

Chinese Bulletin of Botany ›› 2023, Vol. 58 ›› Issue (6): 982-997.DOI: 10.11983/CBB22216 cstr: 32102.14.CBB22216
• SPECIAL TOPICS • Previous Articles Next Articles
Xinhai Zeng1,2, Rui Chen1,2, Yu Shi1,2, Chaoyue Gai3, Kai Fan2,3, Zhaowei Li1,2,*( )
)
Received:2022-09-07
Accepted:2023-05-31
Online:2023-11-01
Published:2023-11-27
Contact:
* E-mail: lizw197@163.com
Xinhai Zeng, Rui Chen, Yu Shi, Chaoyue Gai, Kai Fan, Zhaowei Li. Research Advances in Biological Functions of Plant SPL Transcription Factors[J]. Chinese Bulletin of Botany, 2023, 58(6): 982-997.
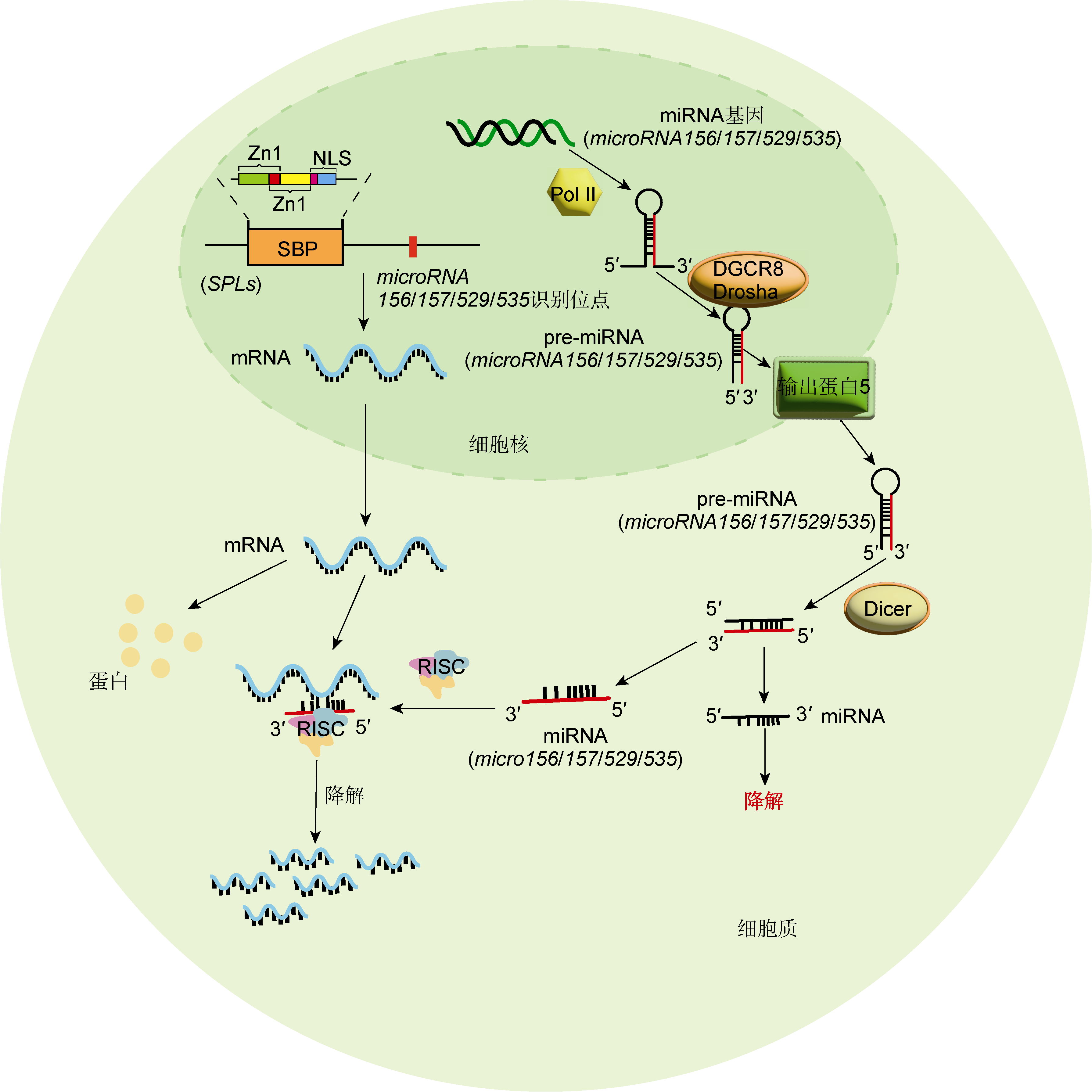
Figure 1 Schematic diagram of transcriptional regulation of SPLs by microRNA SBP: SQUAMOSA promoter binding protein; NLS: Nuclear localization signal; RISC: RNA-induced silencing complex
| 物种 | 拉丁名 | SPL名称 | 功能 | 参考文献 | |||
|---|---|---|---|---|---|---|---|
| 水稻 | Oryza sativa | OsSPLs | SPLs和NL1调控PLA1表达进而调节营养生长向生殖生长转换; OsSPL2、OsSPL4、OsSPL16和OsSPL17通过microRNA156-SPLs模型影响花粉育性。OsSPL4、OsSPL13和OsSPL16通过调控细胞分裂素影响水稻粒宽 | Wang et al., 2021a | |||
| OsSPL3 | MicroRNA156-OsSPL3-OsMADS50互作模块调控不定根发育 | Shao et al., | |||||
| OsSPL9 | OsSPL9激活幼穗分化早期的花分生组织基因RCN1, 调控穗分枝和穗粒数 | Hu et al., | |||||
| OsSPL12 | OsSPL12调控粒宽基因GW5表达进而负调节粒宽 | Zhang et al., | |||||
| OsSPL14 | 缩短叶片生长期, 调节叶片性状与厚度; 在生殖生长期, 促进穗分枝, 提高稻谷产量 | Miura et al., Lian et al., | |||||
| OsSPL17 | 正调控类黄酮含量, 负调控APX1表达进而提高雄花育性 | Sun et al., | |||||
| OsSPL18 | 结合直立密穗控制基因DEP1的启动子增强其表达, 调控穗型 | Yuan et al., | |||||
| 拟南芥 | Arabidopsis thaliana | AtSPLs | AtSPL2、AtSPL9、AtSPL10、AtSPL11、AtSPL13和AtSPL15等协同调控幼苗营养生长向生殖生长转换; AtSPLs通过N端结合TCP抑制其功能, 调控雌蕊和胚珠发育; SPLs抑制AHL15的表达进而抑制营养生长, 加速从幼年营养生长向成年生殖生长转变和开花。atspl2/atspl10/atspl11三突变体延迟开花 | Wang et al., Wei et al., Xu et al., Yao et al., Rahimi et al., | |||
| AtSPL3 | AtSPL3直接提高FUL和AP1的转录, 调节拟南芥花期 | Wu and Poethig, Yamaguchi et al., | |||||
| AtSPL4 | AtSPL4和AtSPL5促进FUL与SOC1的转录, 调节植物花期 | Birkenbihl et al., | |||||
| AtSPL8 | 拟南芥产孢组织正常发育所必需, 受micro156/157特异性调节, 调控花发育早期的细胞分裂与分化 | Xing et al., | |||||
| AtSPL9 | AtSPL9正向调控FUL、SOC1和AGL42, 促进开花 | Birkenbihl et al., | |||||
| AtSPL10 | AtSPL10与MED25共同调控FUL和LFY, 调节开花 | Barrera-Rojas et al., | |||||
| 柳枝稷 | Panicum vir- gatum | PvSPLs | PvSPL1和PvSPL2共同调控植株侧向分蘖, 从而提高生物量; PvSPL6、PvSPL7和PvSPL8调控柳枝稷开花 | Wu et al., | |||
| 黄花蒿 | Artemisia an- nua | AaSPL9 | AaSPL9正调控AaHD1的表达, 调节黄花蒿腺毛的发育起始 | He et al., | |||
| 小麦 | Triticum aes- tivum | TaSPL8 | TaSPL8通过调节生长素和油菜素内酯信号途径调控叶夹角 | Liu et al., | |||
| TaSPL13-2B | TaSPL13-2B参与小麦的小花分化与发育调控 | Li et al., | |||||
| TaSPL13 | TaSPL13的microRNA156识别元件MRE突变, 导致TaSPL13的转录本增多, 植株开花时间缩短, 分蘖数减少且株高降低, 籽粒大小和粒数增加 | Gupta et al., | |||||
| 苜蓿 | Medicago sa- tiva | MsSPL08 | MsSPL08基因突变致使苜蓿幼苗叶片数目增加, 叶片边缘锯齿消失 | Min et al., | |||
| MsSPL20 | MsSPL20通过调控小花发育基因HD3A、FTIP1、TEM1和HST1的表达推迟苜蓿开花 | Ma et al., | |||||
| 棉花 | Gossypium hirsutum | GhSPLs | MicroRNA157/SPL模型抑制生长素信号转导途径, 减缓花器官发育 | Liu et al., | |||
| 黄瓜 | Cucumis sa- tivus | CsSPLs | 参与调节黄瓜花药和胚珠发育 | Liu et al., | |||
| 枇杷 | Eriobotrya ja- ponica | EjSPLs | EjSPL3、EjSPL4、EjSPL5和EjSPL9等SPL转录因子参与花期调控 | Jiang et al., | |||
| 蓝莓 | Vaccinium corymbosum | VcSPLs | VcSPL35、VcSPL40、VcSPL45和VcSPL53基因在蓝莓的胚芽过渡阶段(从营养生长到花芽分化)发挥关键作用 | Feng et al., | |||
| 牡丹 | Paeonia × su- ffruticosa | PsSPLs | PsSPL2、PsSPL13和PsSPL14正调控牡丹籽粒大小、数量与荚果分枝 | Wang et al., | |||
| 番茄 | Solanum lyco- persicum | SlySBP | SlySBP与microRNA156协同调节子房分生组织发育, 并起始肉质果实发育 | Ferreira et al., | |||
| SlySPL13 | SlySPL13参与果实发育 | Cui et al., | |||||
| 二穗短 柄草 | Brachypodium distachyon | BdSBP9 | BdSBP9参与穗发育 | Tripathi et al., | |||
| 樱桃 | Prunus avium | PavSPLs | 9个SPL基因调控樱桃的果实发育和成熟过程 | Sun et al., | |||
Table 1 The SPLs are involved in the regulation of plant growth and development
| 物种 | 拉丁名 | SPL名称 | 功能 | 参考文献 | |||
|---|---|---|---|---|---|---|---|
| 水稻 | Oryza sativa | OsSPLs | SPLs和NL1调控PLA1表达进而调节营养生长向生殖生长转换; OsSPL2、OsSPL4、OsSPL16和OsSPL17通过microRNA156-SPLs模型影响花粉育性。OsSPL4、OsSPL13和OsSPL16通过调控细胞分裂素影响水稻粒宽 | Wang et al., 2021a | |||
| OsSPL3 | MicroRNA156-OsSPL3-OsMADS50互作模块调控不定根发育 | Shao et al., | |||||
| OsSPL9 | OsSPL9激活幼穗分化早期的花分生组织基因RCN1, 调控穗分枝和穗粒数 | Hu et al., | |||||
| OsSPL12 | OsSPL12调控粒宽基因GW5表达进而负调节粒宽 | Zhang et al., | |||||
| OsSPL14 | 缩短叶片生长期, 调节叶片性状与厚度; 在生殖生长期, 促进穗分枝, 提高稻谷产量 | Miura et al., Lian et al., | |||||
| OsSPL17 | 正调控类黄酮含量, 负调控APX1表达进而提高雄花育性 | Sun et al., | |||||
| OsSPL18 | 结合直立密穗控制基因DEP1的启动子增强其表达, 调控穗型 | Yuan et al., | |||||
| 拟南芥 | Arabidopsis thaliana | AtSPLs | AtSPL2、AtSPL9、AtSPL10、AtSPL11、AtSPL13和AtSPL15等协同调控幼苗营养生长向生殖生长转换; AtSPLs通过N端结合TCP抑制其功能, 调控雌蕊和胚珠发育; SPLs抑制AHL15的表达进而抑制营养生长, 加速从幼年营养生长向成年生殖生长转变和开花。atspl2/atspl10/atspl11三突变体延迟开花 | Wang et al., Wei et al., Xu et al., Yao et al., Rahimi et al., | |||
| AtSPL3 | AtSPL3直接提高FUL和AP1的转录, 调节拟南芥花期 | Wu and Poethig, Yamaguchi et al., | |||||
| AtSPL4 | AtSPL4和AtSPL5促进FUL与SOC1的转录, 调节植物花期 | Birkenbihl et al., | |||||
| AtSPL8 | 拟南芥产孢组织正常发育所必需, 受micro156/157特异性调节, 调控花发育早期的细胞分裂与分化 | Xing et al., | |||||
| AtSPL9 | AtSPL9正向调控FUL、SOC1和AGL42, 促进开花 | Birkenbihl et al., | |||||
| AtSPL10 | AtSPL10与MED25共同调控FUL和LFY, 调节开花 | Barrera-Rojas et al., | |||||
| 柳枝稷 | Panicum vir- gatum | PvSPLs | PvSPL1和PvSPL2共同调控植株侧向分蘖, 从而提高生物量; PvSPL6、PvSPL7和PvSPL8调控柳枝稷开花 | Wu et al., | |||
| 黄花蒿 | Artemisia an- nua | AaSPL9 | AaSPL9正调控AaHD1的表达, 调节黄花蒿腺毛的发育起始 | He et al., | |||
| 小麦 | Triticum aes- tivum | TaSPL8 | TaSPL8通过调节生长素和油菜素内酯信号途径调控叶夹角 | Liu et al., | |||
| TaSPL13-2B | TaSPL13-2B参与小麦的小花分化与发育调控 | Li et al., | |||||
| TaSPL13 | TaSPL13的microRNA156识别元件MRE突变, 导致TaSPL13的转录本增多, 植株开花时间缩短, 分蘖数减少且株高降低, 籽粒大小和粒数增加 | Gupta et al., | |||||
| 苜蓿 | Medicago sa- tiva | MsSPL08 | MsSPL08基因突变致使苜蓿幼苗叶片数目增加, 叶片边缘锯齿消失 | Min et al., | |||
| MsSPL20 | MsSPL20通过调控小花发育基因HD3A、FTIP1、TEM1和HST1的表达推迟苜蓿开花 | Ma et al., | |||||
| 棉花 | Gossypium hirsutum | GhSPLs | MicroRNA157/SPL模型抑制生长素信号转导途径, 减缓花器官发育 | Liu et al., | |||
| 黄瓜 | Cucumis sa- tivus | CsSPLs | 参与调节黄瓜花药和胚珠发育 | Liu et al., | |||
| 枇杷 | Eriobotrya ja- ponica | EjSPLs | EjSPL3、EjSPL4、EjSPL5和EjSPL9等SPL转录因子参与花期调控 | Jiang et al., | |||
| 蓝莓 | Vaccinium corymbosum | VcSPLs | VcSPL35、VcSPL40、VcSPL45和VcSPL53基因在蓝莓的胚芽过渡阶段(从营养生长到花芽分化)发挥关键作用 | Feng et al., | |||
| 牡丹 | Paeonia × su- ffruticosa | PsSPLs | PsSPL2、PsSPL13和PsSPL14正调控牡丹籽粒大小、数量与荚果分枝 | Wang et al., | |||
| 番茄 | Solanum lyco- persicum | SlySBP | SlySBP与microRNA156协同调节子房分生组织发育, 并起始肉质果实发育 | Ferreira et al., | |||
| SlySPL13 | SlySPL13参与果实发育 | Cui et al., | |||||
| 二穗短 柄草 | Brachypodium distachyon | BdSBP9 | BdSBP9参与穗发育 | Tripathi et al., | |||
| 樱桃 | Prunus avium | PavSPLs | 9个SPL基因调控樱桃的果实发育和成熟过程 | Sun et al., | |||
| 物种 | 拉丁名 | SPL名称 | 功能 | 参考文献 |
|---|---|---|---|---|
| 拟南芥 | Arabidopsis thaliana | AtSPL9 | 结合ABI5的启动子激活其表达, 促进ABA信号转换和种子中ABA积累, 抑制种子成熟后的荚内发芽 | Dong et al., |
| AtSPL10 | AtSPL10和BEL1通过与生长素转运基因PIN1互作, 调节胚珠生长素与细胞分裂素水平, 进而调控胚珠发育 | Bencivenga et al., | ||
| 水稻 | Oryza sativa | OsSPL12 | 与9个GA信号途径相关基因直接互作, 调控籽粒GA水平, 促进成熟籽粒休眠, 抑制稻谷穗发芽 | Qin et al., |
| OsSPL14 | 激活生长素运输基因OsPIN1b和PILS6b, 参与生长素的极性运输 | Li et al., | ||
| 玉米 | Zea mays | ZmSPL12 | ZmSPL12直接与D1的启动子结合抑制其转录, 降低玉米节间赤霉素含量, 抑制细胞伸长, 使节间缩短, 株高降低 | Zhao et al., |
| 番木瓜 | Carica papaya | CpSPLs | CpmicroRNA156/CpSPL3/CpSPL6/CpSPL11响应ETH/I-MCP (ethep- hon/1-methylcyclopropene)信号, 调节番木瓜着色和成熟 | Xu et al., |
| 柳枝稷 | Panicum virga- tum | PvSPL2 | PvSPL2促进SL生物合成基因PvLBO的表达 | Yang et al., |
Table 2 SPLs are involved in hormone regulation in plants
| 物种 | 拉丁名 | SPL名称 | 功能 | 参考文献 |
|---|---|---|---|---|
| 拟南芥 | Arabidopsis thaliana | AtSPL9 | 结合ABI5的启动子激活其表达, 促进ABA信号转换和种子中ABA积累, 抑制种子成熟后的荚内发芽 | Dong et al., |
| AtSPL10 | AtSPL10和BEL1通过与生长素转运基因PIN1互作, 调节胚珠生长素与细胞分裂素水平, 进而调控胚珠发育 | Bencivenga et al., | ||
| 水稻 | Oryza sativa | OsSPL12 | 与9个GA信号途径相关基因直接互作, 调控籽粒GA水平, 促进成熟籽粒休眠, 抑制稻谷穗发芽 | Qin et al., |
| OsSPL14 | 激活生长素运输基因OsPIN1b和PILS6b, 参与生长素的极性运输 | Li et al., | ||
| 玉米 | Zea mays | ZmSPL12 | ZmSPL12直接与D1的启动子结合抑制其转录, 降低玉米节间赤霉素含量, 抑制细胞伸长, 使节间缩短, 株高降低 | Zhao et al., |
| 番木瓜 | Carica papaya | CpSPLs | CpmicroRNA156/CpSPL3/CpSPL6/CpSPL11响应ETH/I-MCP (ethep- hon/1-methylcyclopropene)信号, 调节番木瓜着色和成熟 | Xu et al., |
| 柳枝稷 | Panicum virga- tum | PvSPL2 | PvSPL2促进SL生物合成基因PvLBO的表达 | Yang et al., |
| 物种 | 拉丁名 | SPL名称 | 功能 | 参考文献 |
|---|---|---|---|---|
| 欧洲山杨 | Populus tremula | PtSPLs | MicroRNA156靶向调控SPL表达进而提高欧洲山杨花青素、黄酮和黄酮醇的生物合成 | Wang et al., |
| 丹参 | Salvia miltiorrhiza | SmSPL6 | SmSPL6通过结合Sm4CL9和SmCYP98A14的启动子激活其表达, 促进SalB和RosA的生物合成 | Cao et al., |
| SmSPL7 | SmSPL7通过直接与SmTAT1和Sm4CL9的启动子结合抑制其表达, 阻断SalB的生物合成 | Chen et al., | ||
| 黄花蒿 | Artemisia annua | AaSPL2 | AaSPL2通过结合青蒿素合成关键基因DBR2的启动子促进其表达, 增强青蒿素的合成 | Lv et al., |
| 广藿香 | Pogostemon cab- lin | PaSPL9 | SPL9通过结合倍半萜合酶基因TPS21的启动子激活其表达, 促进老龄组织中倍半萜的生物合成 | Yu et al., |
| 川桑 | Morus notabilis | MnSPL7 | MnSPL7通过激活儿茶素合成途径MnTT2L2基因的转录, 响应家蚕的取食行为 | Li et al., |
Table 3 SPLs are involved in plant secondary metabolism
| 物种 | 拉丁名 | SPL名称 | 功能 | 参考文献 |
|---|---|---|---|---|
| 欧洲山杨 | Populus tremula | PtSPLs | MicroRNA156靶向调控SPL表达进而提高欧洲山杨花青素、黄酮和黄酮醇的生物合成 | Wang et al., |
| 丹参 | Salvia miltiorrhiza | SmSPL6 | SmSPL6通过结合Sm4CL9和SmCYP98A14的启动子激活其表达, 促进SalB和RosA的生物合成 | Cao et al., |
| SmSPL7 | SmSPL7通过直接与SmTAT1和Sm4CL9的启动子结合抑制其表达, 阻断SalB的生物合成 | Chen et al., | ||
| 黄花蒿 | Artemisia annua | AaSPL2 | AaSPL2通过结合青蒿素合成关键基因DBR2的启动子促进其表达, 增强青蒿素的合成 | Lv et al., |
| 广藿香 | Pogostemon cab- lin | PaSPL9 | SPL9通过结合倍半萜合酶基因TPS21的启动子激活其表达, 促进老龄组织中倍半萜的生物合成 | Yu et al., |
| 川桑 | Morus notabilis | MnSPL7 | MnSPL7通过激活儿茶素合成途径MnTT2L2基因的转录, 响应家蚕的取食行为 | Li et al., |
| 物种 | 拉丁名 | SPL名称 | 功能 | 参考文献 |
|---|---|---|---|---|
| 拟南芥 | Arabidopsis thaliana | AtSPLs | AtSPL1和AtSPL12通过激活ABA受体PYL介导的 ABA信号途径, 提高花序的耐热性, 降低花对热胁迫的敏感性 | Chao et al., |
| AtSPL3 | AtSPL3与Cu响应基因的启动子顺式作用元件结合 | Perea-García et al., | ||
| AtSPL7 | AtSPL7直接与Cu响应基因的启动子CuRE结合, 促进铜还原酶基因FRO4/5以及质膜转运蛋白COPT1/ 2/6表达, 增强对Cu的摄取 | Garcia-Molina et al., | ||
| AtSPL9 | 正调控AtCBF2表达, 参与对低温冻害的耐受性调节; 调节抗菌免疫能力 | Yin et al., | ||
| AtSPL14 | AtSPL14表达量降低会提高对伏马毒素B1的抗性 | Stone et al., | ||
| 水稻 | Oryza sativa | OsSPL3 | OsmicroRNA156-OsSPL3-OsWRKY71互作途径调控抗寒基因对低温胁迫的响应 | Zhou and Tang, |
| OsSPL9 | 参与抗病调节, 增强对水稻条纹叶枯病毒的防御反应 | Jin et al., | ||
| OsSPL10 | 负调节水稻耐盐性 | Lan et al., | ||
| OsSPL14 | 受稻瘟病菌诱导磷酸化, 并与OsWRKY45的启动子结合促进其表达, 增强细胞的免疫反应, 提高对稻瘟病菌的抗性; 改善株型, 增加产量 | Wang et al., | ||
| 甘蓝 | Brassica oleracea var. capitata | BoSPLs | 低温胁迫提高BoSPL9b和BoSPL16b的表达丰度 | Shan et al., |
| 苜蓿 | Medicago sativa | MsSPL9 | MsSPL9的RNAi沉默植株比野生型更适应干旱环境 | Hanly et al., |
| MsSPL13 | MsSPL13负调控苜蓿的耐热性; 适度的microRNA156转录丰度有效抑制MsSPL13的翻译, 促进WD40- 1表达, 进而微调控DFR表达, 增强花青素的合成, 促进脯氨酸和可溶性糖积累, 提高苜蓿的耐旱性 | Matthews et al., yissa et al., | ||
| 木薯 | Manihot esculenta | MeSPL9 | MeSPL9通过调控茉莉酸信号和抗氧化物质含量调节木薯的抗旱能力 | Li et al., |
| 美洲山 核桃 | Carya illinoinensis | CiSPLs | CiSPL基因在干旱和盐胁迫下呈现出时空表达特性, 以应对逆境胁迫 | Wang et al., |
| 苹果 | Malus domestica | MdSPL13 | MicroRNA156/SPL13通过增强MdWRKY100的表达提高苹果的耐盐性 | Ma et al., |
| 柽柳 | Tamarix chinensis | TcSPLs | TcSPLs受TcmicroRNA156靶向调节, 参与植株的盐胁迫响应 | Wang et al., |
| 毛果杨 | Populus trichocarpa | PtSPL3 | PtSPL3和PtSPL4调控Cu响应基因的表达 | Lu et al., |
| 番茄 | Solanum lycopersicum | LeSPLs | LeSPLs通过结合SlNR的启动子抑制其表达, 减弱硝酸还原酶对Cd胁迫诱导NO生成的抑制作用 | Chen et al., |
Table 4 SPLs participate in the response to various stresses
| 物种 | 拉丁名 | SPL名称 | 功能 | 参考文献 |
|---|---|---|---|---|
| 拟南芥 | Arabidopsis thaliana | AtSPLs | AtSPL1和AtSPL12通过激活ABA受体PYL介导的 ABA信号途径, 提高花序的耐热性, 降低花对热胁迫的敏感性 | Chao et al., |
| AtSPL3 | AtSPL3与Cu响应基因的启动子顺式作用元件结合 | Perea-García et al., | ||
| AtSPL7 | AtSPL7直接与Cu响应基因的启动子CuRE结合, 促进铜还原酶基因FRO4/5以及质膜转运蛋白COPT1/ 2/6表达, 增强对Cu的摄取 | Garcia-Molina et al., | ||
| AtSPL9 | 正调控AtCBF2表达, 参与对低温冻害的耐受性调节; 调节抗菌免疫能力 | Yin et al., | ||
| AtSPL14 | AtSPL14表达量降低会提高对伏马毒素B1的抗性 | Stone et al., | ||
| 水稻 | Oryza sativa | OsSPL3 | OsmicroRNA156-OsSPL3-OsWRKY71互作途径调控抗寒基因对低温胁迫的响应 | Zhou and Tang, |
| OsSPL9 | 参与抗病调节, 增强对水稻条纹叶枯病毒的防御反应 | Jin et al., | ||
| OsSPL10 | 负调节水稻耐盐性 | Lan et al., | ||
| OsSPL14 | 受稻瘟病菌诱导磷酸化, 并与OsWRKY45的启动子结合促进其表达, 增强细胞的免疫反应, 提高对稻瘟病菌的抗性; 改善株型, 增加产量 | Wang et al., | ||
| 甘蓝 | Brassica oleracea var. capitata | BoSPLs | 低温胁迫提高BoSPL9b和BoSPL16b的表达丰度 | Shan et al., |
| 苜蓿 | Medicago sativa | MsSPL9 | MsSPL9的RNAi沉默植株比野生型更适应干旱环境 | Hanly et al., |
| MsSPL13 | MsSPL13负调控苜蓿的耐热性; 适度的microRNA156转录丰度有效抑制MsSPL13的翻译, 促进WD40- 1表达, 进而微调控DFR表达, 增强花青素的合成, 促进脯氨酸和可溶性糖积累, 提高苜蓿的耐旱性 | Matthews et al., yissa et al., | ||
| 木薯 | Manihot esculenta | MeSPL9 | MeSPL9通过调控茉莉酸信号和抗氧化物质含量调节木薯的抗旱能力 | Li et al., |
| 美洲山 核桃 | Carya illinoinensis | CiSPLs | CiSPL基因在干旱和盐胁迫下呈现出时空表达特性, 以应对逆境胁迫 | Wang et al., |
| 苹果 | Malus domestica | MdSPL13 | MicroRNA156/SPL13通过增强MdWRKY100的表达提高苹果的耐盐性 | Ma et al., |
| 柽柳 | Tamarix chinensis | TcSPLs | TcSPLs受TcmicroRNA156靶向调节, 参与植株的盐胁迫响应 | Wang et al., |
| 毛果杨 | Populus trichocarpa | PtSPL3 | PtSPL3和PtSPL4调控Cu响应基因的表达 | Lu et al., |
| 番茄 | Solanum lycopersicum | LeSPLs | LeSPLs通过结合SlNR的启动子抑制其表达, 减弱硝酸还原酶对Cd胁迫诱导NO生成的抑制作用 | Chen et al., |
| [1] |
Barrera-Rojas CH, Rocha GHB, Polverari L, Pinheiro Brito DA, Batista DS, Notini MM, Da Cruz ACF, Morea EGO, Sabatini S, Otoni WC, Nogueira FTS (2020). miR156-targeted SPL10 controls Arabidopsis root meristem activity and root-derived de novo shoot regenera-tion via cytokinin responses. J Exp Bot 71, 934-950.
DOI PMID |
| [2] |
Bencivenga S, Simonini S, Benková E, Colombo L (2012). The transcription factors BEL1 and SPL are required for cytokinin and auxin signaling during ovule development in Arabidopsis. Plant Cell 24, 2886-2897.
DOI URL |
| [3] |
Bernal M, Casero D, Singh V, Wilson GT, Grande A, Yang H, Dodani SC, Pellegrini M, Huijser P, Connolly EL, Merchant SS, Krämer U (2012). Transcriptome sequencing identifies SPL7-regulated copper acquisition genes FRO4/FRO5 and the copper dependence of iron homeostasis in Arabidopsis. Plant Cell 24, 738-761.
DOI URL |
| [4] |
Birkenbihl RP, Jach G, Saedler H, Huijser P (2005). Functional dissection of the plant-specific SBP-domain: overlap of the DNA-binding and nuclear localization domains. J Mol Biol 352, 585-596.
DOI PMID |
| [5] |
Bonnet E, He Y, Billiau K, Van De Peer Y (2010). TAPIR, a web server for the prediction of plant microRNA targets, including target mimics. Bioinformatics 26, 1566-1568.
DOI PMID |
| [6] |
Cai JJ, Liu WW, Li WQ, Zhao LJ, Chen G, Bai YY, Ma DM, Fu CX, Wang YM, Zhang XC (2022). Downregu-lation of miR156-targeted PvSPL6 in switchgrass delays flowering and increases biomass yield. Front Plant Sci 13, 834431.
DOI URL |
| [7] |
Cao Y, Chen R, Wang WT, Wang DH, Cao XY (2021). SmSPL6 induces phenolic acid biosynthesis and affects root development in Salvia miltiorrhiza. Int J Mol Sci 22, 7895.
DOI URL |
| [8] |
Cardon GH, Höhmann S, Nettesheim K, Saedler H, Huijser P (1997). Functional analysis of the Arabidopsis thaliana SBP-box gene SPL3: a novel gene involved in the floral transition. Plant J 12, 367-377.
PMID |
| [9] |
Chao LM, Liu YQ, Chen DY, Xue XY, Mao YB, Chen XY (2017). Arabidopsis transcription factors SPL1 and SPL12 confer plant thermotolerance at reproductive stage. Mol Plant 10, 735-748.
DOI URL |
| [10] |
Chen FH, Zhang HM, Li H, Lian L, Wei YD, Lin YL, Wang LN, He W, Cai QH, Xie HG, Zhang H, Zhang JF (2023). IPA1 improves drought tolerance by activating SNAC1 in rice. BMC Plant Biol 23, 55.
DOI |
| [11] |
Chen R, Cao Y, Wang WT, Li YH, Wang DH, Wang SQ, Cao XY (2021). Transcription factor SmSPL7 promotes anthocyanin accumulation and negatively regulates phenolic acid biosynthesis in Salvia miltiorrhiza. Plant Sci 310, 110993.
DOI URL |
| [12] |
Chen WW, Jin JF, Lou HQ, Liu L, Kochian LV, Yang JL (2018). LeSPL-CNR negatively regulates Cd acquisition through repressing nitrate reductase-mediated nitric oxide production in tomato. Planta 248, 893-907.
DOI PMID |
| [13] | Cheng YJ, Shang GD, Xu ZG, Yu S, Wu LY, Zhai D, Tian SL, Gao J, Wang L, Wang JW (2021). Cell division in the shoot apical meristem is a trigger for miR156 decline and vegetative phase transition in Arabidopsis. Proc Natl Acad Sci USA 118, e2115667118. |
| [14] |
Cui L, Zheng FY, Wang JF, Zhang CL, Xiao FM, Ye J, Li CX, Ye ZB, Zhang JH (2020). miR156a-targeted SBP- box transcription factor SlSPL13 regulates inflorescence morphogenesis by directly activating SFT in tomato. Plant Biotechnol J 18, 1670-1682.
DOI URL |
| [15] |
Dai X, Zhao PX (2011). psRNATarget: a plant small RNA target analysis server. Nucleic Acids Res 39, 155-159.
DOI PMID |
| [16] |
Dong HX, Yan SL, Jing YX, Yang RZ, Zhang YW, Zhou Y, Zhu YF, Sun JQ (2021). miR156-targeted SPL9 is phosphorylated by SnRK2s and interacts with ABI5 to enhance ABA responses in Arabidopsis. Front Plant Sci 12, 708573.
DOI URL |
| [17] |
Feng X, Zhou BJ, Wu XL, Wu HL, Zhang SL, Jiang Y, Wang YP, Zhang YQ, Cao M, Guo BS, Su SC, Hou ZX (2023). Molecular characterization of SPL gene family during flower morphogenesis and regulation in blueberry. BMC Plant Biol 23, 40.
DOI PMID |
| [18] |
Ferreirae Silva GF, Silva EM, Da Silva Azevedo M, Guivin MAC, Ramiro DA, Figueiredo CR, Carrer H, Peres LEP, Nogueira FTS (2014). MicroRNA156-targeted SPL/SBP box transcription factors regulate tomato ovary and fruit development. Plant J 78, 604-618.
DOI URL |
| [19] |
Feyissa BA, Amyot L, Nasrollahi V, Papadopoulos Y, Kohalmi SE, Hannoufa A (2021). Involvement of the miR156/SPL module in flooding response in Medicago sativa. Sci Rep 11, 3243.
DOI PMID |
| [20] |
Feyissa BA, Arshad M, Gruber MY, Kohalmi SE, Hannoufa A (2019). The interplay between miR156/SPL13 and DFR/WD40-1 regulate drought tolerance in alfalfa. BMC Plant Biol 19, 434.
DOI PMID |
| [21] |
Gandikota M, Birkenbihl RP, Höhmann S, Cardon GH, Saedler H, Huijser P (2007). The miRNA156/157 recognition element in the 3ʹUTR of the Arabidopsis SBP box gene SPL3 prevents early flowering by translational inhibition in seedlings. Plant J 49, 683-693.
DOI PMID |
| [22] |
Garcia-Molina A, Xing SP, Huijser P (2014). Functional characterisation of Arabidopsis SPL7 conserved protein domains suggests novel regulatory mechanisms in the Cu deficiency response. BMC Plant Biol 14, 231.
DOI PMID |
| [23] |
Gou JQ, Tang CR, Chen NC, Wang H, Debnath S, Sun L, Flanagan A, Tang YH, Jiang QZ, Allen RD, Wang ZY (2019). SPL7 and SPL8 represent a novel flowering regulation mechanism in switchgrass. New Phytol 222, 1610-1623.
DOI URL |
| [24] |
Gou JY, Felippes FF, Liu CJ, Weigel D, Wang JW (2011). Negative regulation of anthocyanin biosynthesis in Arabidopsis by a miR156-targeted SPL transcription factor. Plant Cell 23, 1512-1522.
DOI URL |
| [25] |
Gupta A, Hua L, Zhang ZZ, Yang B, Li WL (2023). CRISPR-induced miRNA156-recognition element mutations in TaSPL13 improve multiple agronomic traits in wheat. Plant Biotechnol J 21, 536-548.
DOI URL |
| [26] |
Hanly A, Karagiannis J, Lu QSM, Tian LN, Hannoufa A (2020). Characterization of the role of SPL9in drought stress tolerance in Medicago sativa. Int J Mol Sci 21, 6003.
DOI URL |
| [27] |
He YL, Fu XQ, Li L, Sun XF, Tang KX, Zhao JY (2022). AaSPL9 affects glandular trichomes initiation by positively regulating expression of AaHD1 in Artemisia annua L. Plant Sci 317, 111172.
DOI URL |
| [28] |
Hou HM, Li J, Gao M, Singer SD, Wang H, Mao LY, Fei ZJ, Wang XP (2013). Genomic organization, phylogenetic comparison and differential expression of the SBP-box family genes in grape. PLoS One 8, e59358.
DOI URL |
| [29] |
Hu JH, Huang LY, Chen GL, Liu H, Zhang YS, Zhang R, Zhang SL, Liu JT, Hu QY, Hu FY, Wang W, Ding Y (2021a). The elite alleles of OsSPL4 regulate grain size and increase grain yield in rice. Rice 14, 90.
DOI |
| [30] |
Hu L, Chen WL, Yang W, Li XL, Zhang C, Zhang XY, Zheng L, Zhu XB, Yin JJ, Qin P, Wang YP, Ma BT, Li SG, Yuan H, Tu B (2021b). OsSPL9 regulates grain number and grain yield in rice. Front Plant Sci 12, 682018.
DOI URL |
| [31] |
Huijser P, Klein J, Lönnig WE, Meijer H, Saedler H, Sommer H (1992). Bracteomania, an inflorescence anomaly, is caused by the loss of function of the MADS-box gene SQUAMOSA in Antirrhinum majus. EMBO J 11, 1239-1249.
DOI PMID |
| [32] |
Jiang YY, Peng JR, Wang M, Su WB, Gan XQ, Jing Y, Yang XH, Lin SQ, Gao YS (2019). The role of EjSPL3, EjSPL4, EjSPL5, and EjSPL9 in regulating flowering in loquat (Eriobotrya japonica Lindl.). Int J Mol Sci 21, 248.
DOI URL |
| [33] |
Jiao YQ, Wang YH, Xue DW, Wang J, Yan MX, Liu GF, Dong GJ, Zeng DL, Lu ZF, Zhu XD, Qian Q, Li JY (2010). Regulation of OsSPL14 by OsmiR156 defines ideal plant architecture in rice. Nat Genet 42, 541-544.
DOI |
| [34] |
Jin B, Zhou XR, Jiang BL, Gu ZM, Zhang PH, Qian Q, Chen XF, Ma BJ (2015). Transcriptome profiling of the spl5 mutant reveals that SPL5 has a negative role in the biosynthesis of serotonin for rice disease resistance. Rice 8, 18.
DOI URL |
| [35] |
Jones-Rhoades MW, Bartel DP, Bartel B (2006). MicroRNAs and their regulatory roles in plants. Annu Rev Plant Biol 57, 19-53.
PMID |
| [36] |
Jung JH, Ju Y, Seo PJ, Lee JH, Park CM (2012). The SOC1-SPL module integrates photoperiod and gibberellic acid signals to control flowering time in Arabidopsis. Plant J 69, 577-588.
DOI URL |
| [37] |
Lan T, Zheng YL, Su ZL, Yu SB, Song HB, Zheng XY, Lin GG, Wu WR (2019). OsSPL10, a SBP-box gene, plays a dual role in salt tolerance and trichome formation in rice (Oryza sativa L.). G3 9, 4107-4114.
DOI URL |
| [38] |
Li HS, Ma B, Luo YW, Wei WQ, Yuan JL, Zhai CX, He NJ (2022a). The mulberry SPL gene family and the response of MnSPL7 to silkworm herbivory through activating the transcription of MnTT2L2 in the catechin biosynthesis pathway. Int J Mol Sci 23, 1141.
DOI URL |
| [39] |
Li L, Shi F, Wang YQ, Yu XF, Zhi JJ, Guan YB, Zhao HY, Chang JL, Chen MJ, Yang GX, Wang YS, He GY (2020). TaSPL13 regulates inflorescence architecture and development in transgenic wheat (Triticum aestivum L.). Plant Sci 296, 110516.
DOI URL |
| [40] |
Li SX, Cheng ZH, Li ZB, Dong SM, Yu XL, Zhao PJ, Liao WB, Yu X, Peng M (2022b). MeSPL9 attenuates drought resistance by regulating JA signaling and protectant metabolite contents in cassava. Theor Appl Genet 135, 817-832.
DOI |
| [41] |
Li Y, He YZ, Liu ZX, Qin T, Wang L, Chen ZH, Zhang BM, Zhang HT, Li HT, Liu L, Zhang J, Yuan WY (2022c). OsSPL14 acts upstream of OsPIN1b and PILS6b to modulate axillary bud outgrowth by fine-tuning auxin transport in rice. Plant J 111, 1167-1182.
DOI URL |
| [42] |
Lian L, Xu HB, Zhang H, He W, Cai QH, Lin YL, Wei LY, Pan LY, Xie XP, Zheng YM, Wei YD, Zhu YS, Xie HA, Zhang JF (2020). Overexpression of OsSPL14results in transcriptome and physiology changes in indica rice ‘MH86’. Plant Growth Regul 90, 265-278.
DOI |
| [43] |
Liu KY, Cao J, Yu KH, Liu XY, Gao YJ, Chen Q, Zhang WJ, Peng HR, Du JK, Xin MM, Hu ZR, Guo WL, Rossi V, Ni ZF, Sun QX, Yao YY (2019). Wheat TaSPL8 modulates leaf angle through auxin and brassinosteroid signaling. Plant Physiol 181, 179-194.
DOI URL |
| [44] |
Liu N, Tu LL, Wang LC, Hu HY, Xu J, Zhang XL (2017). MicroRNA157-targeted SPL genes regulate floral organ size and ovule production in cotton. BMC Plant Biol 17, 7.
DOI URL |
| [45] |
Liu XF, Ning K, Che G, Yan SS, Han LJ, Gu R, Li Z, Weng YQ, Zhang XL (2018). CsSPL functions as an adaptor between HD-ZIP III and CsWUS transcription factors regulating anther and ovule development in Cucumis sativus (cucumber). Plant J 94, 535-547.
DOI URL |
| [46] |
Liu YT, Wu GX, Zhao YP, Wang HH, Dai ZY, Xue WC, Yang J, Wei HB, Shen RX, Wang HY (2021). DWARF53 interacts with transcription factors UB2/UB3/TSH4 to regulate maize tillering and tassel branching. Plant Physiol 187, 947-962.
DOI PMID |
| [47] |
Long JM, Liu CY, Feng MQ, Liu Y, Wu XM, Guo WW (2018). miR156-SPL modules regulate induction of somatic embryogenesis in citrus callus. J Exp Bot 69, 2979-2993.
DOI URL |
| [48] |
Lu SF, Yang CM, Chiang VL (2011). Conservation and diversity of microRNA-associated copper-regulatory networks in Populus trichocarpa. J Integr Plant Biol 53, 879-891.
DOI URL |
| [49] |
Lv ZY, Wang Y, Liu Y, Peng BW, Zhang L, Tang KX, Chen WS (2019). The SPB-box transcription factor AaSPL2 positively regulates artemisinin biosynthesis in Artemisia annua L. Front Plant Sci 10, 409.
DOI URL |
| [50] |
Ma L, Liu XQ, Liu WH, Wen HY, Zhang YC, Pang YZ, Wang XM (2022). Characterization of Squamosa-promoter binding protein-box family genes reveals the critical role of MsSPL20 in alfalfa flowering time regulation. Front Plant Sci 12, 775690.
DOI URL |
| [51] |
Ma Y, Xue H, Zhang F, Jiang Q, Yang S, Yue PT, Wang F, Zhang YY, Li LG, He P, Zhang ZH (2021). The miR156/SPL module regulates apple salt stress tolerance by activating MdWRKY100 expression. Plant Biotechnol J 19, 311-323.
DOI URL |
| [52] |
Matthews C, Arshad M, Hannoufa A (2019). Alfalfa response to heat stress is modulated by microRNA156. Physiol Plant 165, 830-842.
DOI PMID |
| [53] |
Min XY, Luo K, Liu WX, Zhou KY, Li JY, Wei ZW (2022). Molecular characterization of the miR156/ MsSPL model in regulating the compound leaf development and abiotic stress response in alfalfa. Genes 13, 331.
DOI URL |
| [54] |
Miura K, Ikeda M, Matsubara A, Song XJ, Ito M, Asano K, Matsuoka M, Kitano H, Ashikari M (2010). OsSPL14 promotes panicle branching and higher grain productivity in rice. Nat Genet 42, 545-549.
DOI |
| [55] |
Morea EGO, Da Silva EM, Ferreirae Silva GF, Valente GT, Barrera Rojas CH, Vincentz M, Nogueira FTS (2016). Functional and evolutionary analyses of the miR156 and miR529 families in land plants. BMC Plant Biol 16, 40.
DOI PMID |
| [56] |
Ning K, Chen S, Huang HJ, Jiang J, Yuan HM, Li HY (2017). Molecular characterization and expression analysis of the SPL gene family with BpSPL9 transgenic lines found to confer tolerance to abiotic stress in Betula platyphylla Suk. Plant Cell Tissue Organ Cult 130, 469-481.
DOI URL |
| [57] |
Perea-García A, Andrés-Bordería A, Huijser P, Peñarrubia L (2021). The copper-microRNA pathway is integrated with developmental and environmental stress responses in Arabidopsis thaliana. Int J Mol Sci 22, 9547.
DOI URL |
| [58] |
Qin MM, Zhang Y, Yang YM, Miao CB, Liu SK (2020). Seed-specific overexpression of SPL12 and IPA1 improves seed dormancy and grain size in rice. Front Plant Sci 11, 532771.
DOI URL |
| [59] |
Rahimi A, Karami O, Balazadeh S, Offringa R (2022). miR156-independent repression of the ageing pathway by longevity-promoting AHL proteins in Arabidopsis. New Phytol 235, 2424-2438.
DOI URL |
| [60] |
Rhoades MW, Reinhart BJ, Lim LP, Burge CB, Bartel B, Bartel DP (2002). Prediction of plant microRNA targets. Cell 110, 513-520.
DOI PMID |
| [61] |
Riechmann JL, Ratcliffe OJ (2000). A genomic perspective on plant transcription factors. Curr Opin Plant Biol 3, 423-434.
PMID |
| [62] |
Schwab R, Palatnik JF, Riester M, Schommer C, Schmid M, Weigel D (2005). Specific effects of microRNAs on the plant transcriptome. Dev Cell 8, 517-527.
DOI PMID |
| [63] |
Schwechheimer C, Zourelidou M, Bevan MW (1998). Plant transcription factor studies. Annu Rev Plant Physiol Plant Mol Biol 49, 127-150.
DOI URL |
| [64] |
Shan X, Zhang W, Huang JX, Yu FW, Qin WB, Li JB, Wang SY, Dai ZL (2021). Identification and characterization of SPL transcription factor family reveals organization and chilling-responsive patterns in cabbage (Brassica oleracea var. capitata L.). Agronomy 11, 1445.
DOI URL |
| [65] |
Shao YL, Zhou HZ, Wu YR, Zhang H, Lin J, Jiang XY, He QJ, Zhu JS, Li Y, Yu H, Mao CZ (2019). OsSPL3, an SBP-domain protein, regulates crown root development in rice. Plant Cell 31, 1257-1275.
DOI |
| [66] |
Si LZ, Chen JY, Huang XH, Gong H, Luo JH, Hou QQ, Zhou TY, Lu TT, Zhu JJ, Shangguan YY, Chen EW, Gong CX, Zhao Q, Jing YF, Zhao Y, Li Y, Cui LL, Fan DL, Lu YQ, Weng QJ, Wang YC, Zhan QL, Liu KY, Wei XH, An K, An G, Han B (2016). OsSPL13 controls grain size in cultivated rice. Nat Genet 48, 447-456.
DOI |
| [67] |
Song XG, Lu ZF, Yu H, Shao GN, Xiong JS, Meng XB, Jing YH, Liu GF, Xiong GS, Duan JB, Yao XF, Liu CM, Li HQ, Wang YH, Li JY (2017). IPA1 functions as a downstream transcription factor repressed by D53 in strigolactone signaling in rice. Cell Res 27, 1128-1141.
DOI PMID |
| [68] |
Stone JM, Liang XW, Nekl ER, Stiers JJ (2005). Arabidopsis AtSPL14, a plant-specific SBP-domain transcription factor, participates in plant development and sensitivity to fumonisin B1. Plant J 41, 744-754.
DOI URL |
| [69] |
Sun YJ, Fu M, Wang L, Bai YX, Fang XL, Wang Q, He Y, Zeng HL (2022). OsSPLs regulate male fertility in response to different temperatures by flavonoid biosynthesis and tapetum PCD in PTGMS rice. Int J Mol Sci 23, 3744.
DOI URL |
| [70] |
Sun YT, Wang YY, Xiao YQ, Zhang X, Du BY, Turupu M, Wang C, Yao QS, Gai SL, Huang J, Tong S, Li TH (2023). Genome-wide identification of the SQUAMOSA promoter-binding protein-like (SPL) transcription factor family in sweet cherry fruit. Int J Mol Sci 24, 2880.
DOI URL |
| [71] |
Sunkar R, Zhu JK (2004). Novel and stress-regulated microRNAs and other small RNAs from Arabidopsis. Plant Cell 16, 2001-2019.
DOI URL |
| [72] |
Tripathi RK, Overbeek W, Singh J (2020). Global analysis of SBP gene family in Brachypodium distachyon reveals its association with spike development. Sci Rep 10, 15032.
DOI PMID |
| [73] |
Tsuzuki M, Futagami K, Shimamura M, Inoue C, Kunimoto K, Oogami T, Tomita Y, Inoue K, Kohchi T, Yamaoka S, Araki T, Hamada T, Watanabe Y (2019). An early arising role of the microRNA156/529-SPL module in reproductive development revealed by the liverwort Marchantia polymorpha. Curr Biol 29, 3307-3314.
DOI URL |
| [74] | Unte US, Sorensen AM, Pesaresi P, Gandikota M, Leister D, Saedler H, Huijser P (2003). SPL8, an SBP-box gene that affects pollen sac development in Arabidopsis. Plant Cell 15, 1009-1019. |
| [75] |
Vogel JT, Zarka DG, Van Buskirk HA, Fowler SG, Thomashow MF (2005). Roles of the CBF2 and ZAT12 transcription factors in configuring the low temperature transcriptome of Arabidopsis. Plant J 41, 195-211.
DOI URL |
| [76] |
Wang H, Wang HY (2015). The miR156/SPL module, a regulatory hub and versatile toolbox, gears up crops for enhanced agronomic traits. Mol Plant 8, 677-688.
DOI PMID |
| [77] |
Wang J, Zhou L, Shi H, Chern M, Yu H, Yi H, He M, Yin JJ, Zhu XB, Li Y, Li WT, Liu JL, Wang JC, Chen XQ, Qing H, Wang YP, Liu GF, Wang WM, Li P, Wu XJ, Zhu LH, Zhou JM, Ronald PC, Li SC, Li JY, Chen XW (2018). A single transcription factor promotes both yield and immunity in rice. Science 361, 1026-1028.
DOI PMID |
| [78] |
Wang JW, Czech B, Weigel D (2009). miR156-regulated SPL transcription factors define an endogenous flowering pathway in Arabidopsis thaliana. Cell 138, 738-749.
DOI URL |
| [79] |
Wang JW, Ye YJ, Xu M, Feng LG, Xu LA (2019). Roles of the SPL gene family and miR156 in the salt stress responses of tamarisk (Tamarix chinensis). BMC Plant Biol 19, 370.
DOI |
| [80] | Wang L, Ming LC, Liao KY, Xia CJ, Sun SY, Chang Y, Wang HK, Fu DB, Xu CH, Wang ZJ, Li X, Xie WB, Ouyang YD, Zhang QL, Li XH, Zhang QH, Xiao JH, Zhang QF (2021a). Bract suppression regulated by the miR156/529-SPLs-NL1-PLA1 module is required for the transition from vegetative to reproductive branching in rice. Mol Plant 14, 1168-1184. |
| [81] | Wang L, Sun SY, Jin JY, Fu DB, Yang XF, Weng XY, Xu CG, Li XH, Xiao JH, Zhang QF (2015). Coordinated regulation of vegetative and reproductive branching in rice. Proc Natl Acad Sci USA 112, 15504-15509. |
| [82] |
Wang M, Mo ZH, Lin RZ, Zhu CC (2021b). Characterization and expression analysis of the SPL gene family during floral development and abiotic stress in pecan (Carya illinoinensis). Peer J 9, e12490.
DOI URL |
| [83] |
Wang SK, Wu K, Yuan QB, Liu XY, Liu ZB, Lin XY, Zeng RZ, Zhu HT, Dong GJ, Qian Q, Zhang GQ, Fu XD (2012). Control of grain size, shape and quality by OsSPL16 in rice. Nat Genet 44, 950-954.
DOI |
| [84] |
Wang SL, Ren XX, Xue JQ, Xue YQ, Cheng XD, Hou XG, Zhang XX (2020a). Molecular characterization and expression analysis of the SQUAMOSA PROMOTER BINDING PROTEIN-LIKE gene family in Paeonia suffruticosa. Plant Cell Rep 39, 1425-1441.
DOI |
| [85] |
Wang YM, Liu WW, Wang XW, Yang RJ, Wu ZY, Wang H, Wang L, Hu ZB, Guo SY, Zhang HL, Lin JX, Fu CX (2020b). miR156 regulates anthocyanin biosynthesis through SPL targets and other microRNAs in poplar. Hortic Res 7, 118.
DOI |
| [86] |
Wei BY, Zhang JZ, Pang CX, Yu H, Guo DS, Jiang H, Ding MX, Chen ZY, Tao Q, Gu HY, Qu LJ, Qin GJ (2015). The molecular mechanism of SPOROCYTELESS/NOZZLE in controlling Arabidopsis ovule development. Cell Res 25, 121-134.
DOI |
| [87] |
Wu G, Poethig RS (2006). Temporal regulation of shoot development in Arabidopsis thaliana by miR156 and its target SPL3. Development 133, 3539-3547.
DOI URL |
| [88] |
Wu ZY, Cao YP, Yang RJ, Qi TX, Hang YQ, Lin H, Zhou GK, Wang ZY, Fu CX (2016). Switchgrass SBP-box transcription factors PvSPL1 and 2 function redundantly to initiate side tillers and affect biomass yield of energy crop. Biotechnol Biofuels 9, 101.
DOI PMID |
| [89] |
Xing SP, Salinas M, Höhmann S, Berndtgen R, Huijser P (2010). miR156-targeted and nontargeted SBP-box transcription factors act in concert to secure male fertility in Arabidopsis. Plant Cell 22, 3935-3950.
DOI URL |
| [90] |
Xu ML, Hu TQ, Zhao JF, Park MY, Earley KW, Wu G, Yang L, Poethig RS (2016). Developmental functions of miR156-regulated SQUAMOSA PROMOTER BINDING PROTEIN-LIKE (SPL) genes in Arabidopsis thaliana. PLoS Genet 12, e1006263.
DOI URL |
| [91] |
Xu YJ, Xu HX, Wall MM, Yang JZ (2020). Roles of transcription factor SQUAMOSA promoter binding protein-like gene family in papaya (Carica papaya) development and ripening. Genomics 112, 2734-2747.
DOI URL |
| [92] |
Yamaguchi A, Wu MF, Yang L, Wu G, Poethig RS, Wagner D (2009). The microRNA-regulated SBP-Box transcription factor SPL3 is a direct upstream activator of LEAFY, FRUITFULL, and APETALA1. Dev Cell 17, 268-278.
DOI PMID |
| [93] |
Yamasaki H, Hayashi M, Fukazawa M, Kobayashi Y, Shikanai T (2009). SQUAMOSA promoter binding protein-like7 is a central regulator for copper homeostasis in Arabidopsis. Plant Cell 21, 347-361.
DOI PMID |
| [94] |
Yamasaki K, Kigawa T, Inoue M, Tateno M, Yamasaki T, Yabuki T, Aoki M, Seki E, Matsuda T, Nunokawa E, Ishizuka Y, Terada T, Shirouzu M, Osanai T, Tanaka A, Seki M, Shinozaki K, Yokoyama S (2004). A novel zinc-binding motif revealed by solution structures of DNA-binding domains of Arabidopsis SBP-family transcription factors. J Mol Biol 337, 49-63.
DOI URL |
| [95] |
Yang RJ, Liu WW, Sun Y, Sun ZC, Wu ZY, Wang YM, Wang MQ, Wang HL, Bai SQ, Fu CX (2022). LATERAL BRANCHING OXIDOREDUCTASE, one novel target gene of Squamosa promoter binding protein-like 2, regulates tillering in switchgrass. New Phytol 235, 563-575.
DOI PMID |
| [96] |
Yao SZ, Yang ZR, Yang RX, Huang Y, Guo G, Kong XY, Lan Y, Zhou T, Wang H, Wang WM, Cao XF, Wu JG, Li Y (2019a). Transcriptional regulation of miR528 by OsSPL9 orchestrates antiviral response in rice. Mol Plant 12, 1114-1122.
DOI URL |
| [97] |
Yao T, Park BS, Mao HZ, Seo JS, Ohama N, Li Y, Yu N, Mustafa NFB, Huang CH, Chua NH (2019b). Regulation of flowering time by SPL10/MED25 module in Arabidopsis. New Phytol 224, 493-504.
DOI URL |
| [98] |
Yin HB, Hong GJ, Li LY, Zhang XY, Kong YZ, Sun ZT, Li JM, Chen JP, He YQ (2019). miR156/SPL9 regulates reactive oxygen species accumulation and immune response in Arabidopsis thaliana. Phytopathology 109, 632-642.
DOI URL |
| [99] |
Yu ZX, Wang LJ, Zhao B, Shan CM, Zhang YH, Chen DF, Chen XY (2015). Progressive regulation of sesquiterpene biosynthesis in Arabidopsis and patchouli (Pogostemon cablin) by the miR156-targeted SPL transcription factors. Mol Plant 8, 98-110.
DOI URL |
| [100] |
Yuan H, Qin P, Hu L, Zhan SJ, Wang SF, Gao P, Li J, Jin MY, Xu ZY, Gao Q, Du AP, Tu B, Chen WL, Ma BT, Wang YP, Li SG (2019). OsSPL18 controls grain weight and grain number in rice. J Genet Genomics 46, 41-51.
DOI PMID |
| [101] |
Yue EK, Li C, Li Y, Liu Z, Xu JH (2017). miR529a modulates panicle architecture through regulating SQUAMOSA PROMOTER BINDING-LIKE genes in rice (Oryza sativa). Plant Mol Biol 94, 469-480.
DOI PMID |
| [102] |
Yun JX, Sun ZX, Jiang Q, Wang YN, Wang C, Luo YQ, Zhang FR, Li X (2022). The miR156b-GmSPL9d module modulates nodulation by targeting multiple core nodulation genes in soybean. New Phytol 233, 1881-1899.
DOI URL |
| [103] |
Zhang LL, Huang YY, Zheng YP, Liu XX, Zhou SX, Yang XM, Liu SL, Li Y, Li JL, Zhao SL, Wang H, Ji YP, Zhang JW, Pu M, Zhao ZX, Fan J, Wang WM (2022). Osa- miR535 targets SQUAMOSA promoter binding protein-like 4 to regulate blast disease resistance in rice. Plant J 110, 166-178.
DOI URL |
| [104] |
Zhang XF, Yang CY, Lin HX, Wang JW, Xue HW (2021). Rice SPL12 coevolved with GW5 to determine grain shape. Sci Bull 66, 2353-2357.
DOI URL |
| [105] |
Zhang Y, Schwarz S, Saedler H, Huijser P (2007). SPL8, a local regulator in a subset of gibberellin-mediated developmental processes in Arabidopsis. Plant Mol Biol 63, 429-439.
DOI PMID |
| [106] |
Zhao BB, Xu MY, Zhao YP, Li YY, Xu H, Li CY, Kong DX, Xie YR, Zheng ZG, Wang BB, Wang HY (2022a). Over-expression of ZmSPL12 confers enhanced lodging resistance through transcriptional regulation of D1 in maize. Plant Biotechnol J 20, 622-624.
DOI URL |
| [107] | Zhao JL, Shi M, Yu J, Guo CK (2022b). SPL9 mediates freezing tolerance by directly regulating the expression of CBF2 in Arabidopsis thaliana. BMC Plant Biol 22, 59. |
| [108] |
Zhou MQ, Tang W (2019). MicroRNA156 amplifies transcription factor-associated cold stress tolerance in plant cells. Mol Genet Genomics 294, 379-393.
DOI PMID |
| [1] | Xiong Lianglin, Liang Guolu, Guo Qigao, Jing Danlong. Advances in the Regulation of Alternative Splicing of Genes in Plants in Response to Abiotic Stress [J]. Chinese Bulletin of Botany, 2025, 60(3): 435-448. |
| [2] | Chen Pengxiang, Wang Bo, Wang Zijun, Han Rong. The Regulatory Roles of the Transcription Factors in Plant's Response to UV-B Radiation [J]. Chinese Bulletin of Botany, 2025, 60(3): 449-459. |
| [3] | Yaping Wang, Wenquan Bao, Yu’e Bai. Advances in the Application of Single-cell Transcriptomics in Plant Growth, Development and Stress Response [J]. Chinese Bulletin of Botany, 2025, 60(1): 101-113. |
| [4] | SUN Jia-Mei, AN Bing-Er, LIU Wei, WANG Jing, PAN Qing-Min. Propagule regulation technique in grasslands: cultivation and transplantation of “propagule island” [J]. Chin J Plant Ecol, 2025, 49(1): 129-137. |
| [5] | LIANG Yi-Xian, WANG Chuan-Kuan, ZANG Miao-Han, SHANGGUAN Hong-Yu, LIU Yi-Xiao, QUAN Xian-Kui. Responses of radial growth and biomass allocation of Larix gmelinii to climate warming [J]. Chin J Plant Ecol, 2024, 48(4): 459-468. |
| [6] | ZANG Miao-Han, WANG Chuan-Kuan, LIANG Yi-Xian, LIU Yi-Xiao, SHANGGUAN Hong-Yu, QUAN Xian-Kui. Stoichiometric characteristics of leaf, branch and root in Larix gmelinii in response to climate warming based on latitudinal transplantation [J]. Chin J Plant Ecol, 2024, 48(4): 469-482. |
| [7] | Min Kang, Meiying Zhang, Xiushuang Qi, Ningning Tong, Yang Li, Qingyan Shu, Zheng’an Liu, Changping Lü, Liping Peng. Establishment of a Fast Breeding System for Itoh Hybrid ‘He Xie’ in Tissue Culture [J]. Chinese Bulletin of Botany, 2024, 59(3): 441-451. |
| [8] | Wen Chen, Yingying Zhou, Ping Luo, Yongyi Cui. Molecular Mechanism of Petal Doubling of Flower in Angiosperm [J]. Chinese Bulletin of Botany, 2024, 59(2): 257-277. |
| [9] | Yanan Xu, Jiarong Yan, Xin Sun, Xiaomei Wang, Yufeng Liu, Zhouping Sun, Mingfang Qi, Tianlai Li, Feng Wang. Red and Far-red Light Regulation of Plant Growth, Development, and Abiotic Stress Responses [J]. Chinese Bulletin of Botany, 2023, 58(4): 622-637. |
| [10] | Yu Miao, Ruan Chengjiang, Ding Jian, Li Jingbin, Lu Shunguang, Wen Xiufeng. Hrh-miRn458 Regulates Oil Biosynthesis of Sea Buckthorn via Targeting Transcription Factor WRI1 [J]. Chinese Bulletin of Botany, 2022, 57(5): 635-648. |
| [11] | Hao Xuefeng, Wu Zhangjing, Ma Tian, Jin Zhuping, Pei Yanxi. Alternative Splicing of Plant Genes: Full of Change, Sail with Wind [J]. Chinese Bulletin of Botany, 2022, 57(5): 661-672. |
| [12] | Jingwen Wang, Xingjun Wang, Changle Ma, Pengcheng Li. A Review on the Mechanism of Ribosome Stress Response in Plants [J]. Chinese Bulletin of Botany, 2022, 57(1): 80-89. |
| [13] | Fangfang Cai, Changsheng Shao, Yuqiang Sun. The Role of Alternative Splicing in Floral Transition [J]. Chinese Bulletin of Botany, 2022, 57(1): 69-79. |
| [14] | Dong Liu. Managing Both Internal and Foreign Affairs—A PHR-centered Gene Network Regulates Plant-mycorrhizal Symbiosis [J]. Chinese Bulletin of Botany, 2021, 56(6): 647-650. |
| [15] | Tianxingzi Wang, Zheng Zhu, Yue Chen, Yuqing Liu, Gaowei Yan, Shan Xu, Tong Zhang, Jinjiao Ma, Shijuan Dou, Liyun Li, Guozhen Liu. Rice OsWRKY42 is a Novel Element in Xa21-mediated Resistance Pathway Against Bacterial Leaf Blight [J]. Chinese Bulletin of Botany, 2021, 56(6): 687-698. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||