0.86 bc | |
| R(630)3B7 | 0.71±0.05 a | 43.16±2.70 a | 32.94±0.77 ab | 0.73±0.04 bc | 46.04±2.37 bc | 28.98±1.09 a | |
| R(630)2B8 | 0.80±0.06 ab | 45.01±3.61 a | 30.94±0.78 a | 0.76±0.05 bcd | 43.65±2.53 bc | 32.53±0.85 b | |
| BLUE | 0.79±0.05 ab | 53.30±3.35 abcd | 39.68±0.89 f | 0.74±0.04 bc | 41.88±2.46 ab | 37.79±1.01 de | |
| RED(660) | 0.83±0.05 abc | 47.80±2.49 ab | 37.41±0.90 def | 0.89±0.05 d | 53.17±7.59 c | 42.78±1.26 f | |
| R(660)7B3 | 1.04±0.05 e | 56.86±1.77 bcde | 37.18±0.92 def | 0.68±0.04 b | 40.16±2.74 ab | 32.92±1.13 b | |
| R(660)5B5 | 0.95±0.05 bcde | 58.26±3.12 cde | 32.93±0.76 ab | 0.83±0.05 cd | 42.41±2.14 ab | 34.37±1.14 bc | |
| CW5R(630)5 | 0.99±0.05 cde | 56.80±3.06 bcde | 34.94±0.96 bcd | 0.75±0.04 bcd | 47.30±2.94 bc | 38.83±1.14 de | |
| Warm W | 1.04±0.06 e | 60.25±3.61 de | 36.37±1.08 cde | 0.81±0.05 bc | 41.76±2.27 ab | 40.70±1.19 ef | |
| R(630)6B3FR1 | 0.88±0.05 abcde | 50.44±2.82 abcd | 34.96±0.98 bcd | 0.70±0.04 bc | 43.67±2.11 bc | 32.41±0.87 b | |
| R(630)4B4FR2 | 1.04±0.08 de | 63.37±4.68 e | 34.47±1.22 bcd | 0.81±0.03 bcd | 49.82±2.56 bc | 33.02±1.16 b | |
| R(630)3B6FR1 | 0.86±0.04 abcd | 51.89±2.46 abcd | 34.69±0.91 bcd | 0.69±0.04 bc | 42.69±1.96 abc | 31.55±0.98 ab | |
| WFL | 0.87±0.06 abcde | 50.63±3.17 abcd | 33.74±0.83 abc | 0.53±0.03 a | 32.59±1.60 a | 31.32±0.98 ab | |
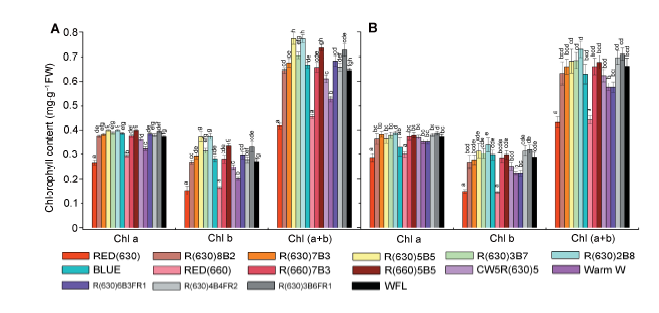
Figure 1 Effects of different light qualities of LED on chlorophyll content of Phalaenopsis leaves (A) Chlorophyll content of Green Bear (B) Chlorophyll content of Big Chilli. WFL: white fluorescent tube
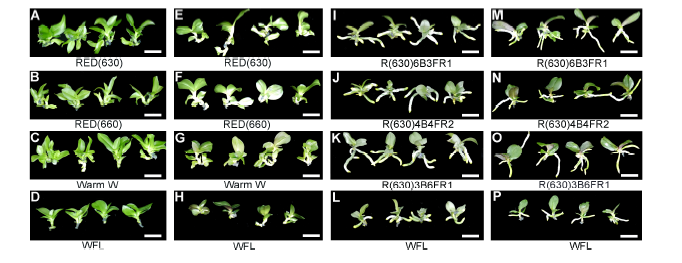
Figure 2 The growth conditions of partial Phalaenopsis’s plantlet under different light quality of LED(A)-(D) Multiple shoots of Green Bear after 60 day’s multiplication culture; (E)-(H) Multiple shoots of Big Chilli after 60 day’s multiplication culture; (I)-(L) Plantlet of Green Bear after 60 day’s rooting culture; (M)-(P) Plantlet of Big Chilli after 60 day’s rooting culture. Bar=2 cm
| Light treatment | Green Bear | Big Chilli | ||||||
|---|---|---|---|---|---|---|---|---|
| Root length (mm) | Number of root | Root activity (mg·g-1·h-1) | Root length (mm) | Number of root | Root activity (mg·g-1·h-1) | |||
| RED(630) | 19.11±0.76 a | 3.78±0.22 cde | 92.08±12.84 a | 12.50±0.89 a | 2.67±0.16 a | 118.74±15.12 a | ||
| R(630)8B2 | 20.34±0.99 ab | 3.36±0.20 abcd | 106.1±16.47 ab | 25.33±1.39 b | 2.68±0.11 a | 177.04±22.40 abc | ||
| R(630)7B3 | 20.50±0.86 ab | 3.92±0.18 de | 115.53±10.06 abc | 30.65±1.42 cd | 3.02±0.14 abc | 185.91±20.58 abc | ||
| R(630)5B5 | 21.77±0.94 abc | 2.88±0.16 a | 148.8±26.45 abcd | 37.38±1.88 fg | 3.30±0.12 cde | 201.35±30.95 abc | ||
| R(630)3B7 | 23.65±0.91 bcde | 4.03±0.19 e | 180.87±17.96 cd | 36.20±1.38 efg | 3.43±0.12 def | 201.76±38.83 abc | ||
| R(630)2B8 | 21.08±1.02 abc | 3.73±0.23 cde | 211.99±18.74 de | 36.56±1.75 efg | 3.25±0.14 cde | 271.84±49.93 cde | ||
| BLUE | 22.80±1.18 bcd | 3.07±0.20 ab | 253.49±11.79 e | 36.50±1.73 efg | 3.42±0.14 def | 323.20±73.13 de | ||
| RED(660) | 20.93±0.97 ab | 3.76±0.19 cde | 166.72±3.95 bcd | 33.77±1.51 def | 3.82±0.14 f | 148.01±16.17 ab | ||
| R(660)7B3 | 23.69±1.27 bcde | 3.25±0.18 abc | 182.35±25.72 cd | 27.40±1.46 bc | 3.19±0.14 cd | 183.29±12.02 abc | ||
| R(660)5B5 | 20.82±0.95 ab | 3.71±0.22 cde | 259.68±29.63 e | 31.12±2.77 cde | 3.09±0.20 bc | 261.83±39.75 bcde | ||
| CW5R(630)5 | 20.74±0.97 ab | 3.53±0.16 bcde | 171.98±33.48 bcd | 31.54±1.90 cde | 3.46±0.15 def | 188.82±20.36 abc | ||
| Warm W | 22.29±0.92 abcd | 3.73±0.21 cde | 211.05±17.48 de | 30.66±2.00 cd | 3.57±0.14 def | 225.28±40.13 abcd | ||
| R(630)6B3FR1 | 24.27±1.01 cde | 3.86±0.19 cde | 260.63±29.06 e | 39.22±1.83 fg | 3.67±0.11 ef | 351.65±24.40 ef | ||
| R(630)4B4FR2 | 26.40±1.29 e | 3.75±0.15 cde | 349.41±31.61 f | 39.88±2.17 g | 3.56±0.15 def | 366.20±29.03 ef | ||
| R(630)3B6FR1 | 25.04±0.96 de | 3.74±0.19 cde | 369.49±36.35 f | 40.35±2.08 g | 3.58±0.15 def | 449.72±57.66 f | ||
| WFL | 20.53±1.06 ab | 3.49±0.18 bcde | 172.52±11.89 bcd | 24.58±1.61 b | 2.64±0.13 a | 171.67±23.38 abc | ||
Table 4 Effects of different light qualities of LED on rooting of Phalaenopsis plantlet
| Light treatment | Green Bear | Big Chilli | ||||||
|---|---|---|---|---|---|---|---|---|
| Root length (mm) | Number of root | Root activity (mg·g-1·h-1) | Root length (mm) | Number of root | Root activity (mg·g-1·h-1) | |||
| RED(630) | 19.11±0.76 a | 3.78±0.22 cde | 92.08±12.84 a | 12.50±0.89 a | 2.67±0.16 a | 118.74±15.12 a | ||
| R(630)8B2 | 20.34±0.99 ab | 3.36±0.20 abcd | 106.1±16.47 ab | 25.33±1.39 b | 2.68±0.11 a | 177.04±22.40 abc | ||
| R(630)7B3 | 20.50±0.86 ab | 3.92±0.18 de | 115.53±10.06 abc | 30.65±1.42 cd | 3.02±0.14 abc | 185.91±20.58 abc | ||
| R(630)5B5 | 21.77±0.94 abc | 2.88±0.16 a | 148.8±26.45 abcd | 37.38±1.88 fg | 3.30±0.12 cde | 201.35±30.95 abc | ||
| R(630)3B7 | 23.65±0.91 bcde | 4.03±0.19 e | 180.87±17.96 cd | 36.20±1.38 efg | 3.43±0.12 def | 201.76±38.83 abc | ||
| R(630)2B8 | 21.08±1.02 abc | 3.73±0.23 cde | 211.99±18.74 de | 36.56±1.75 efg | 3.25±0.14 cde | 271.84±49.93 cde | ||
| BLUE | 22.80±1.18 bcd | 3.07±0.20 ab | 253.49±11.79 e | 36.50±1.73 efg | 3.42±0.14 def | 323.20±73.13 de | ||
| RED(660) | 20.93±0.97 ab | 3.76±0.19 cde | 166.72±3.95 bcd | 33.77±1.51 def | 3.82±0.14 f | 148.01±16.17 ab | ||
| R(660)7B3 | 23.69±1.27 bcde | 3.25±0.18 abc | 182.35±25.72 cd | 27.40±1.46 bc | 3.19±0.14 cd | 183.29±12.02 abc | ||
| R(660)5B5 | 20.82±0.95 ab | 3.71±0.22 cde | 259.68±29.63 e | 31.12±2.77 cde | 3.09±0.20 bc | 261.83±39.75 bcde | ||
| CW5R(630)5 | 20.74±0.97 ab | 3.53±0.16 bcde | 171.98±33.48 bcd | 31.54±1.90 cde | 3.46±0.15 def | 188.82±20.36 abc | ||
| Warm W | 22.29±0.92 abcd | 3.73±0.21 cde | 211.05±17.48 de | 30.66±2.00 cd | 3.57±0.14 def | 225.28±40.13 abcd | ||
| R(630)6B3FR1 | 24.27±1.01 cde | 3.86±0.19 cde | 260.63±29.06 e | 39.22±1.83 fg | 3.67±0.11 ef | 351.65±24.40 ef | ||
| R(630)4B4FR2 | 26.40±1.29 e | 3.75±0.15 cde | 349.41±31.61 f | 39.88±2.17 g | 3.56±0.15 def | 366.20±29.03 ef | ||
| R(630)3B6FR1 | 25.04±0.96 de | 3.74±0.19 cde | 369.49±36.35 f | 40.35±2.08 g | 3.58±0.15 def | 449.72±57.66 f | ||
| WFL | 20.53±1.06 ab | 3.49±0.18 bcde | 172.52±11.89 bcd | 24.58±1.61 b | 2.64±0.13 a | 171.67±23.38 abc | ||
| 1 | 白宝璋, 金锦子, 白崧, 黄丽萍 (1994). 玉米根系活力TTC测定法的改良. 玉米科学 2, 44-47. |
| 2 | 陈志, 孙庆丽, 汪一婷, 牟豪杰, 吕永平, 周迪江, 陈剑平 (2012). 不同光质对甘蔗组培苗的影响. 农业工程 2, 51-57. |
| 3 | 戴艳娇, 王琼丽, 张欢, 代大勇, 文民操, 徐志刚 (2010). 不同光谱的LEDs对蝴蝶兰组培苗生长的影响. 江苏农业科学 5, 227-231. |
| 4 | 邸秀茹, 焦学磊, 崔瑾, 刘晓英, 孔燕, 徐志刚 (2008). 新型光源LED辐射的不同光质配比光对菊花组培苗生长的影响. 植物生理学通讯 44, 661-664. |
| 5 | 杜建芳, 廖祥儒, 叶步青, 李萌 (2002). 光质对油菜幼苗生长及抗氧化酶活性的影响. 植物学通报 19, 743-745. |
| 6 | 李韶山, 潘瑞炽 (1994). 蓝光对水稻幼苗生长效应的研究. 中国水稻科学 8, 115-118. |
| 7 | 刘晓青, 苏家乐, 陈尚平, 李畅, 项立平, 何丽斯 (2013). 高山杜鹃叶片再生和试管苗生长对不同LED光质的响应特征. 江苏农业学报 29, 1451-1455. |
| 8 | 王学奎 (2006). 植物生理生化实验原理和技术(第2版). 北京: 高等教育出版社. pp. 134-136. |
| 9 | 闫新房, 丁林波, 丁义, 何松林 (2009). LED光源在植物组织培养中的应用. 中国农学通报 25, 42-45. |
| 10 | 张玉娟, 朱根发 (2011). 蝴蝶兰. 北京: 中国农业出版社. pp. 1-3. |
| 11 | 郑子松, 王红, 王神云, 于利, 李建斌, 刁阳隆 (2013). LED光源对结球甘蓝(Brassica oleracea var. capitatal)不定芽再生的影响. 天津农学院学报 20, 6-10. |
| 12 | Christenson EA (2001). Phalaenopsis, A Monograph. London: Timber Press. pp. 294-320. |
| 13 | Hahn EJ, Kozai T, Paek KY (2000). Blue and red light- emitting diodes with or without sucrose and ventilation affect in vitro growth of Rehmannia glutinosa plantlets.J Plant Biol 43, 247-250. |
| 14 | Hsu HC, Chen CC (2010). The effect of light spectrum on the growth characteristics of in vitro cultures of Phalae- nopsis.Propag Ornam Plants 10, 3-8. |
| 15 | Kim SJ, Hahn EJ, Heo JW, Paek KY (2004). Effects of LEDs on net photosynthetic rate, growth and leaf stomata of chrysanthemum plantlets in vitro.Sci Hortic 101, 143-151. |
| 16 | Lu YX, Godo T, Fujiwara K, Guan KY, Mii M (2013). Effect of nitrogen source and wavelength of LED-light on organo- genesis from leaf and shoot tip cultures in Lysionotus pauciflorus Maxim.Propag Ornam Plants 13, 174-180. |
| 17 | Massa GD, Kim HH, Wheeler RM, Mitchell CA (2008). Plant productivity in response to LED lighting.Hortscience 7, 1951-1956. |
| 18 | Tanaka M, Takamura T, Watanabe H, Endo M, Yanagi T, Okamoto K (1998). In vitro growth of Cymbidium plantlets cultured under super bright red and blue light-emitting diodes (LEDs).J Hortic Sci Biotech 73, 39-44. |
| [1] | SHANGGUAN Yao-Yao, SU Shi-Ping, GU Xue-Dan, ZHANG Zheng-Zhong, ZHAO Hu, LI Yi, WEI Xing-Yu. Response of Reaumuria songorica seedlings to photoperiod and light quality ratio [J]. Chin J Plant Ecol, 2025, 49(5): 788-800. |
| [2] |
Tong Li, Churan Li, Zhiyu Zhang, Xiaoman Fu, Yun Liu, Yingjun Zhang, Liying Yang, Ping Zhao.
A Preliminary Study on Tissue Culture and Rapid Propagation Technology of Phyllanthus acidus [J]. Chinese Bulletin of Botany, 2025, 60(4): 1-0. |
| [3] | Yuze Liu, Yifei Wang, Weizhen Ren, Hao Li, Bin Lu, Bingshe Lu, Xiaoyue Yu. Establishment of Immature Embryo Rescue and Regeneration System for Pyrus calleryana cv. ‘Cleveland’ [J]. Chinese Bulletin of Botany, 2024, 59(5): 800-809. |
| [4] | Yan Luo, Qiyuan Liu, Yuanbing Lü, Yue Wu, Yaoyu Tian, Tian An, Zhenhua Li. Photothermal Sensitivity of Phytochrome Mutants During Seed Germination in Arabidopsis thaliana [J]. Chinese Bulletin of Botany, 2024, 59(5): 752-762. |
| [5] | Wen Feng, Yuguo Wang. Establishment of an In Vitro Regeneration System for Stem Segments of Cultivated Dioscorea polystachya [J]. Chinese Bulletin of Botany, 2024, 59(5): 792-799. |
| [6] | Hao Zeng, Peifang Li, Zhihui Guo, Chunlin Liu, Ying Ruan. Establishment of a Regeneration System for Lunaria annua [J]. Chinese Bulletin of Botany, 2024, 59(3): 433-440. |
| [7] | Shangwen Zhang, Shiyu Huang, Tianwei Yang, Ting Li, Xiangjun Zhang, Manrong Gao. Establishment of a Tissue Culture and Rapid Propagation System for Erythropalum scandens Based on Orthogonal Test [J]. Chinese Bulletin of Botany, 2024, 59(1): 99-109. |
| [8] | Chungang Xie, Zhe Liu, Shusheng Zhang, Haitao Hu. Establishment of In Vitro Regeneration System of Citrus australasica [J]. Chinese Bulletin of Botany, 2023, 58(6): 926-934. |
| [9] | Yefei Liu, Haixia Zhao, Xiping Jiang, Rui Qiu, Xinyue Zhou, Yan Zhao, Chunxiang Fu. Establishment of Highly Efficient Tissue Culture and Agrobacterium-mediated Callus Infection Systems for Hordeum brevisubulatum [J]. Chinese Bulletin of Botany, 2023, 58(3): 440-448. |
| [10] | Jinchun Lu, Lina Cao, Guanjie Tong, Xinying Wang, Liying Zhang, Xin Yu, Huifang Li, Yanhui Li. Establishment of Callus Induction and Regeneration System of Anemone silvestris [J]. Chinese Bulletin of Botany, 2022, 57(2): 217-226. |
| [11] | Mengyue Li, Liu Liu, Yan Liu, Xiaoman Zhang. Establishment of Tissue Culture System for Axillary Bud Regeneration of Primula × pubescens [J]. Chinese Bulletin of Botany, 2021, 56(6): 732-739. |
| [12] | Qian Luo, Yansha Zhang, Jing Ou. Callus Induction and Plant Regeneration of Cerasus serrulata var. lannesiana cv. ‘Grandiflora’ [J]. Chinese Bulletin of Botany, 2021, 56(4): 451-461. |
| [13] | Hong Luo, Xiaohui Wen, Yuanyuan Zhou, Silan Dai. Establishment of In Vitro Regeneration System of Helenium aromaticum [J]. Chinese Bulletin of Botany, 2020, 55(3): 318-328. |
| [14] | Sha Deng, Yanni Wu, Kunlin Wu, Lin Fang, Lin Li, Songjun Zeng. Breeding characteristics and artificial propagation of 14 species of Wild Plant with Extremely Small Populations (WPESP) in China [J]. Biodiv Sci, 2020, 28(3): 385-400. |
| [15] | Wenting Zhang,Yanhong He,Ning Shu,Jingjing Xing,Baojun Liu,Manzhu Bao,Guofeng Liu. Plant Regeneration and Rapid Propagation System of Lilium bakerianum var. aureum [J]. Chinese Bulletin of Botany, 2019, 54(6): 773-778. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||