

Chinese Bulletin of Botany ›› 2023, Vol. 58 ›› Issue (5): 770-782.DOI: 10.11983/CBB22263 cstr: 32102.14.CBB22263
• SPECIAL TOPICS • Previous Articles Next Articles
Yuan Yuan1, Enhebayaer1,*( ), Qi Yanhua1,2,*(
), Qi Yanhua1,2,*( )
)
Received:2022-11-17
Accepted:2023-02-13
Online:2023-09-01
Published:2023-09-21
Contact:
*E-mail: nmsdenhe@imnu.edu.cn; qyhjp@zju.edu.cn
Yuan Yuan, Enhebayaer, Qi Yanhua. Research Advances in Biological Functions of GH3 Gene Family in Plants[J]. Chinese Bulletin of Botany, 2023, 58(5): 770-782.
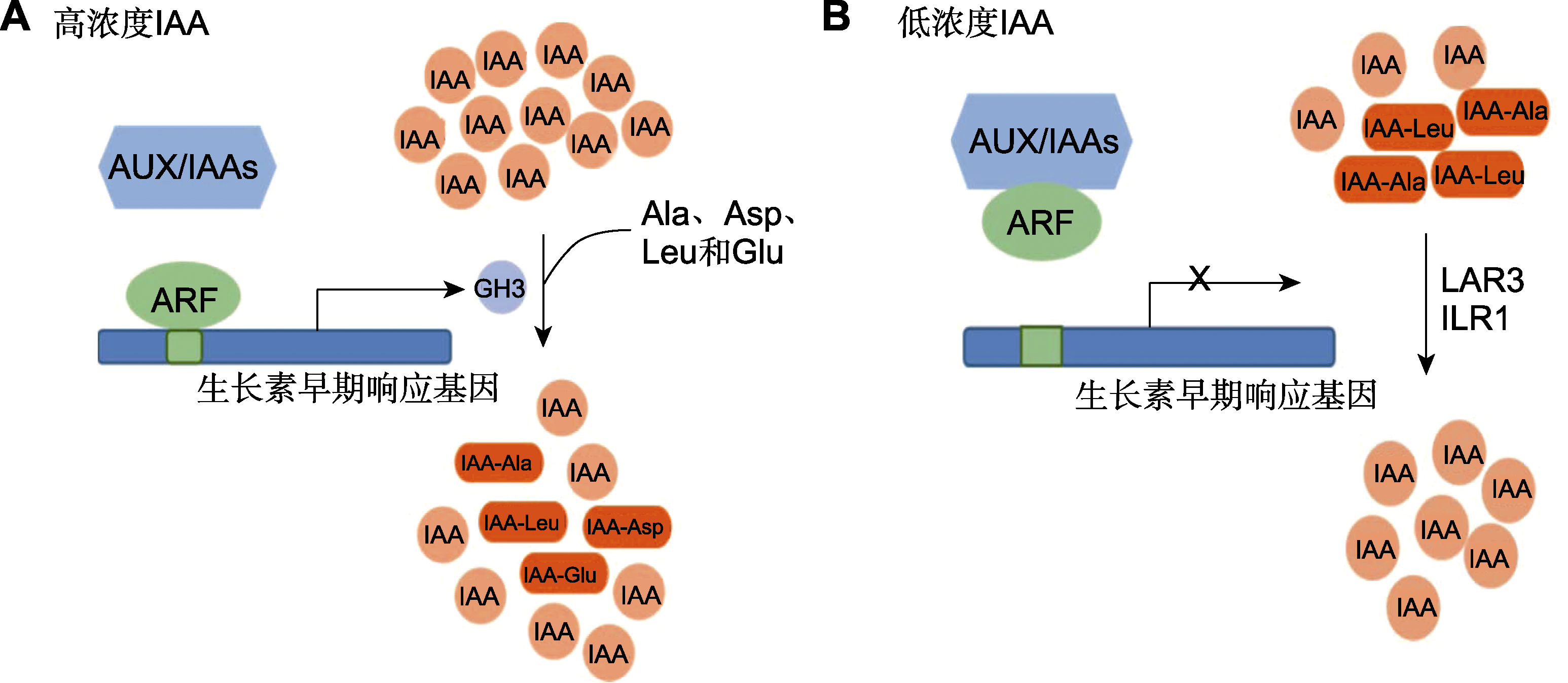
Figure 2 Auxin (IAA) dynamic equilibrium in plants (refer to Hagen and Guilfoyle, 2002; Woodward and Bartel, 2005) (A) Under higher IAA concentration, ARF splits with Aux/IAAs dimer, and ARF binds to AuxREs and activates the expression of GH3, which catalyzes the binding of IAA to amino acids; (B) Under lower IAA concentration, ARF forms a dimer with Aux/IAAs and turns off AuxREs, GH3 gene transcription is inhibited, and IAA-Ala and IAA-Leu, which are auxin reservoirs, are hydrolyzed by amidohydrolase to release IAA again.
| 基因编号 | 基因名称 | 功能 | 参考文献 |
|---|---|---|---|
| At2g14960.1 | AtGH3.1 | 调节吲哚-3-乙酸(IAA)动态平衡 | Staswick et al., |
| At4g37390.1 | AtGH3.2/YDK1 | 调控株高、主根伸长、侧根数与顶端优势 | Takase et al., |
| At4g27260.1 | AtGH3.5/AtGH3a/WES1 | 调控主根伸长及侧根数, 参与水杨酸(SA)与光信号途径 | Staswick et al., |
| At5g54510.1 | AtGH3.6/DFL1 | 调控生长素含量, 影响株高、叶形态、侧根数及非生物胁迫响应, 参与光信号途径, 影响下胚轴伸长 | Nakazawa et al., |
| At2g47750.1 | AtGH3.9 | 调控株高、花器官以及果实发育 | 周苹等, |
| At4g03400.1 | AtGH3.10/DFL2 | 参与光信号途径, 影响下胚轴伸长 | Takase et al., |
| At2g46370.1 | AtGH3.11/JAR1/FIN219 | 调控茉莉酸(JA)水平, 影响抗病性, 参与光信号转导途径 | Staswick et al., |
| At5g13320.1 | AtGH3.12/PBS3/GDG1 | 调控SA水平, 影响植物抗病性 | Jagadeeswaran et al., |
| At5g13370.1 | AtGH3.15 | 调节吲哚丁酸(IBA)水平, 影响主根伸长与侧根数 | Sherp et al., |
| At1g28130.1 | AtGH3.17 | 调控株高、根系发育及叶片形态, 参与油菜素内酯(BL)信号途径 | 周淑瑶等, |
Table 1 Functions of GH3 genes in Arabidopsis thaliana
| 基因编号 | 基因名称 | 功能 | 参考文献 |
|---|---|---|---|
| At2g14960.1 | AtGH3.1 | 调节吲哚-3-乙酸(IAA)动态平衡 | Staswick et al., |
| At4g37390.1 | AtGH3.2/YDK1 | 调控株高、主根伸长、侧根数与顶端优势 | Takase et al., |
| At4g27260.1 | AtGH3.5/AtGH3a/WES1 | 调控主根伸长及侧根数, 参与水杨酸(SA)与光信号途径 | Staswick et al., |
| At5g54510.1 | AtGH3.6/DFL1 | 调控生长素含量, 影响株高、叶形态、侧根数及非生物胁迫响应, 参与光信号途径, 影响下胚轴伸长 | Nakazawa et al., |
| At2g47750.1 | AtGH3.9 | 调控株高、花器官以及果实发育 | 周苹等, |
| At4g03400.1 | AtGH3.10/DFL2 | 参与光信号途径, 影响下胚轴伸长 | Takase et al., |
| At2g46370.1 | AtGH3.11/JAR1/FIN219 | 调控茉莉酸(JA)水平, 影响抗病性, 参与光信号转导途径 | Staswick et al., |
| At5g13320.1 | AtGH3.12/PBS3/GDG1 | 调控SA水平, 影响植物抗病性 | Jagadeeswaran et al., |
| At5g13370.1 | AtGH3.15 | 调节吲哚丁酸(IBA)水平, 影响主根伸长与侧根数 | Sherp et al., |
| At1g28130.1 | AtGH3.17 | 调控株高、根系发育及叶片形态, 参与油菜素内酯(BL)信号途径 | 周淑瑶等, |
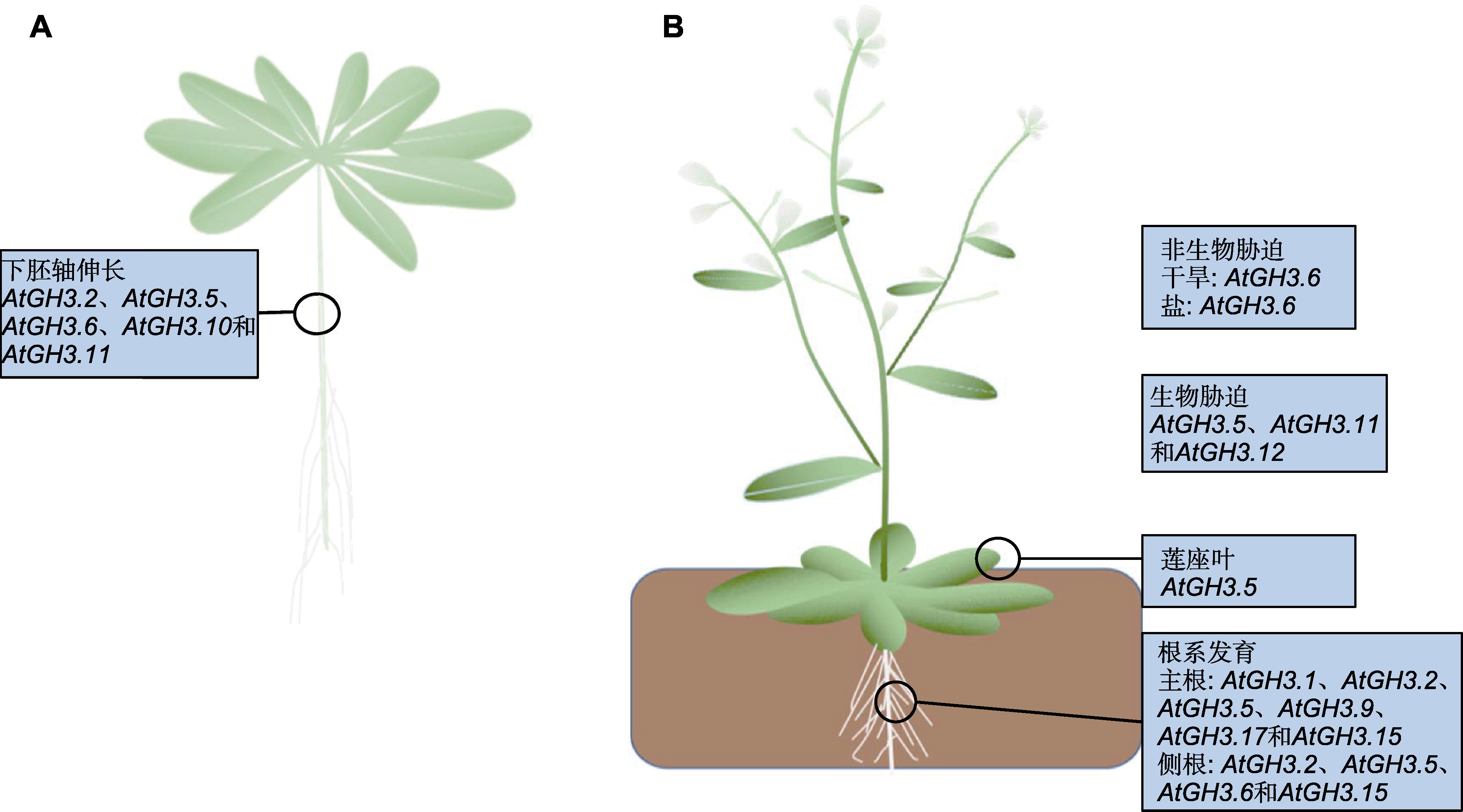
Figure 3 The function of GH3 genes in Arabidopsis growth and development (refer to Staswick et al., 1998, 2005; van Loon et al., 1998; Hsieh et al., 2000; Overmyer et al., 2000; Rao et al., 2000; Nakazawa et al., 2001; Takase et al., 2003, 2004; Park et al., 2007b; Jagadeeswaran et al., 2007; Nobuta et al., 2007; Zhang et al., 2007; 2008; Zhou et al., 2015, in Chinese; Liu et al., 2016, in Chinese; Sherp et al., 2018; Zhou et al., 2023, in Chinese) (A) Arabidopsis seedling; (B) Arabidopsis mature plant
| 基因编号 | 基因名称 | 功能 | 参考文献 |
|---|---|---|---|
| LOC_Os01g57610.1 | OsGH3.1/LC1 | 调控株型, 参与油菜素内酯(BL)信号途径与抗病性 | Domingo et al., |
| LOC_Os01g55940.1 | OsGH3.2 | 调控株型和根系发育, 响应低温和干旱胁迫 | Du et al., |
| LOC_Os01g12160.1 | OsGH3.3 | 参与茉莉酸(JA)信号转导途径 | Hui et al., |
| LOC_Os05g50890.1 | OsGH3.5/OsJAR1 | 调控水稻株高和叶夹角, 参与光信号途径 | Riemann et al., |
| LOC_Os05g05180.2 | OsGH3.6 | 参与JA信号转导途径 | Hui et al., |
| LOC_Os07g40290.1 | OsGH3.8 | 调控株型及根系发育, 影响种子发芽率和可育性, 参与抗病性 | Ding et al., |
| LOC_Os11g08340.1 | OsGH3.12 | 参与JA信号转导途径 | Hui et al., |
| LOC_Os11g32520.1 | OsGH3.13 | 调控株型及根系发育, 响应干旱胁迫 | Zhang et al., |
Table 2 Functions of GH3 genes in rice
| 基因编号 | 基因名称 | 功能 | 参考文献 |
|---|---|---|---|
| LOC_Os01g57610.1 | OsGH3.1/LC1 | 调控株型, 参与油菜素内酯(BL)信号途径与抗病性 | Domingo et al., |
| LOC_Os01g55940.1 | OsGH3.2 | 调控株型和根系发育, 响应低温和干旱胁迫 | Du et al., |
| LOC_Os01g12160.1 | OsGH3.3 | 参与茉莉酸(JA)信号转导途径 | Hui et al., |
| LOC_Os05g50890.1 | OsGH3.5/OsJAR1 | 调控水稻株高和叶夹角, 参与光信号途径 | Riemann et al., |
| LOC_Os05g05180.2 | OsGH3.6 | 参与JA信号转导途径 | Hui et al., |
| LOC_Os07g40290.1 | OsGH3.8 | 调控株型及根系发育, 影响种子发芽率和可育性, 参与抗病性 | Ding et al., |
| LOC_Os11g08340.1 | OsGH3.12 | 参与JA信号转导途径 | Hui et al., |
| LOC_Os11g32520.1 | OsGH3.13 | 调控株型及根系发育, 响应干旱胁迫 | Zhang et al., |
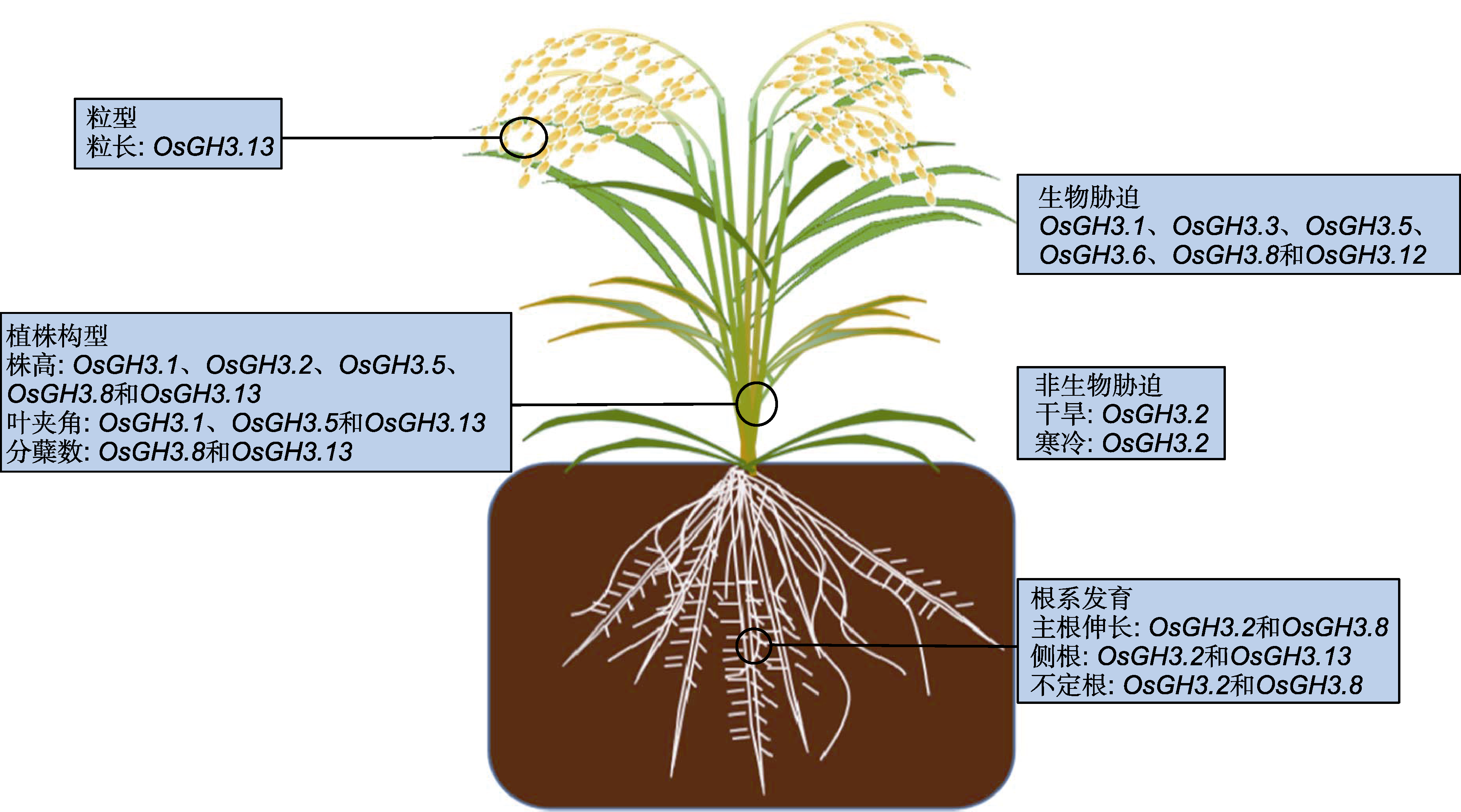
Figure 4 The function of GH3 gene in rice during growth and development (refer to Yang et al., 2006; Qiu et al., 2007; Ding et al., 2008; Domingo et al., 2009; Tao et al., 2009; Zhang et al., 2009, 2015; Du et al., 2012; Zhao et al., 2013; Dai et al., 2018; Hui et al., 2019; Liu et al., 2022)
| 物种名称 | 基因编号 | 基因名称 | 功能 | 参考文献 |
|---|---|---|---|---|
| 番茄 | Solyc01g107390.4.1 | SlGH3.2 | 调控株高、结实率及种子大小 | 王慧敏, |
| Solyc02g092820.4.1 | SlGH3.4 | 调节吲哚-3-乙酸(IAA)含量, 影响抗病性 | 陈潇, | |
| Solyc07g054580.3.1 | SlGH3.8 | 调控IAA含量, 影响植物生长发育 | Sun et al., | |
| Solyc12g005310.2.1 | SlGH3.15 | 调控株高、叶片形态及侧根数目 | 艾国, | |
| 马铃薯 | - | StGH3.1/StGH3.5 | 参与茉莉酸(JA)信号转导途径 | 张超, |
| 柑橘 | Cs1g22140 | CrGH3.1 | 调节IAA含量, 影响抗病性 | Chen et al., |
| Cs8g04610 | CrGH3.6 | 调控株高和叶片形态, 影响抗病性 | 邹修平等, | |
| Ciclev10017968m.g/ Ciclev10019393m.g | CrGH3.4/CrGH3.7 | 参与胁迫响应 | 庞少萍等, | |
| 小麦 | TraesCS1A02G425100.1/ TraesCS2B02G210600.1/ TraesCS3A02G301200.1/ TraesCS3B02G335300.1/ TraesCS3D02G300600.1 | TaGH3.2a/ TaGH3.7/ TaGH3.11/ TaGH3.13/ TaGH3.15 | 参与根系发育和非生物胁迫响应 | Jiang et al., |
Table 3 Functions of GH3 genes in other plants
| 物种名称 | 基因编号 | 基因名称 | 功能 | 参考文献 |
|---|---|---|---|---|
| 番茄 | Solyc01g107390.4.1 | SlGH3.2 | 调控株高、结实率及种子大小 | 王慧敏, |
| Solyc02g092820.4.1 | SlGH3.4 | 调节吲哚-3-乙酸(IAA)含量, 影响抗病性 | 陈潇, | |
| Solyc07g054580.3.1 | SlGH3.8 | 调控IAA含量, 影响植物生长发育 | Sun et al., | |
| Solyc12g005310.2.1 | SlGH3.15 | 调控株高、叶片形态及侧根数目 | 艾国, | |
| 马铃薯 | - | StGH3.1/StGH3.5 | 参与茉莉酸(JA)信号转导途径 | 张超, |
| 柑橘 | Cs1g22140 | CrGH3.1 | 调节IAA含量, 影响抗病性 | Chen et al., |
| Cs8g04610 | CrGH3.6 | 调控株高和叶片形态, 影响抗病性 | 邹修平等, | |
| Ciclev10017968m.g/ Ciclev10019393m.g | CrGH3.4/CrGH3.7 | 参与胁迫响应 | 庞少萍等, | |
| 小麦 | TraesCS1A02G425100.1/ TraesCS2B02G210600.1/ TraesCS3A02G301200.1/ TraesCS3B02G335300.1/ TraesCS3D02G300600.1 | TaGH3.2a/ TaGH3.7/ TaGH3.11/ TaGH3.13/ TaGH3.15 | 参与根系发育和非生物胁迫响应 | Jiang et al., |
| [1] | 艾国 (2017). 番茄SlGH3-15基因的功能解析. 硕士论文. 武汉: 华中农业大学. pp. 7-8. |
| [2] | 陈潇 (2017). 番茄吲哚-3-乙酸氨基合成酶基因SlGH3.4在丛枝菌根共生中的功能和调控机制研究. 博士论文. 南京: 南京农业大学. pp. 62-63. |
| [3] | 李超, 牛宇鹏, 吴俊彩 (2021). 马铃薯生全粉护色工艺研究. 安徽农业科学 49, 188-190. |
| [4] | 黎家, 李传友 (2019). 新中国成立70年来植物激素研究进展. 中国科学: 生命科学 49, 1227-1281. |
| [5] | 李梦莎, 阎秀峰 (2014). 植物的环境信号分子茉莉酸及其生物学功能. 生态学报 34, 6779-6788. |
| [6] |
刘晓东, 王若仲, 焦彬彬, 代培红, 李月 (2016). 拟南芥IAA酰胺合成酶GH3-6负调控干旱和盐胁迫的反应. 植物学报 51, 586-593.
DOI |
| [7] |
庞少萍, 谢让金, 马岩岩, 钱春, 何绍兰, 易时来, 吕强, 郑永强, 邓烈 (2015). 柑橘CjGH3.4和CjGH3.7基因的生物信息学分析及表达分析. 园艺学报 42, 2362-2372.
DOI |
| [8] | 王慧敏 (2015). 番茄SlGH3.2的表达特征及在水稻中的功能研究分析. 硕士论文. 南京: 南京农业大学. pp. 6-7. |
| [9] | 谢小芳, 黄勤怡, 吴为人 (2010). 植物GH3基因家族的生物信息学分析. 基因组学与应用生物学 29, 829-837. |
| [10] | 张超 (2021). 茉莉酸调控基因GH3家族的鉴定及在马铃薯中抗病及损伤分析. 博士论文. 杨凌: 西北农林科技大学. pp. 26-62. |
| [11] | 周苹, 唐冬英, 郭明, 谭振华, 赵小英, 刘选明 (2015). 拟南芥GH3.9基因的过表达及其表型分析. 西北植物学报 35, 454-458. |
| [12] |
周淑瑶, 李建明, 毛娟 (2023). AtGH3.17调控拟南芥生长素和油菜素甾醇的响应. 植物学报 58, 373-384.
DOI |
| [13] |
邹修平, 龙俊宏, 彭爱红, 陈敏, 龙琴, 陈善春 (2019). 超量表达CsGH3.6通过抑制生长素信号转导增强柑橘溃疡病抗性. 中国农业科学 52, 3806-3818.
DOI |
| [14] | Bierfreund NM, Tintelnot S, Reski R, Decker EL (2004). Loss of GH3 function does not affect phytochrome-mediated development in a moss, Physcomitrella patens. J Plant Physiol 161, 823-835. |
| [15] |
Chang KH, Xiang H, Dunaway-Mariano D (1997). Acyladenylate motif of the acyl-adenylate/thioester-forming enzyme superfamily: a site-directed mutagenesis study with the Pseudomonas sp. strain CBS3 4-chlorobenzoate: coenzyme A ligase. Biochemistry 36, 15650-15659.
PMID |
| [16] | Chen CY, Ho SS, Kuo TY, Hsieh HK, Cheng YS (2017). Structural basis of jasmonate-amido synthetase FIN219 in complex with glutathione S-transferase FIP1 during the JA signal regulation. Proc Natl Acad Sci USA 114, E1815- E1824. |
| [17] | Chen M, He YR, Xu LZ, Peng AH, Lei TG, Yao LX, Li Q, Zhou PF, Bai XJ, Duan MJ, Jiang XY, Jia RR, Zou XP, Chen SC (2016). Cloning and expression analysis of citrus genes CsGH3.1 and CsGH3.6 responding to Xanthomonas axonopodis pv. citri infection. Hortic Plant J 2, 193-202. |
| [18] |
Conner TW, Goekjian VH, La Fayette PR, Key JL (1990). Structure and expression of two auxin-inducible genes from Arabidopsis. Plant Mol Biol 15, 623-632.
PMID |
| [19] |
Dai ZY, Wang J, Yang XF, Lu H, Miao XX, Shi ZY (2018). Modulation of plant architecture by the miR156f-OsSPL7- OsGH3.8 pathway in rice. J Exp Bot 69, 5117-5130.
DOI URL |
| [20] |
Ding CH, Lin XH, Zuo Y, Yu ZL, Baerson SR, Pan ZQ, Zeng RS, Song YY (2021). Transcription factor OsbZIP49 controls tiller angle and plant architecture through the induction of indole-3-acetic acid-amido synthetases in rice. Plant J 108, 1346-1364.
DOI URL |
| [21] |
Ding XH, Cao YL, Huang LL, Zhao J, Xu CG, Li XH, Wang SP (2008). Activation of the indole-3-acetic acid-amido synthetase GH3-8 suppresses expansin expression and promotes salicylate- and jasmonate-independent basal immunity in rice. Plant Cell 20, 228-240.
DOI PMID |
| [22] |
Domingo C, Andrés F, Tharreau D, Iglesias DJ, Talón M (2009). Constitutive expression of OsGH3.1 reduces auxin content and enhances defense response and resistance to a fungal pathogen in rice. Mol Plant Microbe Interact 22, 201-210.
DOI URL |
| [23] |
Du H, Wu N, Fu J, Wang SP, Li XH, Xiao JH, Xiong LZ (2012). A GH3 family member, OsGH3-2, modulates auxin and abscisic acid levels and differentially affects drought and cold tolerance in rice. J Exp Bot 63, 6467-6480.
DOI PMID |
| [24] |
Fu J, Yu HH, Li XH, Xiao JH, Wang SP (2011). Rice GH3 gene family: regulators of growth and development. Plant Signal Behav 6, 570-574.
DOI URL |
| [25] |
Gulick AM (2009). Conformational dynamics in the Acyl-CoA synthetases, adenylation domains of non-ribosomal peptide synthetases, and firefly luciferase. ACS Chem Biol 4, 811-827.
DOI PMID |
| [26] |
Hagen G, Guilfoyle T (2002). Auxin-responsive gene expression: genes, promoters and regulatory factors. Plant Mol Biol 49, 373-385.
PMID |
| [27] |
Hagen G, Guilfoyle TJ (1985). Rapid induction of selective transcription by auxins. Mol Cell Biol 5, 1197-1203.
DOI PMID |
| [28] |
Hagen G, Kleinschmidt A, Guilfoyle T (1984). Auxin-regulated gene expression in intact soybean hypocotyl and excised hypocotyl sections. Planta 162, 147-153.
DOI PMID |
| [29] |
Hsieh HL, Okamoto H, Wang ML, Ang LH, Matsui M, Goodman H, Deng XW (2000). FIN219, an auxin-regulated gene, defines a link between phytochrome A and the downstream regulator COP1 in light control of Arabidopsis development. Genes Dev 14, 1958-1970.
DOI URL |
| [30] |
Hui SG, Hao MY, Liu HB, Xiao JH, Li XH, Yuan M, Wang SP (2019). The group I GH3 family genes encoding JA-Ile synthetase act as positive regulator in the resistance of rice to Xanthomonas oryzae pv. oryzae. Biochem Biophys Res Commun 508, 1062-1066.
DOI URL |
| [31] |
Jagadeeswaran G, Raina S, Acharya BR, Maqbool SB, Mosher SL, Appel HM, Schultz JC, Klessig DF, Raina R (2007). Arabidopsis GH3-LIKE DEFENSE GENE 1is required for accumulation of salicylic acid, activation of defense responses and resistance to Pseudomonas syringae. Plant J 51, 234-246.
PMID |
| [32] |
Jiang WQ, Yin JL, Zhang HT, He YQ, Shuai SM, Chen SH, Cao SL, Li W, Ma DF, Chen HG (2020). Genome-wide identification, characterization analysis and expression profiling of auxin-responsive GH3 family genes in wheat (Triticum aestivum L.). Mol Biol Rep 47, 3885-3907.
DOI |
| [33] |
Khan S, Stone JM (2007). Arabidopsis thaliana GH3.9 influences primary root growth. Planta 226, 21-34.
DOI URL |
| [34] |
Kumar R, Agarwal P, Tyagi AK, Sharma AK (2012). Genome-wide investigation and expression analysis suggest diverse roles of auxin-responsive GH3 genes during development and response to different stimuli in tomato (Solanum lycopersicum). Mol Genet Genom 287, 221-235.
DOI URL |
| [35] | Lee HM, Park JS, Kim SJ, Kim SG, Park YD (2022). Using transcriptome analysis to explore gray mold resistance- related genes in onion (Allium cepa L.). Genes (Basel) 13, 542. |
| [36] |
Liu CC, Liu YN, Cheng JF, Guo R, Tian L, Wang B (2022). Dual roles of OsGH3.2 in modulating rice root morphology and affecting arbuscular mycorrhizal symbiosis. Front Plant Sci 13, 853435.
DOI URL |
| [37] |
Liu KD, Kang BC, Jiang H, Moore SL, Li HX, Watkins CB, Setter TL, Jahn MM (2005). A GH3-like gene, CcGH3, isolated from Capsicum chinense L. fruit is regulated by auxin and ethylene. Plant Mol Biol 58, 447-464.
DOI URL |
| [38] |
Liu ZB, Ulmasov T, Shi X, Hagen G, Guilfoyle TJ (1994). Soybean GH3 promoter contains multiple auxin-inducible elements. Plant Cell 6, 645-657.
PMID |
| [39] | Lowder LG, Paul III JW, Qi YP (2017). Multiplexed transcriptional activation or repression in plants using CRISPR- dCas9-based systems. In: Kaufmann K, Mueller-Roeber B, eds. Plant Gene Regulatory Networks. New York: Humana Press. pp. 167-184. |
| [40] |
Lowder LG, Zhou JP, Zhang YX, Malzahn A, Zhong ZH, Hsieh TF, Voytas DF, Zhang Y, Qi YP (2018). Robust transcriptional activation in plants using multiplexed CRISPR-Act2.0 and mTALE-act systems. Mol Plant 11, 245-256.
DOI PMID |
| [41] |
Mallory AC, Bartel DP, Bartel B (2005). MicroRNA-directed regulation of Arabidopsis AUXIN RESPONSE FACTOR 17 is essential for proper development and modulates expression of early auxin response genes. Plant Cell 17, 1360-1375.
DOI URL |
| [42] |
McNellis TW, von Arnim AG, Deng XW (1994). Overexpression of Arabidopsis COP1 results in partial suppression of light-mediated development: evidence for a light- inactivable repressor of photomorphogenesis. Plant Cell 6, 1391-1400.
DOI PMID |
| [43] |
Nakazawa M, Yabe N, Ichikawa T, Yamamoto YY, Yoshizumi T, Hasunuma K, Matsui M (2001). DFL1, an auxin-responsive GH3gene homologue, negatively regulates shoot cell elongation and lateral root formation, and positively regulates the light response of hypocotyl length. Plant J 25, 213-221.
PMID |
| [44] |
Nobuta K, Okrent RA, Stoutemyer M, Rodibaugh N, Kempema L, Wildermuth MC, Innes RW (2007). The GH3 acyl adenylase family member PBS3 regulates salicylic acid-dependent defense responses in Arabidopsis. Plant Physiol 144, 1144-1156.
PMID |
| [45] |
Okrent RA, Brooks MD, Wildermuth MC (2009). Arabidopsis GH3.12 (PBS3) conjugates amino acids to 4-substituted benzoates and is inhibited by salicylate. J Biol Chem 284, 9742-9754.
DOI URL |
| [46] |
Osterlund MT, Ang LH, Deng XW (1999). The role of COP1 in repression of Arabidopsis photomorphogenic development. Trends Cell Biol 9, 113-118.
PMID |
| [47] |
Overmyer K, Tuominen H, Kettunen R, Betz C, Langebartels C, Sandermann H, Kangasjärvi J (2000). Ozone- sensitive Arabidopsis rcd1 mutant reveals opposite roles for ethylene and jasmonate signaling pathways in regulating superoxide-dependent cell death. Plant Cell 12, 1849-1862.
PMID |
| [48] |
Park JE, Park JY, Kim YS, Staswick PE, Jeon J, Yun J, Kim SY, Kim J, Lee YH, Park CM (2007a). GH3-mediated auxin homeostasis links growth regulation with stress adaptation response in Arabidopsis. J Biol Chem 282, 10036-10046.
DOI URL |
| [49] |
Park JE, Seo PJ, Lee AK, Jung JH, Kim YS, Park CM (2007b). An Arabidopsis GH3 gene, encoding an auxin- conjugating enzyme, mediates phytochrome B-regulated light signals in hypocotyl growth. Plant Cell Physiol 48, 1236-1241.
DOI URL |
| [50] |
Qin GJ, Gu HY, Zhao YD, Ma ZQ, Shi GL, Yang Y, Pichersky E, Chen HD, Liu MH, Chen ZL, Qu LJ (2005). An indole-3-acetic acid carboxyl methyltransferase regulates Arabidopsis leaf development. Plant Cell 17, 2693-2704.
DOI URL |
| [51] |
Qiu DY, Xiao J, Ding XH, Xiong M, Cai M, Cao YL, Li XH, Xu CG, Wang SP (2007). OsWRKY13mediates rice disease resistance by regulating defense-related genes in salicylate- and jasmonate-dependent signaling. Mol Plant Microbe Interact 20, 492-499.
DOI URL |
| [52] |
Rao MV, Lee H, Creelman RA, Mullet JE, Davis KR (2000). Jasmonic acid signaling modulates ozone-induced hypersensitive cell death. Plant Cell 12, 1633-1646.
PMID |
| [53] |
Riemann M, Riemann M, Takano M (2008). Rice JASMONATE RESISTANT 1is involved in phytochrome and jasmonate signaling. Plant Cell Environ 31, 783-792.
DOI URL |
| [54] |
Round A, Brown E, Marcellin B, Kapp U, Westfall CS, Jez JM, Zubieta C (2013). Determination of the GH3.12 protein conformation through HPLC-integrated SAXS measurements combined with X-ray crystallography. Acta Crystallogr Sect D Biol Crystallogr 69, 2072-2080.
DOI URL |
| [55] |
Sherp AM, Westfall CS, Alvarez S, Jez JM (2018). Arabidopsis thaliana GH3.15 acyl acid amido synthetase has a highly specific substrate preference for the auxin precursor indole-3-butyric acid. J Biol Chem 293, 4277-4288.
DOI URL |
| [56] |
Staswick PE, Serban B, Rowe M, Tiryaki I, Maldonado MT, Maldonado MC, Suza W (2005). Characterization of an Arabidopsis enzyme family that conjugates amino acids to indole-3-acetic acid. Plant Cell 17, 616-627.
DOI PMID |
| [57] |
Staswick PE, Tiryaki I, Rowe ML (2002). Jasmonate response locus JAR1 and several related Arabidopsis genes encode enzymes of the firefly luciferase superfamily that show activity on jasmonic, salicylic, and indole-3- acetic acids in an assay for adenylation. Plant Cell 14, 1405-1415.
PMID |
| [58] |
Staswick PE, Yuen GY, Lehman CC (1998). Jasmonate signaling mutants of Arabidopsis are susceptible to the soil fungus Pythium irregular. Plant J 15, 747-754.
DOI PMID |
| [59] |
Sun MH, Li H, Li YB, Xiang HZ, Liu YD, He Y, Qi MF, Li TL (2020). Tomato YABBY2b controls plant height through regulating indole-3-acetic acid-amido synthetase (GH3.8) expression. Plant Sci 297, 110530.
DOI URL |
| [60] |
Takase T, Nakazawa M, Ishikawa A, Kawashima M, Ichikawa T, Takahashi N, Shimada H, Manabe K, Matsui M (2004). ydk1-D, an auxin-responsive GH3 mutant that is involved in hypocotyl and root elongation. Plant J 37, 471-483.
DOI URL |
| [61] |
Takase T, Nakazawa M, Ishikawa A, Manabe K, Matsui M (2003). DFL2, a new member of the Arabidopsis GH3gene family, is involved in red light-specific hypocotyl elongation. Plant Cell Physiol 44, 1071-1080.
DOI URL |
| [62] |
Tao Z, Liu HB, Qiu DY, Zhou Y, Li XH, Xu CG, Wang SP (2009). A pair of allelic WRKY genes play opposite roles in rice-bacteria interactions. Plant Physiol 151, 936-948.
DOI URL |
| [63] |
Terol J, Domingo C, Talón M (2006). The GH3 family in plants: genome wide analysis in rice and evolutionary history based on EST analysis. Gene 371, 279-290.
DOI PMID |
| [64] |
Ulmasov T, Hagen G, Guilfoyle TJ (1997). ARF1, a transcription factor that binds to auxin response elements. Science 276, 1865-1868.
DOI PMID |
| [65] |
Ulmasov T, Liu ZB, Hagen G, Guilfoyle TJ (1995). Composite structure of auxin response elements. Plant Cell 7, 1611-1623.
PMID |
| [66] |
van Loon LC, Bakker PAHM, Pieterse CMJ (1998). Systemic resistance induced by rhizosphere bacteria. Annu Rev Phytopathol 36, 453-483.
PMID |
| [67] |
Wasternack C, Hause B (2013). Jasmonates: biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann Bot 111, 1021-1058.
DOI URL |
| [68] |
Westfall CS, Herrmann J, Chen QF, Wang P, Jez JM (2010). Modulating plant hormones by enzyme action: the GH3 family of acyl acid amido synthetases. Plant Signal Behav 5, 1607-1612.
DOI PMID |
| [69] |
Westfall CS, Zubieta C, Herrmann J, Kapp U, Nanao MH, Jez JM (2012). Structural basis for prereceptor modulation of plant hormones by GH3 proteins. Science 336, 1708-1711.
DOI PMID |
| [70] |
Wojtaczka P, Ciarkowska A, Starzynska E, Ostrowski M (2022). The GH3 amidosynthetases family and their role in metabolic crosstalk modulation of plant signaling compounds. Phytochemistry 194, 113039.
DOI URL |
| [71] |
Woodward AW, Bartel B (2005). Auxin: regulation, action, and interaction. Ann Bot 95, 707-735.
DOI URL |
| [72] |
Xie KB, Minkenberg B, Yang YN (2015). Boosting CRISPR/ Cas9 multiplex editing capability with the endogenous tRNA-processing system. Proc Natl Acad Sci USA 112, 3570-3575.
DOI URL |
| [73] |
Xu F, He SB, Zhang JY, Mao ZL, Wang WX, Li T, Hua J, Du SS, Xu PB, Li L, Lian HL, Yang HQ (2018). Photoactivated CRY1 and phyB interact directly with AUX/IAA proteins to inhibit auxin signaling in Arabidopsis. Mol Plant 11, 523-541.
DOI URL |
| [74] |
Yan JB, Yao RF, Chen L, Li SH, Gu M, Nan FJ, Xie DX (2018). Dynamic perception of jasmonates by the F-box protein COI1. Mol Plant 11, 1237-1247.
DOI PMID |
| [75] |
Yang JH, Han SJ, Yoon EK, Lee WS (2006). Evidence of an auxin signal pathway, microRNA167-ARF8-GH3, and its response to exogenous auxin in cultured rice cells. Nucleic Acids Res 34, 1892-1899.
DOI URL |
| [76] |
Zhang SN, Wang SK, Xu YX, Yu CL, Shen CJ, Qian Q, Geisler M, Jiang DA, Qi YH (2015). The auxin response factor, OsARF19, controls rice leaf angles through positively regulating OsGH3-5 and OsBRI1. Plant Cell Environ 38, 638-654.
DOI URL |
| [77] |
Zhang SW, Li CH, Cao J, Zhang YC, Zhang SQ, Xia YF, Sun DY, Sun Y (2009). Altered architecture and enhanced drought tolerance in rice via the down-regulation of indole-3-acetic acid by TLD1/OsGH3.13 activation. Plant Physiol 151, 1889-1901.
DOI URL |
| [78] | Zhang ZQ, Li Q, Li ZM, Staswick PE, Wang MY, Zhu Y, He ZH (2007). Dual regulation role of GH3.5 in salicylic acid and auxin signaling during Arabidopsis-Pseudomonas syringae interaction. Plant Physiol 145, 450-464. |
| [79] |
Zhang ZQ, Wang MY, Li ZM, Li Q, He ZH (2008). Arabidopsis GH3.5 regulates salicylic acid-dependent and both NPR1-dependent and independent defense responses. Plant Signal Behav 3, 537-542.
DOI URL |
| [80] |
Zhao SQ, Xiang JJ, Xue HW (2013). Studies on the rice LEAF INCLINATION 1 (LC1), an IAA-amido synthetase, reveal the effects of auxin in leaf inclination control. Mol Plant 6, 174-187.
DOI URL |
| [1] | Can Ye, Linbo Yao, Ying Jin, Rong Gao, Qi Tan, Xuying Li, Yanjun Zhang, Xifeng Chen, Bojun Ma, Wei Zhang, Kewei Zhang. Establishment and Application of a High-throughput Screening Method for Salicylic Acid Metabolic Mutants in Rice [J]. Chinese Bulletin of Botany, 2025, 60(4): 1-0. |
| [2] | Hongmei Wang, Wei Yuan, Fang Xue, Zhaocong Zhang, Kun Liu, Silong Che. The functions of plant SWEETs and its regulatory mechanisms involved in stress responses [J]. Chinese Bulletin of Botany, 2025, 60(4): 1-0. |
| [3] | Tao Xie, Yifan Zhang, Yunhui Liu, Huiyu You, Jibenben Xia, Rong Ma, Chunni Zhang, Xuejun Hua. Research progress of iron-sulfur cluster synthesis system and regulation in plant mitochondria [J]. Chinese Bulletin of Botany, 2025, 60(4): 1-0. |
| [4] | Yang Li, Qu Xitong, Chen Zihang, Zou Tingting, Wang Quanhua, Wang Xiaoli. Identification of the Spinach AT-hook Gene Family and Analysis of Expression Profiles [J]. Chinese Bulletin of Botany, 2025, 60(3): 377-392. |
| [5] | Yaping Wang, Wenquan Bao, Yu’e Bai. Advances in the Application of Single-cell Transcriptomics in Plant Growth, Development and Stress Response [J]. Chinese Bulletin of Botany, 2025, 60(1): 101-113. |
| [6] | Duxian Lu, Yanyan Zhang, Yan Liu, Yanjun Li, Xinxiu Zuo, Jinxing Lin, Yaning Cui. Recent Advances of Non-coding RNA in Plant Growth, Development and Stress Response [J]. Chinese Bulletin of Botany, 2024, 59(5): 709-725. |
| [7] | Yuying Zhou, Hui Chen, Simu Liu. Research Progress on Auxin Responsive Non-canonical Aux/IAA Proteins in Plants [J]. Chinese Bulletin of Botany, 2024, 59(4): 651-658. |
| [8] | Zhiye Du, Mingyu Li, Ji Chen, Jin Huang. Research Advances in Plant Stress Associated Protein Functions [J]. Chinese Bulletin of Botany, 2024, 59(1): 110-121. |
| [9] | Xinhai Zeng, Rui Chen, Yu Shi, Chaoyue Gai, Kai Fan, Zhaowei Li. Research Advances in Biological Functions of Plant SPL Transcription Factors [J]. Chinese Bulletin of Botany, 2023, 58(6): 982-997. |
| [10] | Xiangpei Kong, Mengyue Zhang, Zhaojun Ding. There Is a Way Out-new Breakthroughs in Extracellular Auxin Sensing [J]. Chinese Bulletin of Botany, 2023, 58(6): 861-865. |
| [11] | Shuyao Zhou, Jianming Li, Juan Mao. AtGH3.17-mediated Regulation of Auxin and Brassinosteroid Response in Arabidopsis thaliana [J]. Chinese Bulletin of Botany, 2023, 58(3): 373-384. |
| [12] | Ye Qing, Yan Xiaoyan, Chen Huize, Feng Jinlin, Han Rong. Effect of Nitrogen-doped Graphene Quantum Dots on Growth Direction of Primary Root in Arabidopsis thaliana [J]. Chinese Bulletin of Botany, 2022, 57(5): 623-634. |
| [13] | Lixia Jia, Yanhua Qi. Advances in the Regulation of Rice (Oryza sativa) Grain Shape by Auxin Metabolism, Transport and Signal Transduction [J]. Chinese Bulletin of Botany, 2022, 57(3): 263-275. |
| [14] | Binqi Li, Jiahui Yan, Hao Li, Wei Xin, Yunhe Tian, Zhenbiao Yang, Wenxin Tang. Changes of Small GTPases Activity During Cucumber Tendril Winding [J]. Chinese Bulletin of Botany, 2022, 57(3): 299-307. |
| [15] | Yanyan Li, Yanhua Qi. Advances in Biological Functions of Aux/IAA Gene Family in Plants [J]. Chinese Bulletin of Botany, 2022, 57(1): 30-41. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||