

Chinese Bulletin of Botany ›› 2022, Vol. 57 ›› Issue (5): 623-634.DOI: 10.11983/CBB22048 cstr: 32102.14.CBB22048
Special Issue: 生物安全
• EXPERIMENTAL COMMUNICATIONS • Previous Articles Next Articles
Ye Qing, Yan Xiaoyan, Chen Huize, Feng Jinlin, Han Rong*( )
)
Received:2022-03-14
Accepted:2022-06-28
Online:2022-09-01
Published:2022-09-09
Contact:
Han Rong
About author:*E-mail: hhwrsl@163.comYe Qing, Yan Xiaoyan, Chen Huize, Feng Jinlin, Han Rong. Effect of Nitrogen-doped Graphene Quantum Dots on Growth Direction of Primary Root in Arabidopsis thaliana[J]. Chinese Bulletin of Botany, 2022, 57(5): 623-634.
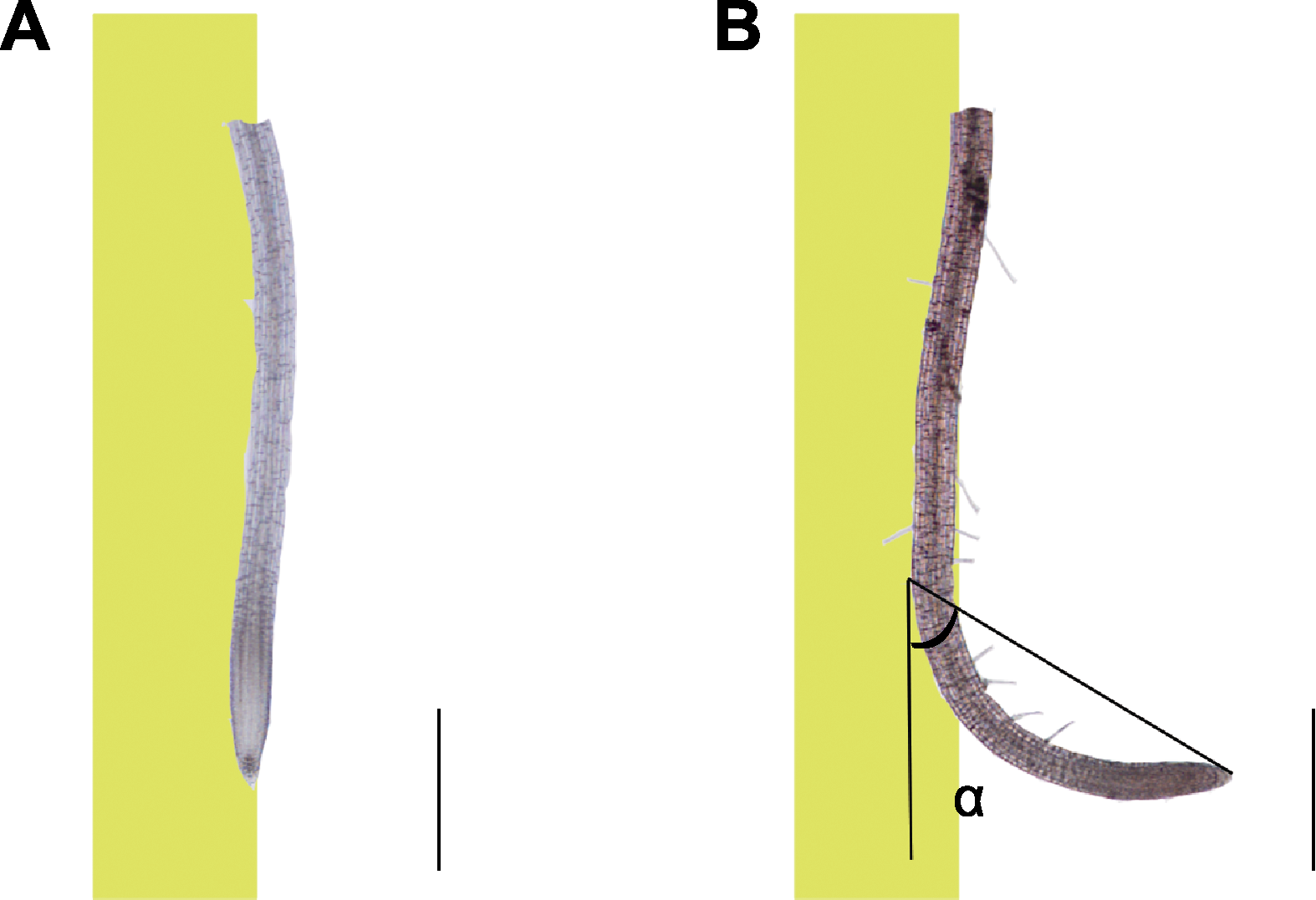
Figure 1 Measurement method for bending angle of Arabidopsis primary root tip after nitrogen-doped graphene quantum dots (N-GQDs) treatment (A) Primary roots of 5-day-old Arabidopsis thaliana seedlings vertically cultured on 1/4MS medium; (B) The seedlings in (A) were transferred to 1/4MS medium containing 50 mg·L-1 N-GQDs, they were cultured vertically for 3 d, the roots were bent towards the direction distant from the medium. The bending angle α of primary roots between root growth direction and gravity direction was determined. The yellow part at the left side in figure meant the seedlings were perpendicularly placed at one side of the medium, and the white part at the right side denoted that the seedlings were placed at the air side distant from the medium. Bars=500 μm
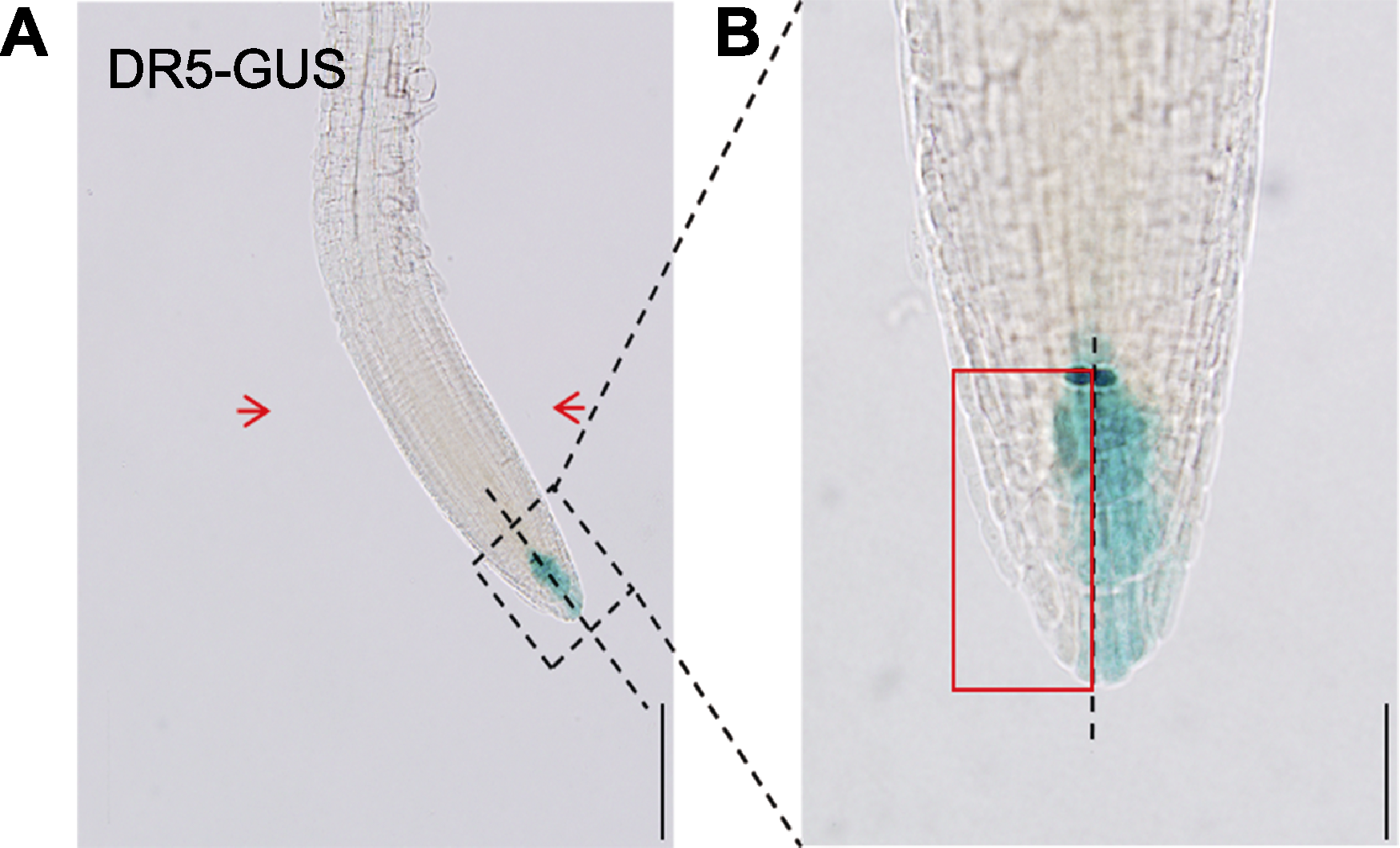
Figure 2 Measurement method of GUS activity at two sides of Arabidopsis thaliana primary root tip after nitrogen-doped graphene quantum dots (N-GQDs) treatment (A) After 5-day-old Arabidopsis thaliana DR5-GUS plant was perpendicularly cultured in 1/4MS medium containing 50 mg·L-1 N-GQDs for 3 d, the root was bent towards a direction distant from the medium, and the GUS activity value at the left and right sides (close to the culture medium/distant from the medium) of primary root tips were determined, the red arrow at the left side represents the side close to the medium and that at the right side denotes the side distant from the medium (bar=200 μm); (B) Measurement method of GUS activity at the left and right sides of primary root tip of DR5-GUS plant, the red box in the figure was the determined left region (bar=50 μm).

Figure 3 The characterization of nitrogen-doped graphene quantum dots (N-GQDs) (A) Transmission electron microscope graph of N-GQDs, the red arrows represent N-GQDs (bar=20 nm); (B) Particle size distribution diagram of N-GQDs; (C) Photoluminescence (PL) spectrogram of N-GQDs
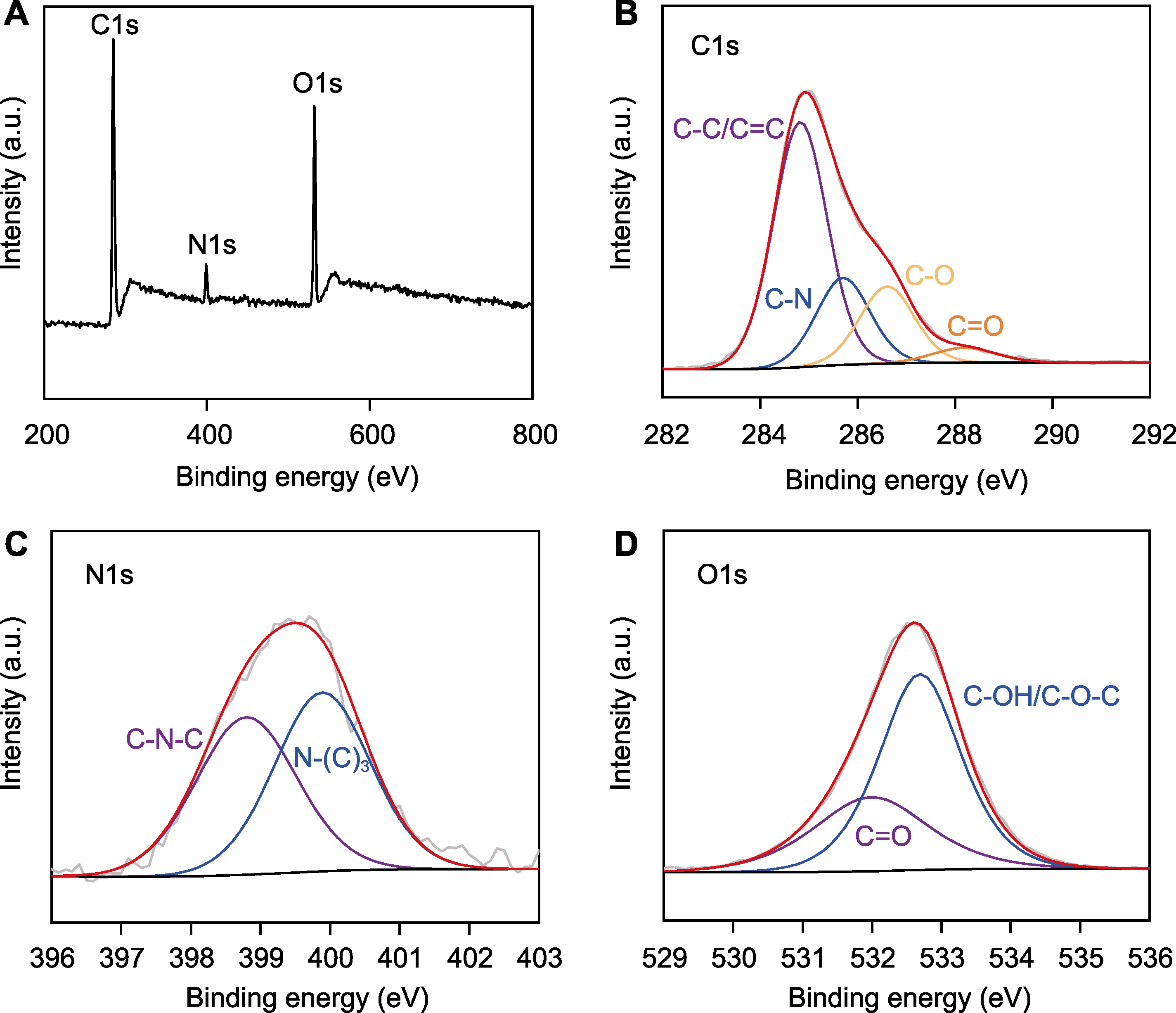
Figure 4 X-ray photoelectron spectroscopy (XPS) spectrum of nitrogen-doped graphene quantum dots (N-GQDs) (A) Full-scan XPS spectrum of N-GQDs; (B) High resolution C1s spectrum; (C) N1s spectrum; (D) O1s spectrum
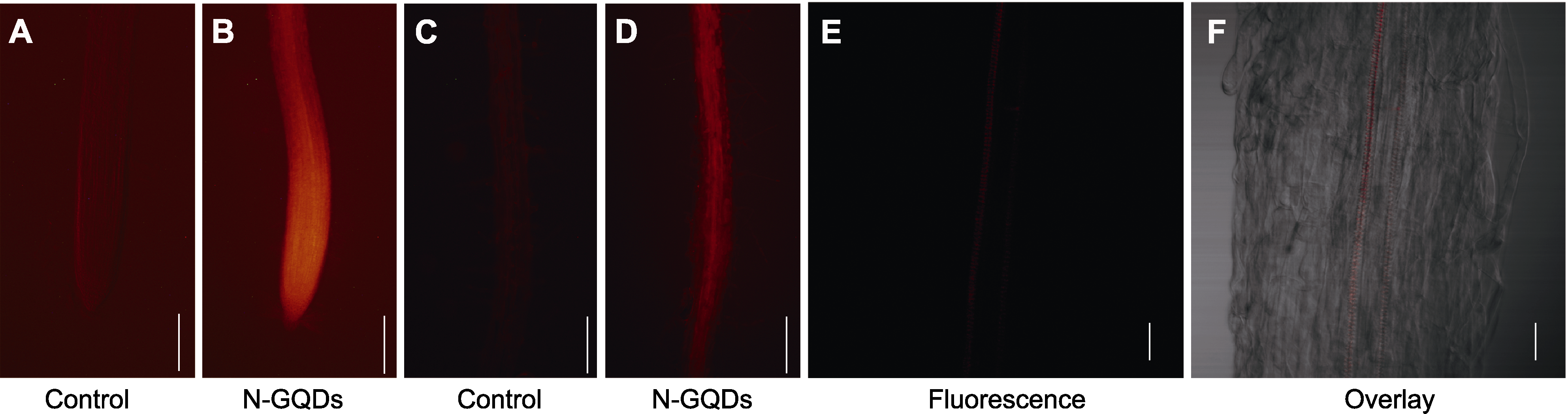
Figure 5 Distribution of nitrogen-doped graphene quantum dots (N-GQDs) in primary roots of Arabidopsis thaliana seedlings (A), (C) Fluorescence micro images of meristem zone and elongation zone (A), and root hair zone (C) of primary roots in Arabidopsis thaliana seedlings untreated with N-GQDs (bars=200 μm); (B), (D) Fluorescence micro images of meristem zone and elongation zone (B), and root hair zone (D) of primary roots under N-GQDs treatment for 3 days (bars=200 μm), the N-GQDs presented red fluorescence under a fluorescence microscope; (E), (F) Confocal images of vascular bundles in the root hair zone under N-GQDs treatment for 3 days (bars=30 μm)
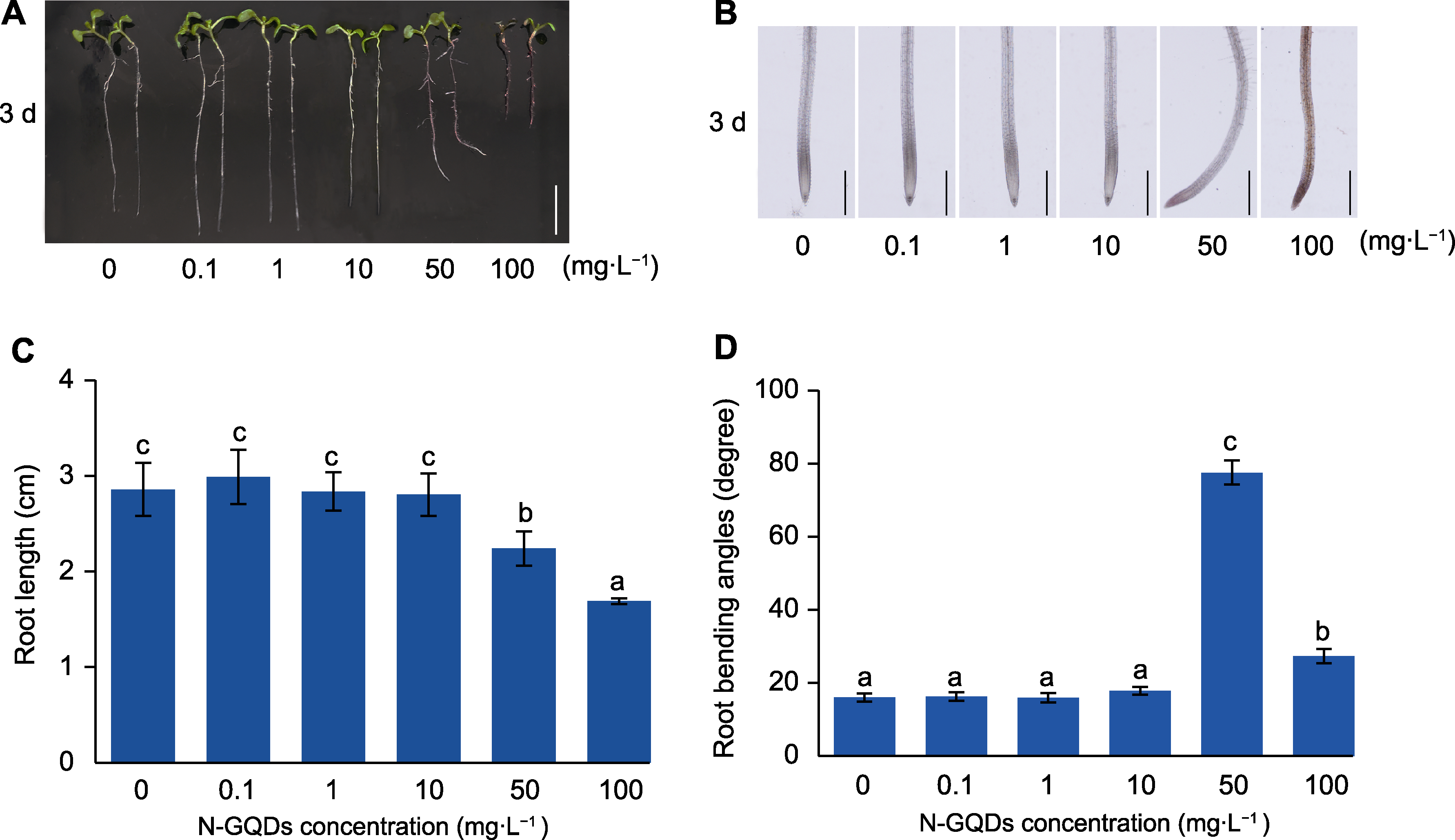
Figure 6 Effects of nitrogen-doped graphene quantum dots (N-GQDs) on growth direction of primary root of Arabidopsis thaliana (A) Primary root phenotypes of Arabidopsis thaliana under different concentrations of N-GQDs for 3 days (bar=1 cm); (B) The growth direction of primary roots under optical microscope (bars=500 μm); (C) Statistical analysis of primary roots length; (D) Statistical analysis of primary roots bending angles. The values are presented as means±SD of triplicate samples (n=30). Different lowercase letters represent significant differences (P<0.05).
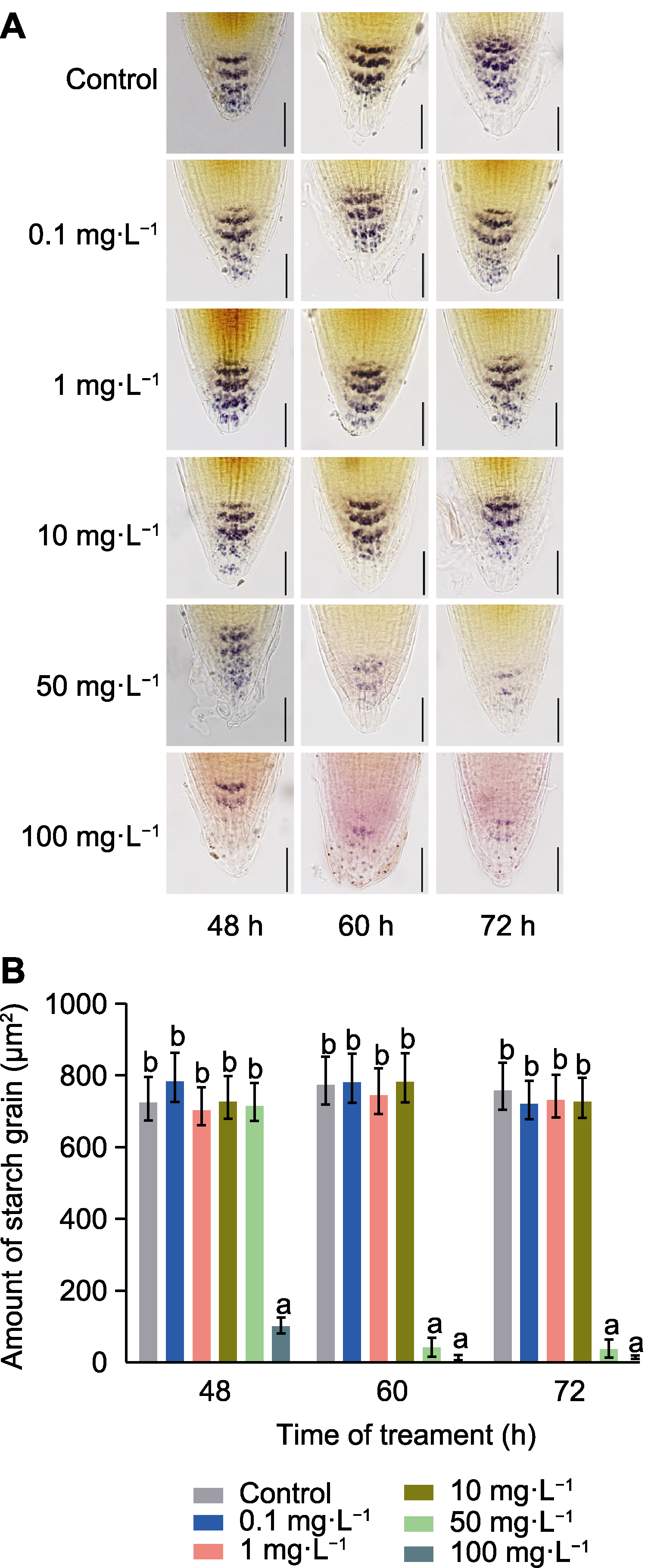
Figure 7 Nitrogen-doped graphene quantum dots (N-GQDs) reduce the accumulation of starch grains in Arabidopsis thaliana primary root tips (A) Changes of starch grains content in A. thaliana primary root tips by staining with different concentrations of N-GQDs treatment for different time (bars=50 μm); (B) Statistical analysis of starch grains amount in primary root tips. The values are presented as means±SD of triplicate samples (n=15). Different lowercase letters represent significant differences (P<0.05).

Figure 8 Nitrogen-doped graphene quantum dots (N-GQDs) disrupt the auxin distribution in Arabidopsis thaliana root tips (A) The GUS activity on both sides of the primary roots were observed by staining after N-GQDs treatment for different time, the red arrow at the left side represents the side close to the culture medium (bars=50 μm); (B) Statistical analysis of GUS activity ratio at the left and right sides of primary roots; the left side corresponds to one side in (A) close to the culture medium while the right side corresponds to the side in (A) distant from the culture medium. The values are presented as means±SD of triplicate samples (n=15). Different lowercase letters represent significant differences (P<0.05).
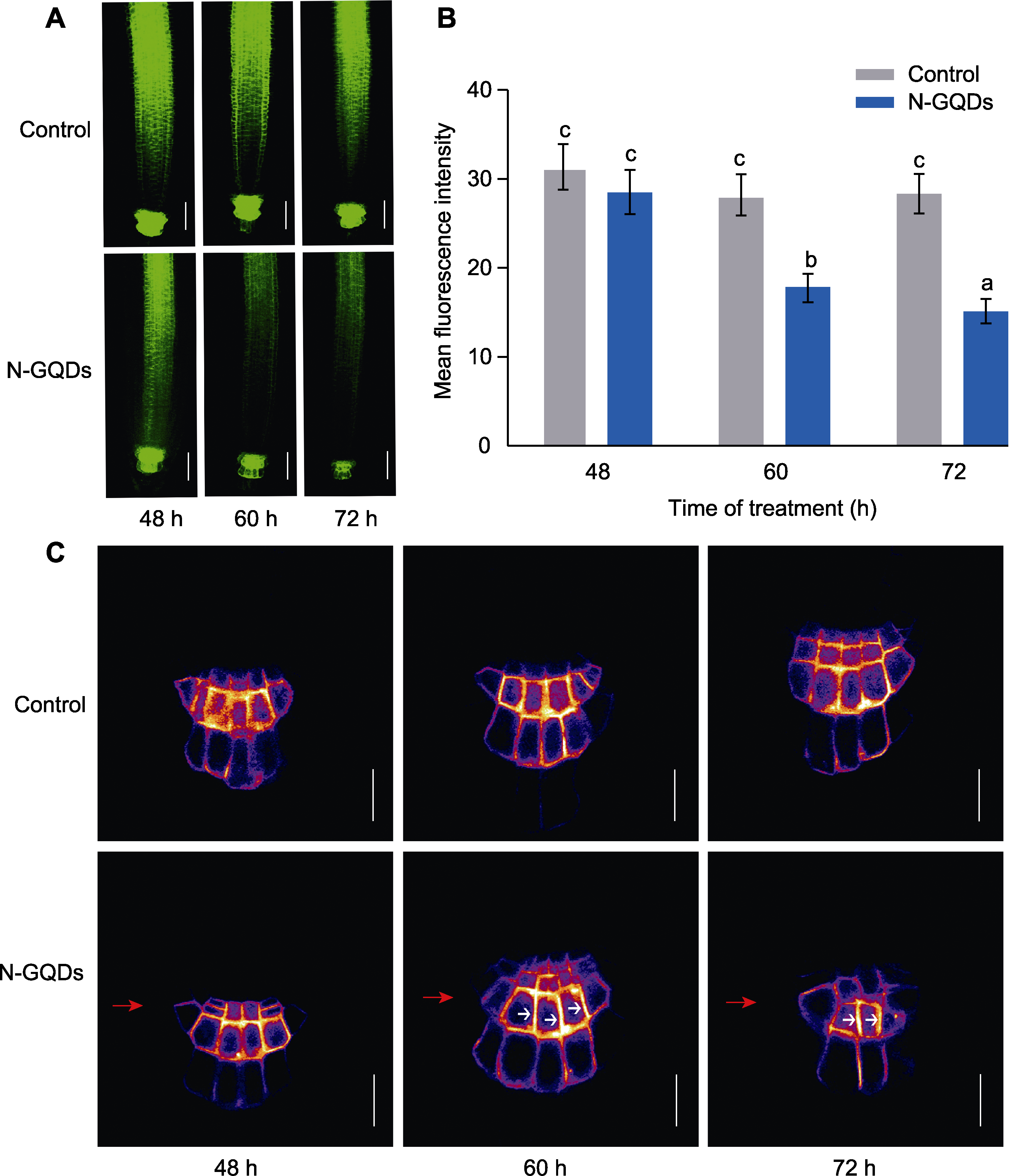
Figure 9 Nitrogen-doped graphene quantum dots (N-GQDs) disrupt the abundance and distribution of PIN3 in Arabidopsis thaliana primary roots (A) GFP fluorescence of PIN3-GFP in the primary roots after N-GQDs treatment for different time (bars=50 μm); (B) Quantitative analysis of fluorescence intensity of PIN3-GFP in the primary roots; (C) Distribution of PIN3 in columnar cells (bars=20 μm). The red arrows represent the side close to the culture medium. The white arrows in the columnar cells indicate the polarization directions of PIN3. The values are presented as means±SD of triplicate samples (n=15). Different lowercase letters represent significant differences (P<0.05).
| [1] | 高坤, 常金科, 黎家 (2018). 植物根向水性反应研究进展. 植物学报 53, 154-163. |
| [2] | 韩雯, 韩榕 (2015). 不同时间的UV-B辐射对拟南芥幼苗生长的影响. 植物学报 50, 40-46. |
| [3] | 李晓阳, 陈慧泽, 韩榕 (2013). UV-B辐射对拟南芥种子萌发和幼苗生长的影响. 植物学报 48, 52-58. |
| [4] | Baldwin KL, Strohm AK, Masson PH (2013). Gravity sensing and signal transduction in vascular plant primary roots. Am J Bot 100, 126-142. |
| [5] | Band LR, Wells DM, Larrieu A, Sun JY, Middleton AM, French AP, Brunoud G, Sato EM, Wilson MH, Péret B, Oliva M, Swarup R, Sairanen I, Parry G, Ljung K, Beeckman T, Garibaldi JM, Estelle M, Owen MR, Vissenberg K, Hodgman TC, Pridmore TP, King JR, Vernoux T, Bennett MJ (2012). Root gravitropism is regulated by a transient lateral auxin gradient controlled by a tipping-point mechanism. Proc Natl Acad Sci USA 109, 4668-4673. |
| [6] | Blancaflor EB, Fasano JM, Gilroy S (1998). Mapping the functional roles of cap cells in the response of Arabidopsis primary roots to gravity. Plant Physiol 116, 213-222. |
| [7] | Brunoud G, Wells DM, Oliva M, Larrieu A, Mirabet V, Burrow AH, Beeckman T, Kepinski S, Traas J, Bennett MJ, Vernoux T (2012). A novel sensor to map auxin response and distribution at high spatio-temporal resolution. Nature 482, 103-106. |
| [8] | Caspar T, Pickard BG (1989). Gravitropism in a starchless mutant of Arabidopsis: implications for the starch-statolith theory of gravity sensing. Planta 177, 185-197. |
| [9] | Chakravarty D, Erande MB, Late DJ (2015). Graphene quantum dots as enhanced plant growth regulators: effects on coriander and garlic plants. J Sci Food Agric 95, 2772-2778. |
| [10] | Deng S, Jia PP, Zhang JH, Junaid M, Niu AP, Ma YB, Fu AL, Pei DS (2018). Transcriptomic response and perturbation of toxicity pathways in zebrafish larvae after exposure to graphene quantum dots (GQDs). J Hazard Mater 357, 146-158. |
| [11] | Ding Y, Cheng HH, Zhou C, Fan YQ, Zhu J, Shao HB, Qu LT (2012). Functional microspheres of graphene quantum dots. Nanotechnology 23, 255605. |
| [12] | Feng P, Geng BJ, Cheng Z, Liao XY, Pan DY, Huang JY (2019). Graphene quantum dots-induced physiological and biochemical responses in mung bean and tomato seedlings. Braz J Bot 42, 29-41. |
| [13] | Friml J, Wiśniewska J, Benková E, Mendgen K, Palme K (2002). Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature 415, 806-809. |
| [14] | Galvan-Ampudia CS, Julkowska MM, Darwish E, Gandullo J, Korver RA, Brunoud G, Haring MA, Munnik T, Vernoux T, Testerink C (2013). Halotropism is a response of plant roots to avoid a saline environment. Curr Biol 23, 2044-2050. |
| [15] | Galvan-Ampudia CS, Testerink C (2011). Salt stress signals shape the plant root. Curr Opin Plant Biol 14, 296-302. |
| [16] | Grunewald W, Friml J (2010). The march of the PINs: developmental plasticity by dynamic polar targeting in plant cells. EMBO J 29, 2700-2714. |
| [17] | Guo XQ, Mei N (2014). Assessment of the toxic potential of graphene family nanomaterials. J Food Drug Anal 22, 105-115. |
| [18] | Hasanuzzaman M, Nahar K, Alam M, Roychowdhury R, Fujita M (2013). Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int J Mol Sci 14, 9643-9684. |
| [19] | Hasegawa PM, Bressan RA, Zhu JK, Bohnert HJ (2000). Plant cellular and molecular responses to high salinity. Annu Rev Plant Physiol Plant Mol Biol 51, 463-499. |
| [20] | Hu XG, Zhou QX (2013). Health and ecosystem risks of graphene. Chem Rev 113, 3815-3835. |
| [21] | Ju J, Chen W (2014). Synthesis of highly fluorescent nitrogen-doped graphene quantum dots for sensitive, label- free detection of Fe (III) in aqueous media. Biosens Bioelectron 58, 219-225. |
| [22] | Kleine-Vehn J, Ding ZJ, Jones AR, Tasaka M, Morita MT, Friml J (2010). Gravity-induced PIN transcytosis for polarization of auxin fluxes in gravity-sensing root cells. Proc Natl Acad Sci USA 107, 22344-22349. |
| [23] | Kong Z, Hu W, Jiao FF, Zhang PZ, Shen JW, Cui B, Wang HB, Liang LJ (2020). Theoretical evaluation of DNA genotoxicity of graphene quantum dots: a combination of density functional theory and molecular dynamics simulations. J Phys Chem B 124, 9335-9342. |
| [24] | Ku TT, Hao F, Yang XX, Rao ZY, Liu QS, Sang N, Faiola F, Zhou QF, Jiang GB (2021). Graphene quantum dots disrupt embryonic stem cell differentiation by interfering with the methylation level of Sox2. Environ Sci Technol 55, 3144-3155. |
| [25] | Leitz G, Kang BH, Schoenwaelder ME, Staehelin LA (2009). Statolith sedimentation kinetics and force transduction to the cortical endoplasmic reticulum in gravity- sensing Arabidopsis columella cells. Plant Cell 21, 843-860. |
| [26] | Li XL, Zhou ZH, Lu DJ, Dong XW, Xu MH, Wei LM, Zhang YF (2014). The effect of pristine carbon-based nanomaterial on the growth of green gram sprouts and pH of water. Nanoscale Res Lett 9, 583. |
| [27] | Li Y, Yuan W, Li LC, Miao R, Dai H, Zhang JH, Xu WF (2020). Light-dark modulates root hydrotropism associated with gravitropism by involving amyloplast response in Arabidopsis. Cell Rep 32, 108198. |
| [28] | Li Y, Zhao Y, Cheng HH, Hu Y, Shi GQ, Dai LM, Qu LT (2012). Nitrogen-doped graphene quantum dots with oxygen-rich functional groups. J Am Chem Soc 134, 15-18. |
| [29] | Lin YH, Zhuang SX, Wang YL, Lin S, Hong ZW, Liu Y, Xu L, Li FP, Xu BH, Chen MH, He SW, Liao BQ, Fu XP, Jiang ZQ, Wang HL (2019). The effects of graphene quantum dots on the maturation of mouse oocytes and development of offspring. J Cell Physiol 234, 13820-13831. |
| [30] | Morita MT, Tasaka M (2004). Gravity sensing and signaling. Curr Opin Plant Biol 7, 712-718. |
| [31] | Nan WB, Wang XM, Yang L, Hu YF, Wei YT, Liang XL, Mao LN, Bi YR (2014). Cyclic GMP is involved in auxin signaling during Arabidopsis root growth and development. J Exp Bot 65, 1571-1583. |
| [32] | Ottenschläger I, Wolff P, Wolverton C, Bhalerao RP, Sandberg G, Ishikawa H, Evans M, Palme K (2003). Gravity-regulated differential auxin transport from columella to lateral root cap cells. Proc Natl Acad Sci USA 100, 2987-2991. |
| [33] | Su SH, Gibbs NM, Jancewicz AL, Masson PH (2017). Molecular mechanisms of root gravitropism. Curr Biol 27, R964-R972. |
| [34] | Sun HF, Wang M, Wang J, Wang WP (2022). Surface charge affects foliar uptake, transport and physiological effects of functionalized graphene quantum dots in plants. Sci Total Environ 812, 151506. |
| [35] | Swarup R, Kramer EM, Perry P, Knox K, Leyser HMO, Haseloff J, Beemster GTS, Bhalerao R, Bennett MJ (2005). Root gravitropism requires lateral root cap and epidermal cells for transport and response to a mobile auxin signal. Nat Cell Biol 7, 1057-1065. |
| [36] | Tsugeki R, Fedoroff NV (1999). Genetic ablation of root cap cells in Arabidopsis. Proc Natl Acad Sci USA 96, 12941-12946. |
| [37] | Ulmasov T, Murfett J, Hagen G, Guilfoyle TJ (1997). Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell 9, 1963-1971. |
| [38] | Wang D, Zhu L, Chen JF, Dai LM (2015a). Can graphene quantum dots cause DNA damage in cells? Nanoscale 7, 9894-9901. |
| [39] | Wang ZG, Zhou R, Jiang D, Song JE, Xu Q, Si J, Chen YP, Zhou X, Gan L, Li JZ, Zhang H, Liu B (2015b). Toxicity of graphene quantum dots in zebrafish embryo. Biomed Environ Sci 28, 341-351. |
| [40] | Wen J, Xu YQ, Li HJ, Lu AP, Sun SG (2015). Recent applications of carbon nanomaterials in fluorescence biosensing and bioimaging. Chem Commun 51, 11346-11358. |
| [41] | Wiśniewska J, Xu J, Seifertová D, Brewer PB, Růžička K, Blilou I, Rouquié D, Benková E, Scheres B, Friml J (2006). Polar PIN localization directs auxin flow in plants. Science 312, 883. |
| [42] | Zheng XT, Ananthanarayanan A, Luo KQ, Chen P (2015). Glowing graphene quantum dots and carbon dots: properties, syntheses, and biological applications. Small 11, 1620-1636. |
| [43] | Zhu JK (2016). Abiotic stress signaling and responses in plants. Cell 167, 313-324. |
| [1] | Yuying Zhou, Hui Chen, Simu Liu. Research Progress on Auxin Responsive Non-canonical Aux/IAA Proteins in Plants [J]. Chinese Bulletin of Botany, 2024, 59(4): 651-658. |
| [2] | WU Chen, CHEN Xin-Yi, LIU Yuan-Hao, HUANG Jin-Xue, XIONG De-Cheng. Effects of warming on fine root growth, mortality and turnover: a review [J]. Chin J Plant Ecol, 2023, 47(8): 1043-1054. |
| [3] | WU Fan, WU Chen, ZHANG Yu-Hui, YU Heng, WEI Zhi-Hua, ZHENG Wei, LIU Xiao-Fei, CHEN Shi-Dong, YANG Zhi-Jie, XIONG De-Cheng. Effects of warming on growth, morphology and physiological metabolism characteristics of fine roots in a mature Cunninghamia lanceolata plantation in different seasons [J]. Chin J Plant Ecol, 2023, 47(6): 856-866. |
| [4] | Xiangpei Kong, Mengyue Zhang, Zhaojun Ding. There Is a Way Out-new Breakthroughs in Extracellular Auxin Sensing [J]. Chinese Bulletin of Botany, 2023, 58(6): 861-865. |
| [5] | Yuan Yuan, Enhebayaer, Qi Yanhua. Research Advances in Biological Functions of GH3 Gene Family in Plants [J]. Chinese Bulletin of Botany, 2023, 58(5): 770-782. |
| [6] | Shuyao Zhou, Jianming Li, Juan Mao. AtGH3.17-mediated Regulation of Auxin and Brassinosteroid Response in Arabidopsis thaliana [J]. Chinese Bulletin of Botany, 2023, 58(3): 373-384. |
| [7] | Lixia Jia, Yanhua Qi. Advances in the Regulation of Rice (Oryza sativa) Grain Shape by Auxin Metabolism, Transport and Signal Transduction [J]. Chinese Bulletin of Botany, 2022, 57(3): 263-275. |
| [8] | Binqi Li, Jiahui Yan, Hao Li, Wei Xin, Yunhe Tian, Zhenbiao Yang, Wenxin Tang. Changes of Small GTPases Activity During Cucumber Tendril Winding [J]. Chinese Bulletin of Botany, 2022, 57(3): 299-307. |
| [9] | Jingwen Wang, Xingjun Wang, Changle Ma, Pengcheng Li. A Review on the Mechanism of Ribosome Stress Response in Plants [J]. Chinese Bulletin of Botany, 2022, 57(1): 80-89. |
| [10] | Yanyan Li, Yanhua Qi. Advances in Biological Functions of Aux/IAA Gene Family in Plants [J]. Chinese Bulletin of Botany, 2022, 57(1): 30-41. |
| [11] | Yuqing Lin, Yanhua Qi. Advances in Auxin Efflux Carrier PIN Proteins [J]. Chinese Bulletin of Botany, 2021, 56(2): 151-165. |
| [12] | Rongfeng Huang, Tongda Xu. Auxin Regulates the Lateral Root Development Through MAPK-mediated VLCFAs Biosynthesis [J]. Chinese Bulletin of Botany, 2021, 56(1): 6-9. |
| [13] | Yuting Yao,Jiaqi Ma,Xiaoli Feng,Jianwei Pan,Chao Wang. A Role of Arabidopsis Phosphoinositide Kinase, FAB1, in Root Hair Growth [J]. Chinese Bulletin of Botany, 2020, 55(2): 126-136. |
| [14] | Zhenmei He,Dongming Li,Yanhua Qi. Advances in Biofunctions of the ABCB Subfamily in Plants [J]. Chinese Bulletin of Botany, 2019, 54(6): 688-698. |
| [15] | Shuhui Zhang,Hong Wang,Wenru Wang,Xuelian Wu,Yuansong Xiao,Futian Peng. Effects of Sucrose on Seedling Growth and Development and SnRK1 Activity in Prunus persica [J]. Chinese Bulletin of Botany, 2019, 54(6): 744-752. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||