INTRODUCTION Nitrogen (N) is one of the most essential nutrients for the plant growth and development, and NH4+-N is the main form of N source. Appropriate amount of NH4+ promotes plant growth and increase crop yield. However, when used as the only N source, NH4+ suppresses the growth and production of crops. Wheat (Triticum aestivum) is the third largest cereal crop in China, and its production has a profound impact on the food production. As the indispensable raw material for foods, wheat is crucial to daily life, so studying NH4+ toxicity and mitigation mechanisms are of great significance for wheat production.
RATIONALE NH4+toxicity is a ubiquitous issue in plants. The mechanisms of how NH4+ causes phytotoxicity are not fully understood. To explore the mechanisms of NO3--dependent alleviation of NH4+toxicity in the roots of wheat, both transcriptomic and proteomic approaches were used to investigate the differential expressions of genes and proteins under different nitrogen treatments.
RESULTS Compared with 7.5 mmol·L-1NO3- (control), 7.5 mmol·L-1NH4+treatment inhibited the root growth of wheat seedlings. Transcriptome analysis showed that sole NH4+ treatment upregulated the expressions of genes encoding glycolysis- and fermentation-related enzymes, including pyruvate decarboxylase, alcohol dehydrogenase and lactate dehydrogenase, while downregulated the expressions of genes encoding TCA cycle enzymes and the ATP synthases in the roots compared with control. Expressions of genes encoding the respiratory burst oxidase homologs (Rbohs), alternative oxidase (AOX) and dioxygenases were significantly upregulated, and expression of the PIP-type aquaporin genes were downregulated. The addition of 1 mmol·L-1NO3- to the solution containing 7.5 mmol·L-1NH4+downregulated the expression of genes encoding glycolysis enzymes, fermentation enzymes, Rbohs, AOX and dioxygenases, and increased the expression of genes encoding the TCA cycle enzymes, the ATP synthases and PIP-type aquaporins. Proteome analysis showed that expressions of glycolysis enzymes, fermentation enzymes and AOX were upregulated, while PIP-type aquaporins were downregulated under sole NH4+conditions compared with the control. The addition of NO3- downregulated the expressions of glycolysis enzymes, fermentation enzymes, Rbohs and AOX and upregulated expressions of PIP-type aquaporins.
CONCLUSION In conclusion, sole NH4+ treatment promotes glycolytic and fermentation pathways, inhibits the TCA cycle and energy generation, and ultimately inhibits root growth of wheat seedlings. The inhibition of root growth may be due to the sole NH4+-induced hypoxic stress in the roots. The addition of NO3- inhibits the glycolysis and fermentation pathways, promotes the TCA cycle and energy production, significantly alleviates the hypoxia stress and thereby attenuates the inhibitory effect of NH4+ on root growth.
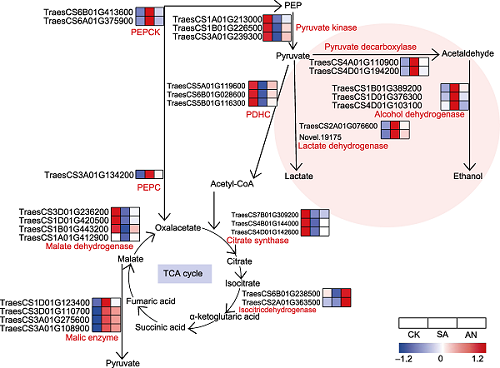
Changes in transcription level of DEGs involved in fermentation pathway and TCA cycle under different N treatments in the roots of wheat seedlings. Sole NH4+ treatment may induce the fermentation pathway, inhibit the capacity of TCA cycle, and ultimately inhibit the root growth in wheat. The addition of NO3- remarkably alleviates the sole NH4+-induced inhibitory effects on the root growth.

 Table of Content
Table of Content
 Home
Home