

Chinese Bulletin of Botany ›› 2019, Vol. 54 ›› Issue (6): 744-752.DOI: 10.11983/CBB19032 cstr: 32102.14.CBB19032
• EXPERIMENTAL COMMUNICATIONS • Previous Articles Next Articles
Shuhui Zhang,Hong Wang,Wenru Wang,Xuelian Wu,Yuansong Xiao,Futian Peng( )
)
Received:2019-02-22
Accepted:2019-05-17
Online:2019-11-01
Published:2020-07-09
Contact:
Futian Peng
Shuhui Zhang,Hong Wang,Wenru Wang,Xuelian Wu,Yuansong Xiao,Futian Peng. Effects of Sucrose on Seedling Growth and Development and SnRK1 Activity in Prunus persica[J]. Chinese Bulletin of Botany, 2019, 54(6): 744-752.
| Gene | Primer name | Primer sequence (5′-3′) |
|---|---|---|
| PpPIN1 (Ppa002944m) | P-PIN1-F | TAACAATACGACAGCGCATTACC |
| P-PIN1-R | TGAAGATCCTTACCACCATCCTC | |
| PpPIN2 (Ppa024134m) | P-PIN2-F | TTCGAATCTCACGGGAGTGG |
| P-PIN2-R | GAATCCACCTTGGAAACTGTTTG | |
| PpPIN3 (Ppa002528m) | P-PIN3-F | ATCTAACCTTACAGGCGCAGAGA |
| P-PIN3-R | GAGTCTCTTCGAAATTTGACGGT | |
| PpYUC2 (Ppa022204m) | P-YUC2-F | GACCCAGCAGTGTTCGATCA |
| P-YUC2-R | CTGCCTCCTCCAATTCTGGCT | |
| PpYUC6 (Ppa005244m) | P-YUC6-F | TCCTCCTCATCACCATCACA |
| P-YUC6-R | CCACAAGAGGCTATGCAATT | |
| PpActin | P-Actin-F | GTTATTCTTCATCGGCGTCTTCG |
| P-Actin-R | CTTCACCATTCCAGTTCCATTGTC |
Table 1 Primers used in this study
| Gene | Primer name | Primer sequence (5′-3′) |
|---|---|---|
| PpPIN1 (Ppa002944m) | P-PIN1-F | TAACAATACGACAGCGCATTACC |
| P-PIN1-R | TGAAGATCCTTACCACCATCCTC | |
| PpPIN2 (Ppa024134m) | P-PIN2-F | TTCGAATCTCACGGGAGTGG |
| P-PIN2-R | GAATCCACCTTGGAAACTGTTTG | |
| PpPIN3 (Ppa002528m) | P-PIN3-F | ATCTAACCTTACAGGCGCAGAGA |
| P-PIN3-R | GAGTCTCTTCGAAATTTGACGGT | |
| PpYUC2 (Ppa022204m) | P-YUC2-F | GACCCAGCAGTGTTCGATCA |
| P-YUC2-R | CTGCCTCCTCCAATTCTGGCT | |
| PpYUC6 (Ppa005244m) | P-YUC6-F | TCCTCCTCATCACCATCACA |
| P-YUC6-R | CCACAAGAGGCTATGCAATT | |
| PpActin | P-Actin-F | GTTATTCTTCATCGGCGTCTTCG |
| P-Actin-R | CTTCACCATTCCAGTTCCATTGTC |
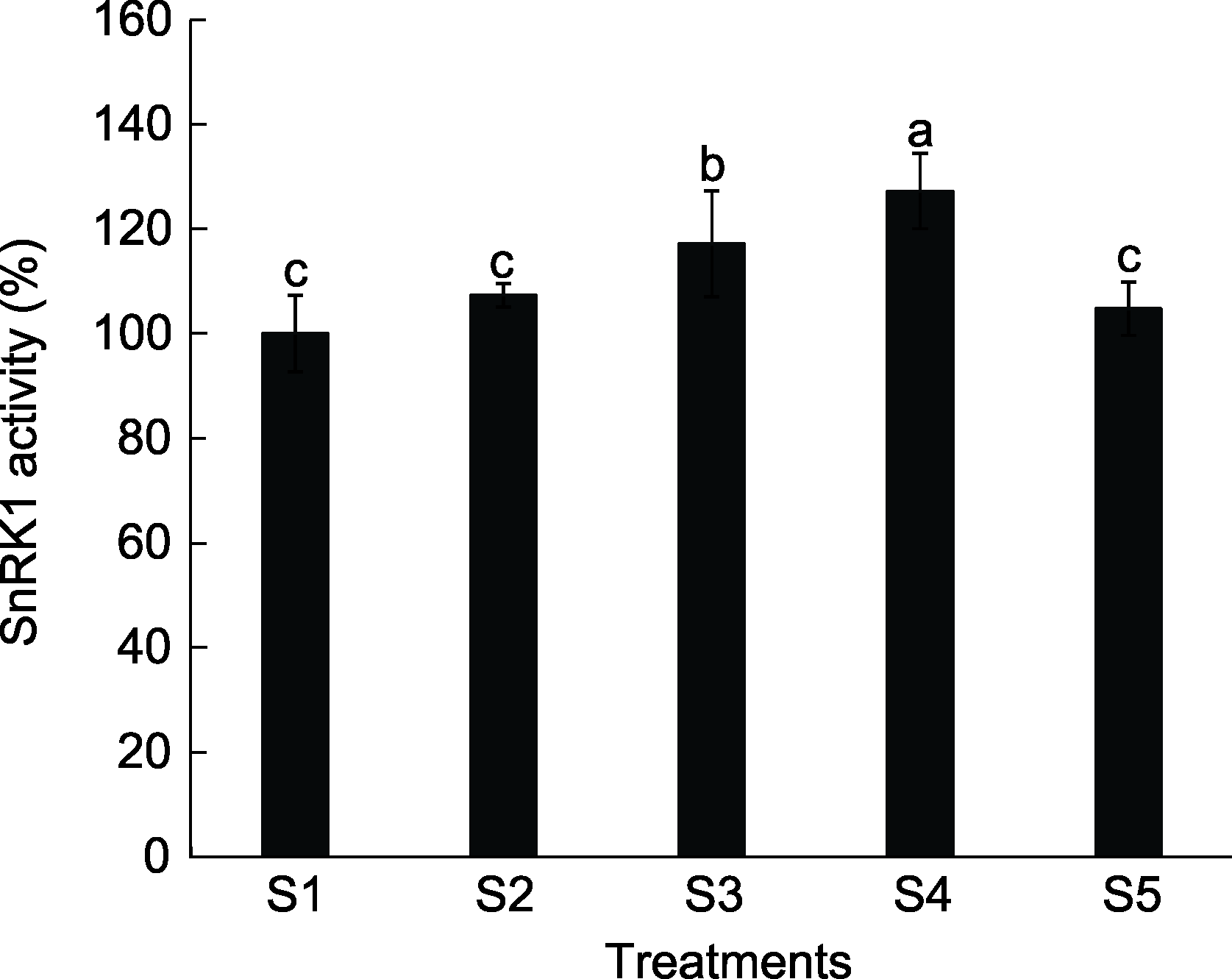
Figure 1 Effects of different sucrose concentrations on SnRK1 activity in peach seedlings S1: Water; S2: 1% sucrose solution; S3: 3% sucrose solution; S4: 5% sucrose solution; S5: 7% sucrose solution. Different lowercase letters above the bars indicate significant differences among different treatmens (P<0.05).
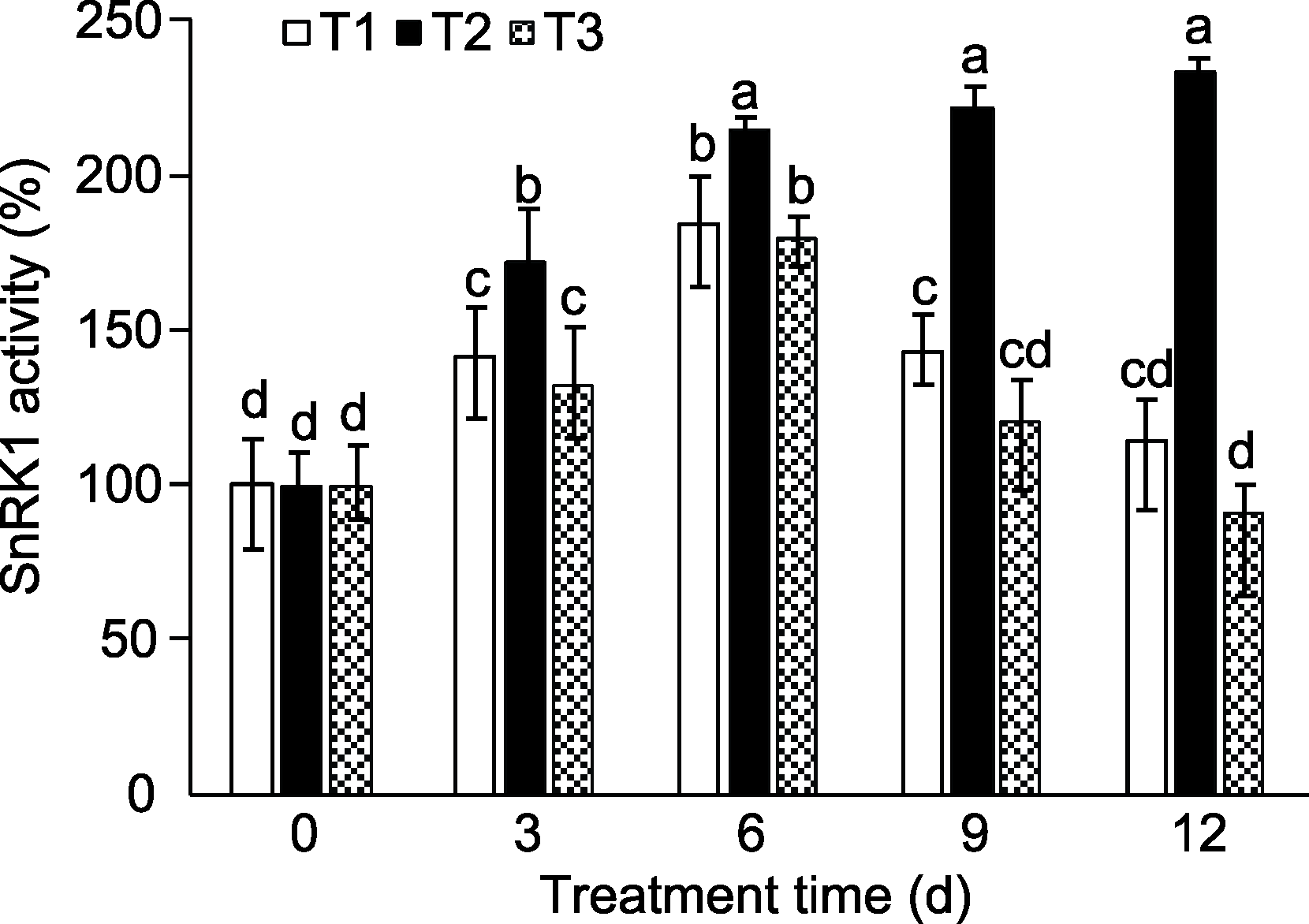
Figure 2 Effects of sucrose treatment on SnRK1 activity in peach seedlings T1: Water; T2: 5% sucrose solution; T3: 5% mannitol solution. Different lowercase letters above the bars indicate significant differences among different treatments (P<0.05).
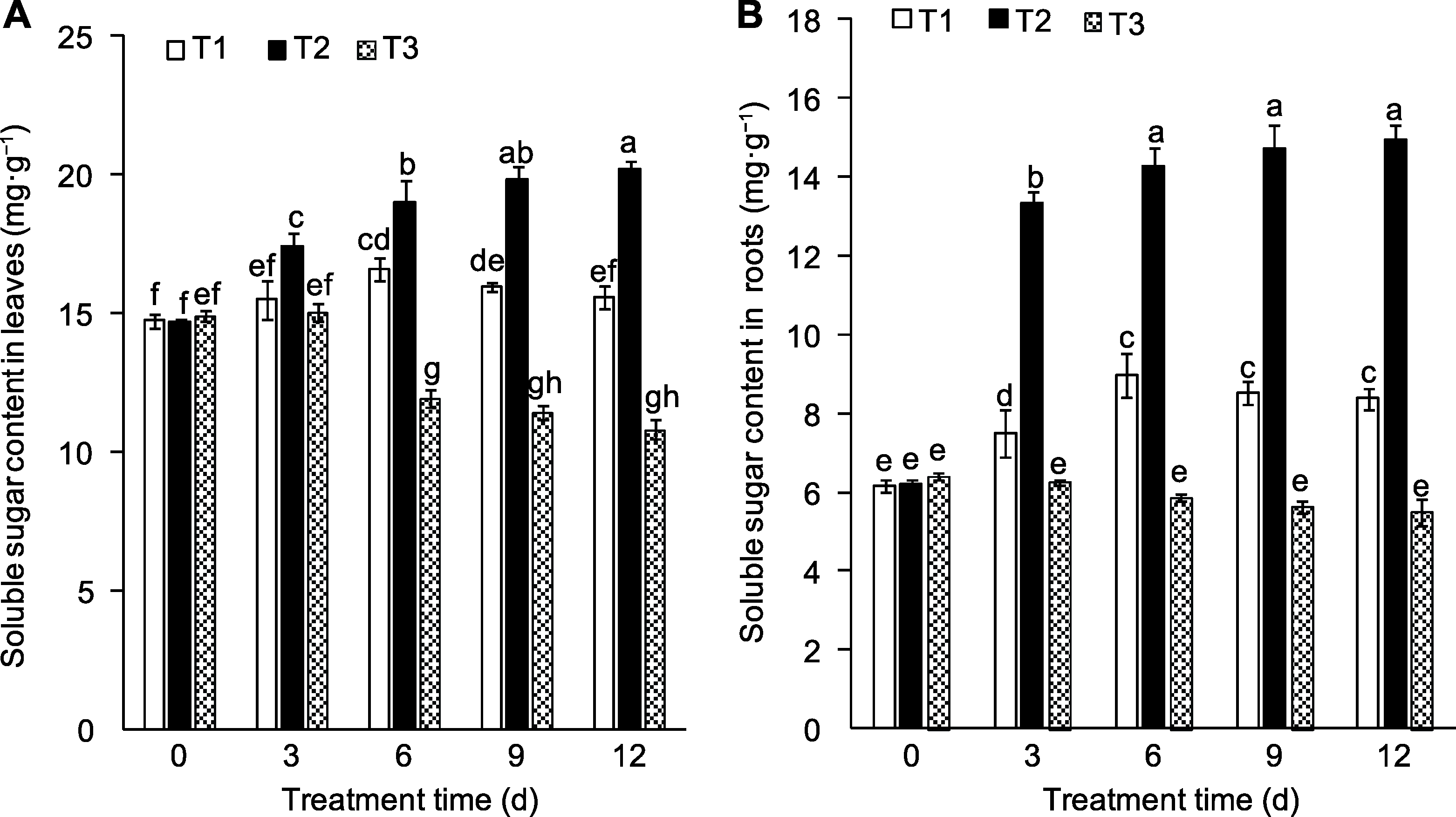
Figure 3 Effects of exogenous sucrose on soluble sugar contents in leaves (A) and roots (B) of peach seedlings T1-T3 see Ffddigure 2. Different lowercase letters above the bars indicate significant differences among different treatments (P<0.05).
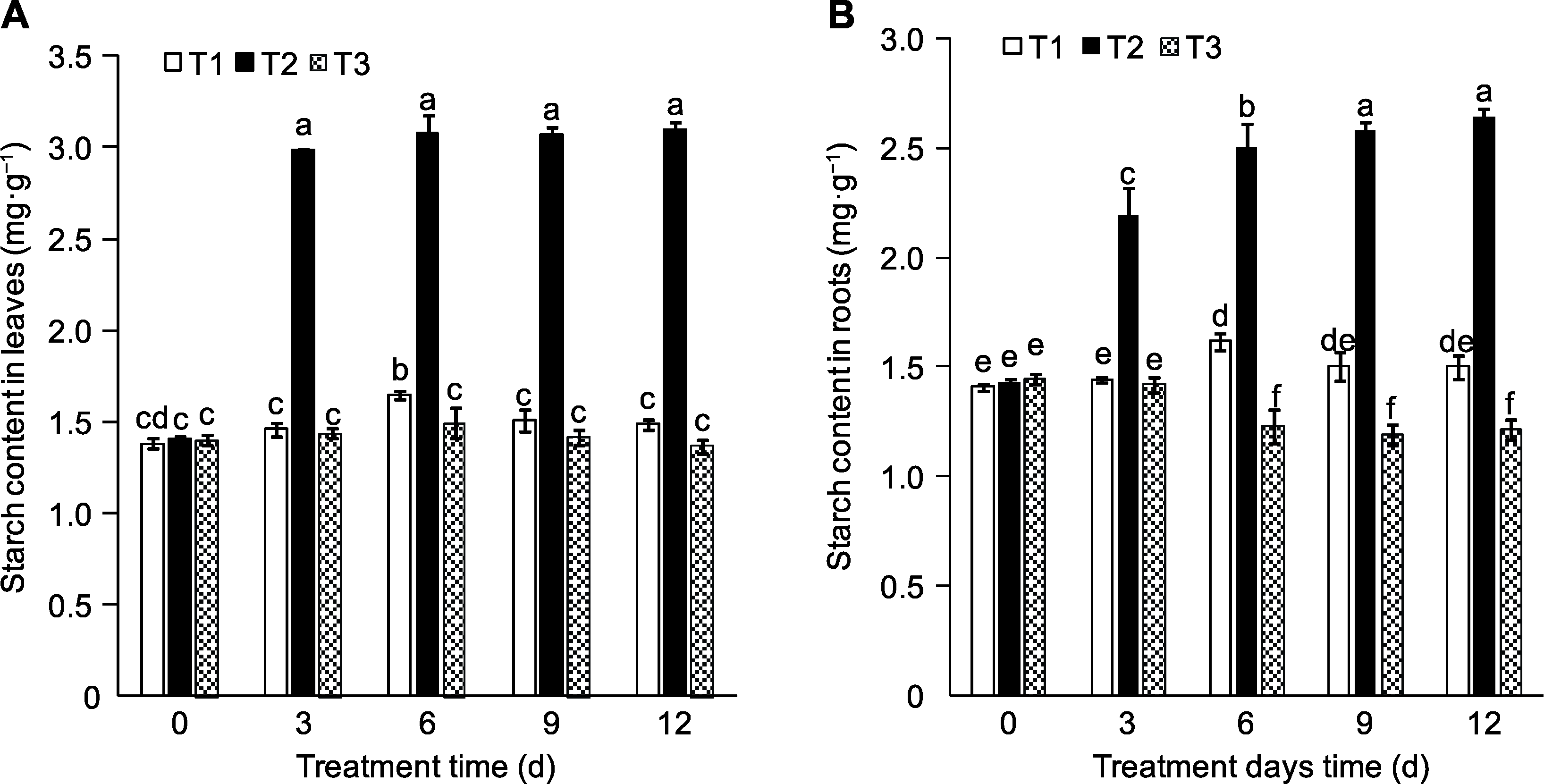
Figure 4 Effects of exogenous sucrose on starch contents in leaves (A) and roots (B) of peach seedlings T1-T3 see Ffddigure 2. Different lowercase letters above the bars indicate significant differences among different treatments (P<0.05).
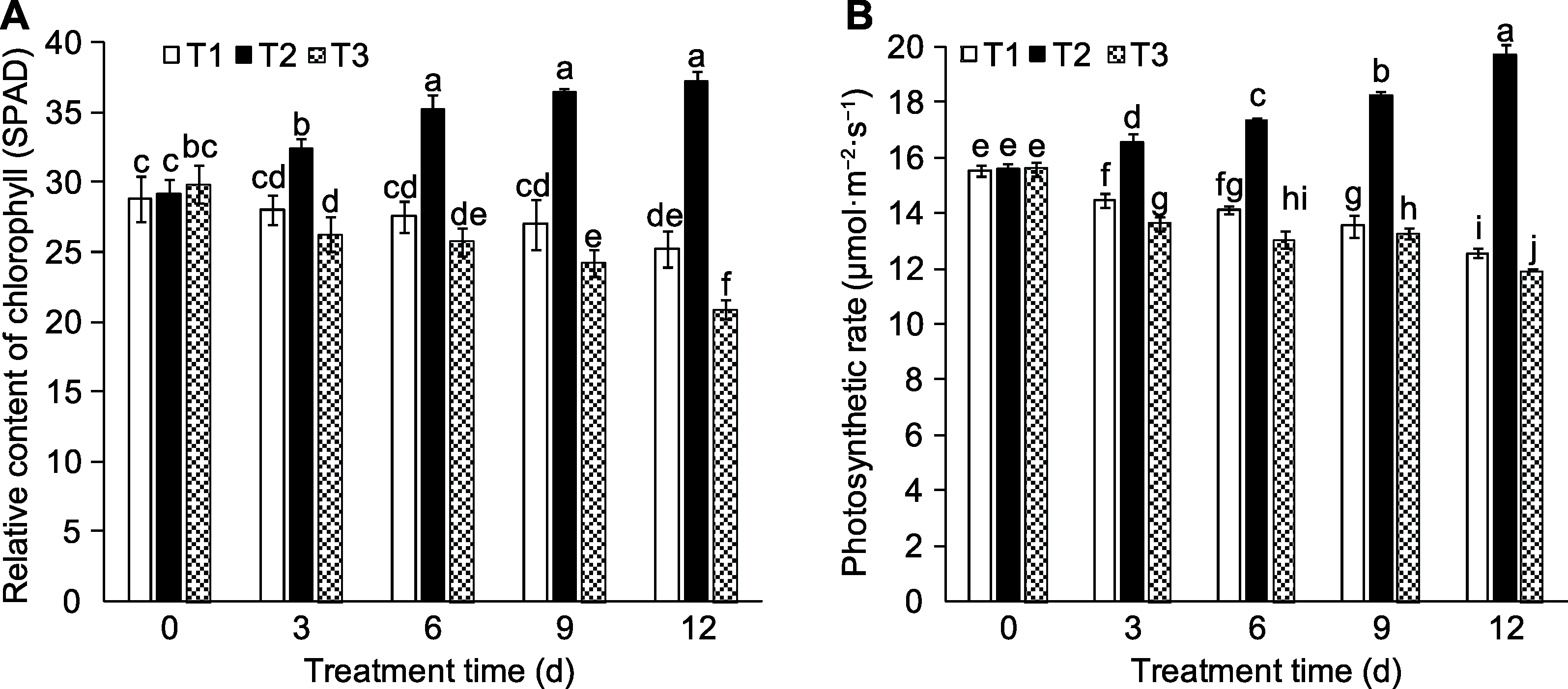
Figure 5 Effects of exogenous sucrose on relative content of chlorophyll (A) and photosynthetic rate (B) of peach leaves T1-T3 see Ffddigure 2. Different lowercase letters above the bars indicate significant differences among different treatments (P<0.05).
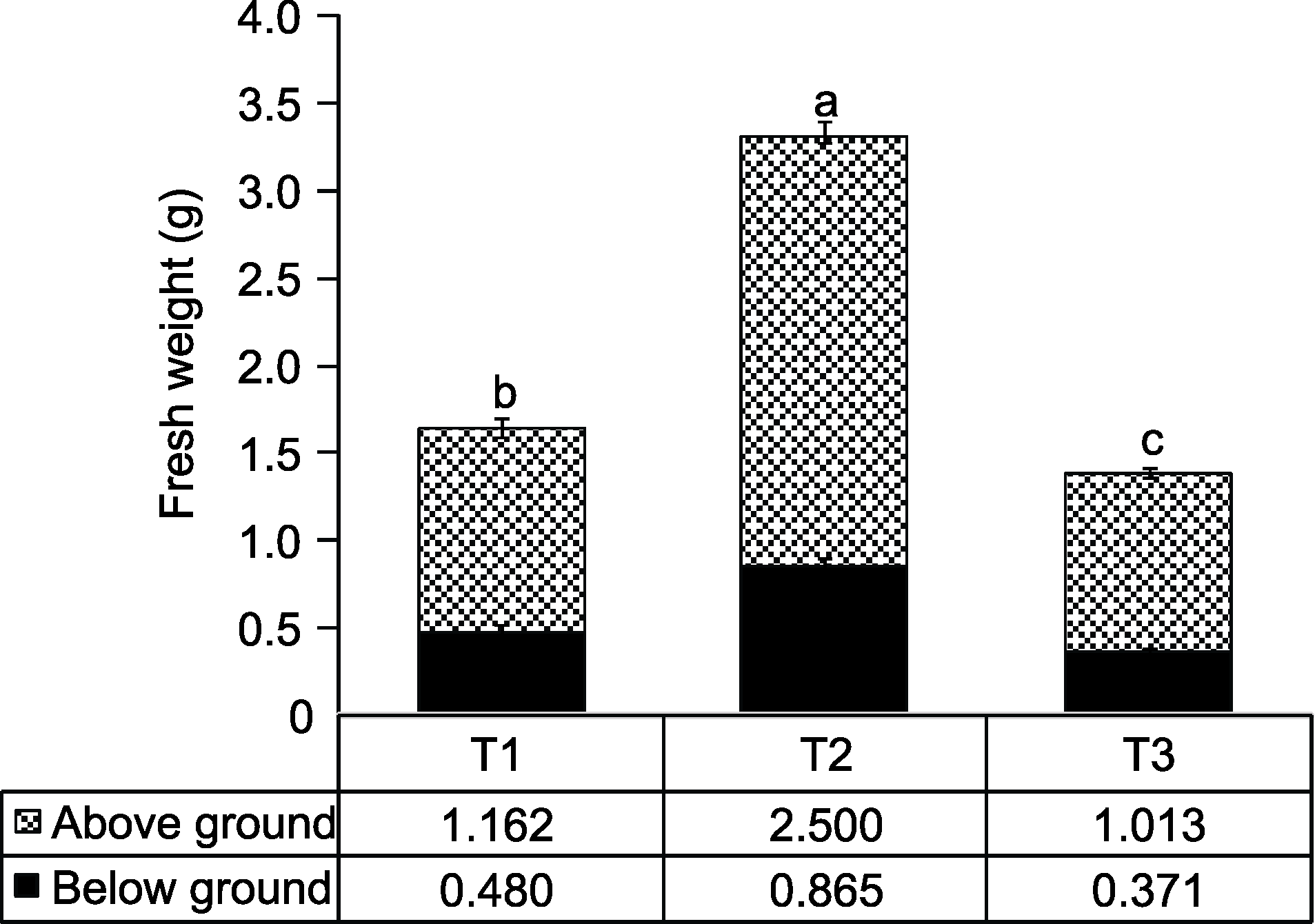
Figure 6 Effects of exogenous sucrose on fresh weight of peach seedlings T1-T3 see Ffddigure 2. Different lowercase letters above the bars indicate significant differences among different treatments (P<0.05).
| Experimental treatments | Number of primary lateral roots | Number of secondary lateral roots | Total root length (cm) | Total root surface area (cm2) |
|---|---|---|---|---|
| T1 | 235.00±14.42 b | 123.67±7.02 b | 86.63±6.01 b | 9.82±1.00 b |
| T2 | 417.33±14.47 a | 338.00±12.77 a | 147.66±14.99 a | 17.82±1.49 a |
| T3 | 107.67±8.96 c | 62.67±7.64 c | 67.67±3.71 c | 6.75±0.54 c |
Table 2 Effects of exogenous sucrose on the root architecture of peach seedlings
| Experimental treatments | Number of primary lateral roots | Number of secondary lateral roots | Total root length (cm) | Total root surface area (cm2) |
|---|---|---|---|---|
| T1 | 235.00±14.42 b | 123.67±7.02 b | 86.63±6.01 b | 9.82±1.00 b |
| T2 | 417.33±14.47 a | 338.00±12.77 a | 147.66±14.99 a | 17.82±1.49 a |
| T3 | 107.67±8.96 c | 62.67±7.64 c | 67.67±3.71 c | 6.75±0.54 c |
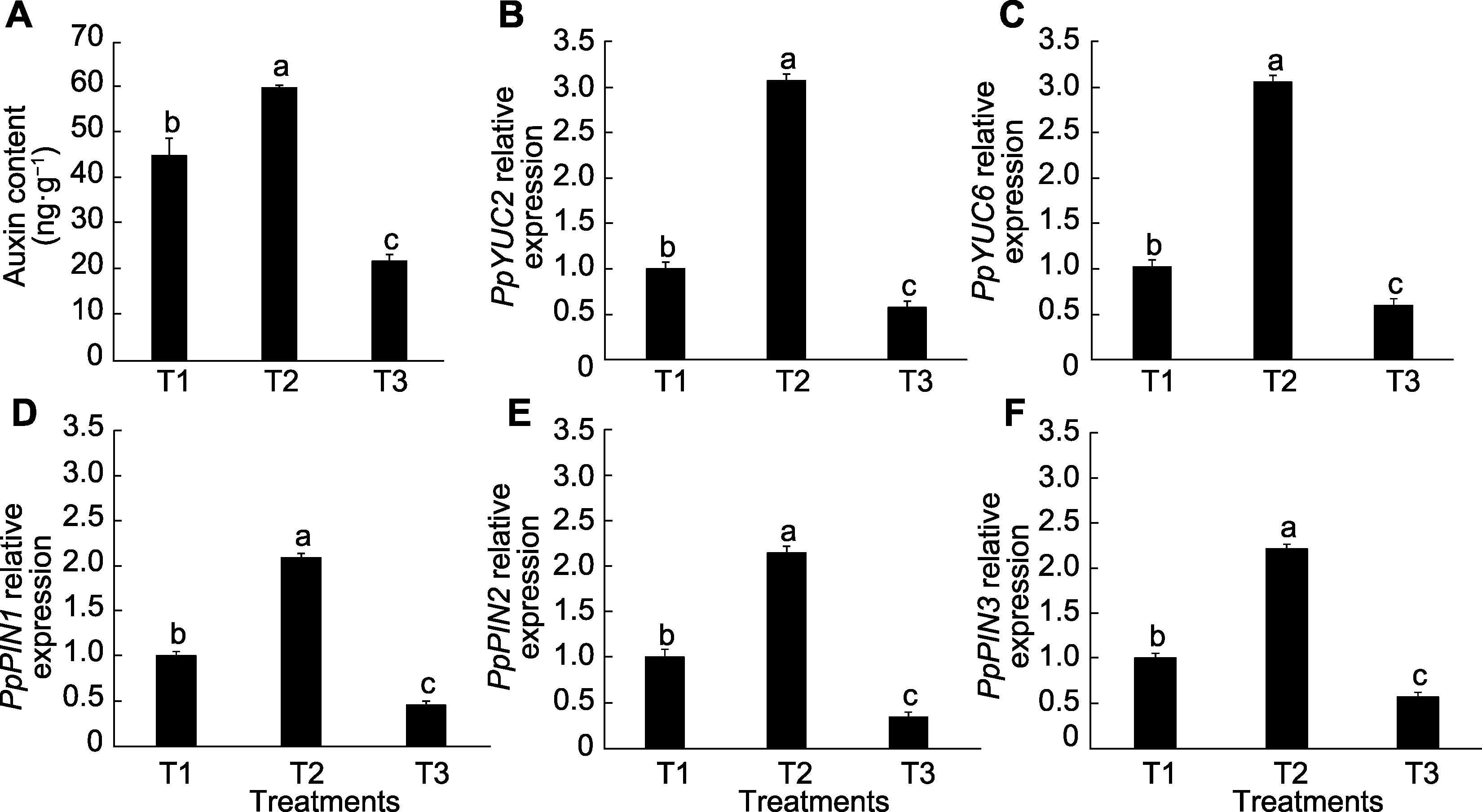
Figure 8 Effects of exogenous sucrose on auxin content (A) and gene expression (B-F) in peach seedling roots T1-T3 see Ffddigure 2. Different lowercase letters above the bars indicate significant differences among different treatments (P<0.05).
| [1] | 董栓泉, 熊茜, 王春幸, 王鸿飞, 邵兴锋, 李和生, 许凤 ( 2016). 蔗糖在延缓青花菜黄化过程中维持其能量和抗氧化力. 园艺学报 43, 1825-1833. |
| [2] | 郭承彬, 董凤丽, 吴明阳 ( 2018). 果树根系生长发育的研究进展及调控应用. 现代园艺 ( 21), 15-17. |
| [3] | 刘丽萍, 臧小云, 袁巧云, 蔡庆生 ( 2006). 外源蔗糖对盐胁迫荞麦幼苗根系生长的缓解效应. 植物生理学通讯 42, 847-850. |
| [4] | 罗静静, 张亚飞, 彭妍, 彭福田, 赵永飞, 于雯 ( 2017). 桃PpSnRK1α在番茄中过表达对营养胁迫下植株生长的影响. 园艺学报 44, 644-652. |
| [5] | 邱念伟, 邓樱 ( 2007). 外源蔗糖显著缓解盐和热胁迫对菠菜PSII颗粒的伤害. 植物学通报 24, 484-489. |
| [6] | 杨凯, 郝锋珍, 续海红, 郭向红, 张鹏飞 ( 2015). 果树根系分布研究进展. 中国农学通报 31(22), 130-135. |
| [7] | 赵世杰, 史国安, 董新纯 (2002). 植物生理学实验指导. 北京: 中国农业科学技术出版社 pp. 84-85, 87-88. |
| [8] | Boriboonkaset T, Theerawitaya C, Yamada N, Pichakum A, Supaibulwatana K, Cha-Um S, Takabe T, Kirdmanee C ( 2013). Regulation of some carbohydrate metabolism-related genes, starch and soluble sugar contents, photosynthetic activities and yield attributes of two contrasting rice genotypes subjected to salt stress. Protoplasma 250, 1157-1167. |
| [9] | Debast S, Nunes-Nesi A, Hajirezaei MR, Hofmann J, Sonnewald U, Fernie AR, Börnke F ( 2011). Altering trehalose-6-phosphate content in transgenic potato tubers growth and alters responsiveness to hormones during sprouting. Plant Physiol 156, 1754-1771. |
| [10] | Emanuelle S, Doblin MS, Stapleton DI, Bacic A, Gooley PR ( 2016). Molecular insights into the enigmatic metabolic regulator, SnRK1. Trends Plant Sci 21, 341-353. |
| [11] | Fujita M, Fujita Y, Noutoshi Y, Takahashi F, Narusaka Y, Yamaguchi-Shinozaki K, Shinozaki K ( 2006). Crosstalk between abiotic and biotic stress responses: a current view from the points of convergence in the stress signaling networks. Curr Opin Plant Biol 9, 436-442. |
| [12] | Geigenberger P, Stitt M, Fernie AR ( 2004). Metabolic control analysis and regulation of the conversion of sucrose to starch in growing potato tubers. Plant Cell Environ 27, 655-673. |
| [13] | Ho SL, Chao YC, Tong WF, Yu SM ( 2001). Sugar coordinately and differentially regulates growth and stress- related gene expression via a complex signal transduction network and multiple control mechanisms. Plant Physiol 125, 877-890. |
| [14] | Jossier M, Bouly JP, Meimoun P, Arjmand A, Lessard P, Hawley S, Hardie DG, Thomas M ( 2009). SnRK1 (SNF1related kinase 1) has a central role in sugar and ABA signaling in Arabidopsis thaliana. Plant J 59, 316-328. |
| [15] | Lastdrager J, Hanson J, Smeekens S ( 2014). Sugar signals and the control of plant growth and development. J Exp Bot 65, 799-807. |
| [16] | Nukarinen E, Nägele T, Pedrotti L, Wurzinger B, Mair A, Landgraf R, Börnke F, Hanson J, Teige M, Baena- Gonzalez E, Dröge-Laser W, Weckwerth W ( 2016). Quantitative phosphoproteomics reveals the role of the AMPK plant ortholog SnRK1 as a metabolic master regulator under energy deprivation. Sci Rep 6, 31697. |
| [17] | Piattoni CV, Bustos DM, Guerrero SA, Iglesias AÁ ( 2011). Nonphosphorylating glyceraldehyde-3-phosphate dehydrogenase is phosphorylated in wheat endosperm at serine-404 by an SNF1-related protein kinase allosterically inhibited by ribose-5-phosphate. Plant Physiol 156, 1337-1350. |
| [18] | Rolland F, Baena-Gonzalez E, Sheen J ( 2006). Sugar sensing and signaling in plants: conserved and novel mechanisms. Ann Rev Plant Biol 57, 675-709. |
| [19] | Smeekens S, Ma JK, Hanson J, Rolland F ( 2010). Sugar signals and molecular networks controlling plant growth. Curr Opin Plant Biol 13, 274-279. |
| [20] | Wurzinger B, Nukarinen E, Nägele T, Weckwerth W, Teige M ( 2018). The SnRK1 kinase as central mediator of energy signaling between different organelles. Plant Physiol 176, 1085-1094. |
| [21] | Yu S, Cao L, Zhou CM, Zhang TQ, Lian H, Sun Y, Wu JQ, Huang JR, Wang GD, Wang JW ( 2013). Sugar is an endogenous cue for juvenile-to-adult phase transition in plants. eLife 2, e00269. |
| [22] | Yu SM, Lo SF, Ho THD ( 2015). Source-sink communication: regulated by hormone, nutrient, and stress cross-signaling. Trends Plant Sci 20, 844-857. |
| [23] | Zhang YH, Primavesi LF, Jhurreea D, Andralojc PJ, Mitchell RAC, Powers SJ, Schluepmann H, Delatte T, Wingler A, Paul MJ ( 2009). Inhibition of SNF1-related protein kinase 1 activity and regulation of metabolic pathways by trehalose-6-phosphate. Plant Physiol 149, 1860-1871. |
| [1] | Yuying Zhou, Hui Chen, Simu Liu. Research Progress on Auxin Responsive Non-canonical Aux/IAA Proteins in Plants [J]. Chinese Bulletin of Botany, 2024, 59(4): 651-658. |
| [2] | Yi Song, Hanghang Chen, Xin Cui, Zhifeng Lu, Shipeng Liao, Yangyang Zhang, Xiaokun Li, Rihuan Cong, Tao Ren, Jianwei Lu. Potassium Nutrient Status-mediated Leaf Growth of Oilseed Rape (Brassica napus) and Its Effect on Phyllosphere Microorganism [J]. Chinese Bulletin of Botany, 2024, 59(1): 54-65. |
| [3] | Xiangpei Kong, Mengyue Zhang, Zhaojun Ding. There Is a Way Out-new Breakthroughs in Extracellular Auxin Sensing [J]. Chinese Bulletin of Botany, 2023, 58(6): 861-865. |
| [4] | Yuan Yuan, Enhebayaer, Qi Yanhua. Research Advances in Biological Functions of GH3 Gene Family in Plants [J]. Chinese Bulletin of Botany, 2023, 58(5): 770-782. |
| [5] | Ziwen Tang, Dongping Zhang. Research Progress on the Molecular Mechanism of Starch Accumulation in Rice Endosperm [J]. Chinese Bulletin of Botany, 2023, 58(4): 612-621. |
| [6] | Shuyao Zhou, Jianming Li, Juan Mao. AtGH3.17-mediated Regulation of Auxin and Brassinosteroid Response in Arabidopsis thaliana [J]. Chinese Bulletin of Botany, 2023, 58(3): 373-384. |
| [7] | Ye Qing, Yan Xiaoyan, Chen Huize, Feng Jinlin, Han Rong. Effect of Nitrogen-doped Graphene Quantum Dots on Growth Direction of Primary Root in Arabidopsis thaliana [J]. Chinese Bulletin of Botany, 2022, 57(5): 623-634. |
| [8] | Li Chen, Liu Jianting, Fan Yongxin, Zhao Xuehui, Xiao Wei, Chen Xiude, Fu Xiling, Li Ling, Li Dongmei. Effects of UV-B on Photosynthetic Function and Chloroplast Ultrastructure of Peach Leaves Grown in Greenhouse [J]. Chinese Bulletin of Botany, 2022, 57(4): 434-443. |
| [9] | Lixia Jia, Yanhua Qi. Advances in the Regulation of Rice (Oryza sativa) Grain Shape by Auxin Metabolism, Transport and Signal Transduction [J]. Chinese Bulletin of Botany, 2022, 57(3): 263-275. |
| [10] | Binqi Li, Jiahui Yan, Hao Li, Wei Xin, Yunhe Tian, Zhenbiao Yang, Wenxin Tang. Changes of Small GTPases Activity During Cucumber Tendril Winding [J]. Chinese Bulletin of Botany, 2022, 57(3): 299-307. |
| [11] | Jingwen Wang, Xingjun Wang, Changle Ma, Pengcheng Li. A Review on the Mechanism of Ribosome Stress Response in Plants [J]. Chinese Bulletin of Botany, 2022, 57(1): 80-89. |
| [12] | Yanyan Li, Yanhua Qi. Advances in Biological Functions of Aux/IAA Gene Family in Plants [J]. Chinese Bulletin of Botany, 2022, 57(1): 30-41. |
| [13] | Yuqing Lin, Yanhua Qi. Advances in Auxin Efflux Carrier PIN Proteins [J]. Chinese Bulletin of Botany, 2021, 56(2): 151-165. |
| [14] | Rongfeng Huang, Tongda Xu. Auxin Regulates the Lateral Root Development Through MAPK-mediated VLCFAs Biosynthesis [J]. Chinese Bulletin of Botany, 2021, 56(1): 6-9. |
| [15] | Yuting Yao,Jiaqi Ma,Xiaoli Feng,Jianwei Pan,Chao Wang. A Role of Arabidopsis Phosphoinositide Kinase, FAB1, in Root Hair Growth [J]. Chinese Bulletin of Botany, 2020, 55(2): 126-136. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||