INTRODUCTION: The phytohormone salicylic acid (SA) plays multiple important roles in plants, such as disease resistance, seed germination, and leaf senescence. Among these, the role of SA in plant disease resistance is the most studied. Since SA promotes disease resistance at the cost of plant growth, plants need to dynamically regulate the content of SA to balance disease resistance and growth. Therefore, fast and accurate measurement of SA content is a critical basis for plant immunity research.
RATIONALE: High-performance liquid chromatography (HPLC)-fluorescence detector is the most popular method for the quantitative measurement of SA. In order to improve the efficiency and sensitivity of current methods, this study optimized the composition, ion concentration, and pH of the mobile phase, as well as the detection wavelength and detection procedure.
RESULTS: The baseline of the chromatogram was more stable when using acetonitrile instead of methanol in the mobile phase. When the pH of the mobile phase was 5.2, the retention time of SA was the shortest, without interference peak near the SA peak, which was preferred for minimizing the detection time. The higher concentration of sodium acetate (100 mmol∙L-1) in the mobile phase was better than that of lower concentration (20-50 mmol∙L-1). Wavelength scanning revealed that the optimal excitation wavelength was 300 nm and the optimal emission wavelength was 405 nm, under which the highest sensitivity for SA detection was obtained. At a flow rate of 2 mL∙min-1, it took 3.5 min for elution, 3.5 min for column wash, and 3 min for column balance, shortening the measurement time per sample from 50 min to 10 min.
CONCLUSION: These optimizations greatly improved the sensitivity, stability, and efficiency of the SA measurement using HPLC, which will contribute to the plant immunity research.
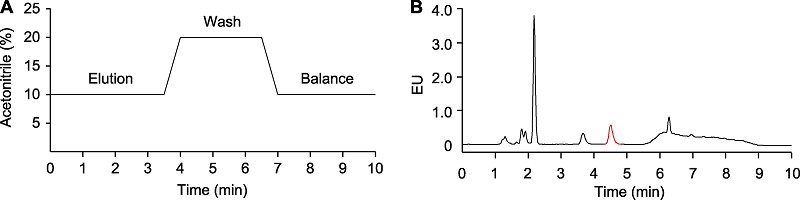
Optimization of salicylic acid (SA) measurement. (A) SA detection procedure; (B) Chromatogram using the optimized condition. The samples are total SA in Arabidopsis without Psm ES4326 infection. EU: Emission units. The peak labeled in red indicates SA.

 Table of Content
Table of Content
 Home
Home