

Chinese Bulletin of Botany ›› 2023, Vol. 58 ›› Issue (1): 108-121.DOI: 10.11983/CBB22155 cstr: 32102.14.CBB22155
Special Issue: 杂粮生物学专辑 (2023年58卷1期)
• EXPERIMENTAL COMMUNICATIONS • Previous Articles Next Articles
Qi Zhang1, Wenjing Zhang1, Xiankai Yuan1, Ming Li1, Qiang Zhao1, Yanli Du1, Jidao Du1,2,*( )
)
Received:2022-07-15
Accepted:2022-10-17
Online:2023-01-01
Published:2023-01-05
Contact:
*E-mail: djdbynd@163.com
Qi Zhang, Wenjing Zhang, Xiankai Yuan, Ming Li, Qiang Zhao, Yanli Du, Jidao Du. The Regulatory Mechanism of Melatonin on Nucleic Acid Repairing of Common Bean (Phaseolus vulgaris) at the Sprout Stage Under Salt Stress[J]. Chinese Bulletin of Botany, 2023, 58(1): 108-121.
| ID | Primer name | Primer sequence (5′-3′) |
|---|---|---|
| 1 | Pvactin11-qF | TGCATACGTTGGTGATGAGG |
| 2 | Pvactin11-qR | AGCCTTGGGGTTAAGAGGAG |
| 3 | Phvul.005G021100F | CGTTCTTCCAGTCTTCGTTC |
| 4 | Phvul.005G021100R | TCTCACTTCCCACACCTCAC |
| 5 | Phvul.005G021200F | TGGAGATGGAGAAGCAAGTG |
| 6 | Phvul.005G021200R | AGGCGAGAAAGAGAAACGG |
| 7 | Phvul.005G045900F | TGACTGTAGGCATAGAGGGT |
| 8 | Phvul.005G045900R | GGTCTTGCTAGAAAGAGGTG |
| 9 | Phvul.006G137800F | ACTATGATTGATATGAACGA |
| 10 | Phvul.006G137800R | TTTGACAGACTAATGGAGAC |
Table 1 qRT-PCR primers used in this study
| ID | Primer name | Primer sequence (5′-3′) |
|---|---|---|
| 1 | Pvactin11-qF | TGCATACGTTGGTGATGAGG |
| 2 | Pvactin11-qR | AGCCTTGGGGTTAAGAGGAG |
| 3 | Phvul.005G021100F | CGTTCTTCCAGTCTTCGTTC |
| 4 | Phvul.005G021100R | TCTCACTTCCCACACCTCAC |
| 5 | Phvul.005G021200F | TGGAGATGGAGAAGCAAGTG |
| 6 | Phvul.005G021200R | AGGCGAGAAAGAGAAACGG |
| 7 | Phvul.005G045900F | TGACTGTAGGCATAGAGGGT |
| 8 | Phvul.005G045900R | GGTCTTGCTAGAAAGAGGTG |
| 9 | Phvul.006G137800F | ACTATGATTGATATGAACGA |
| 10 | Phvul.006G137800R | TTTGACAGACTAATGGAGAC |
| ID | Primer name | Primer sequence (5′-3′) |
|---|---|---|
| 1 | Random primer 1 | GGTGCGGGAA |
| 2 | Random primer 2 | GAGAGCCACC |
| 3 | Random primer 3 | GGGATATCGG |
| 4 | Random primer 4 | TGAGCGGACA |
Table 2 Random amplified polymorphic DNA (RAPD) primers in this study
| ID | Primer name | Primer sequence (5′-3′) |
|---|---|---|
| 1 | Random primer 1 | GGTGCGGGAA |
| 2 | Random primer 2 | GAGAGCCACC |
| 3 | Random primer 3 | GGGATATCGG |
| 4 | Random primer 4 | TGAGCGGACA |
| Treatments | Length (cm) | Surface area (cm2) | Volume (cm3) | Diameter (mm) |
|---|---|---|---|---|
| W | 11.917±0.776 a | 2.945±0.283 b | 0.113±0.007 b | 1.266±0.033 a |
| S | 6.122±0.510 b | 1.652±0.244 c | 0.077±0.013 c | 1.013±0.066 b |
| M+S | 12.096±0.975 a | 3.896±0.212 a | 0.138±0.011 a | 1.293±0.051 a |
Table 3 The phenotypical analysis of common bean sprouts under different treatments
| Treatments | Length (cm) | Surface area (cm2) | Volume (cm3) | Diameter (mm) |
|---|---|---|---|---|
| W | 11.917±0.776 a | 2.945±0.283 b | 0.113±0.007 b | 1.266±0.033 a |
| S | 6.122±0.510 b | 1.652±0.244 c | 0.077±0.013 c | 1.013±0.066 b |
| M+S | 12.096±0.975 a | 3.896±0.212 a | 0.138±0.011 a | 1.293±0.051 a |
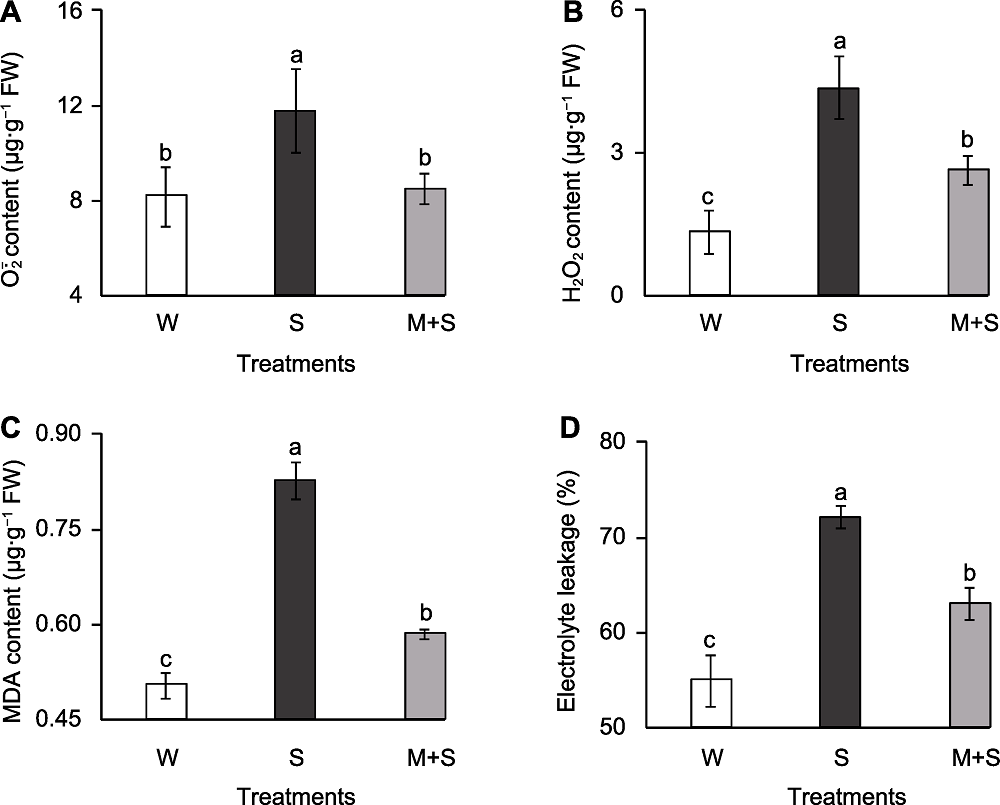
Figure 2 The membrane peroxidation indicators of common bean sprouts (A) O2- . content; (B) H2O2 content; (C) Malondialdehyde (MDA) content; (D) Electrolyte leakage. W, S, and M+S are the same as shown in Figure 1. Different lowercase letters indicate significant differences among different treatments (P<0.05).
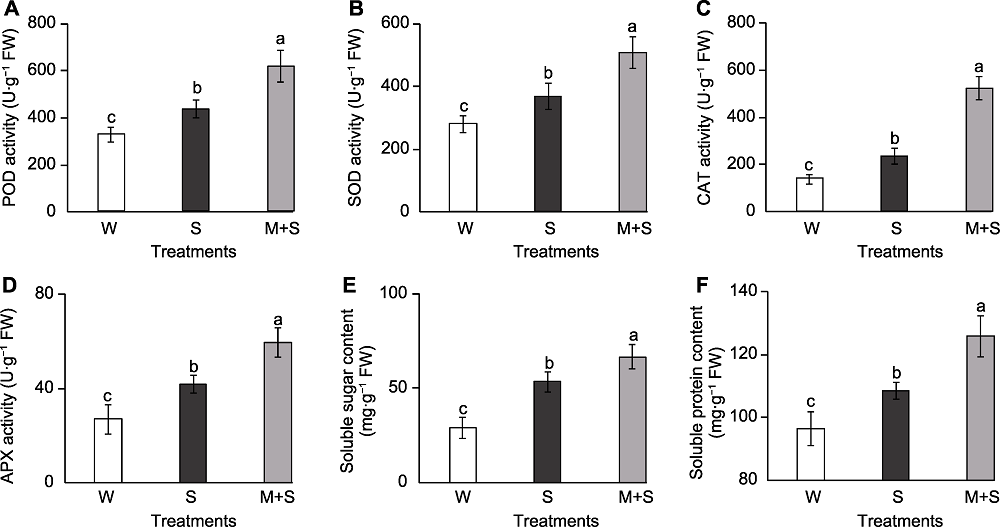
Figure 3 The antioxidant enzyme activity and osmolyte content of common bean sprouts (A) Peroxidase (POD) activity; (B) Superoxide dismutase (SOD) activity; (C) Catalase (CAT) activity; (D) Ascorbate peroxidase (APX) activity; (E) Soluble sugar content; (F) Soluble protein content. W, S, and M+S are the same as shown in Figure 1. Different lowercase letters indicate significant differences among different treatments (P<0.05).
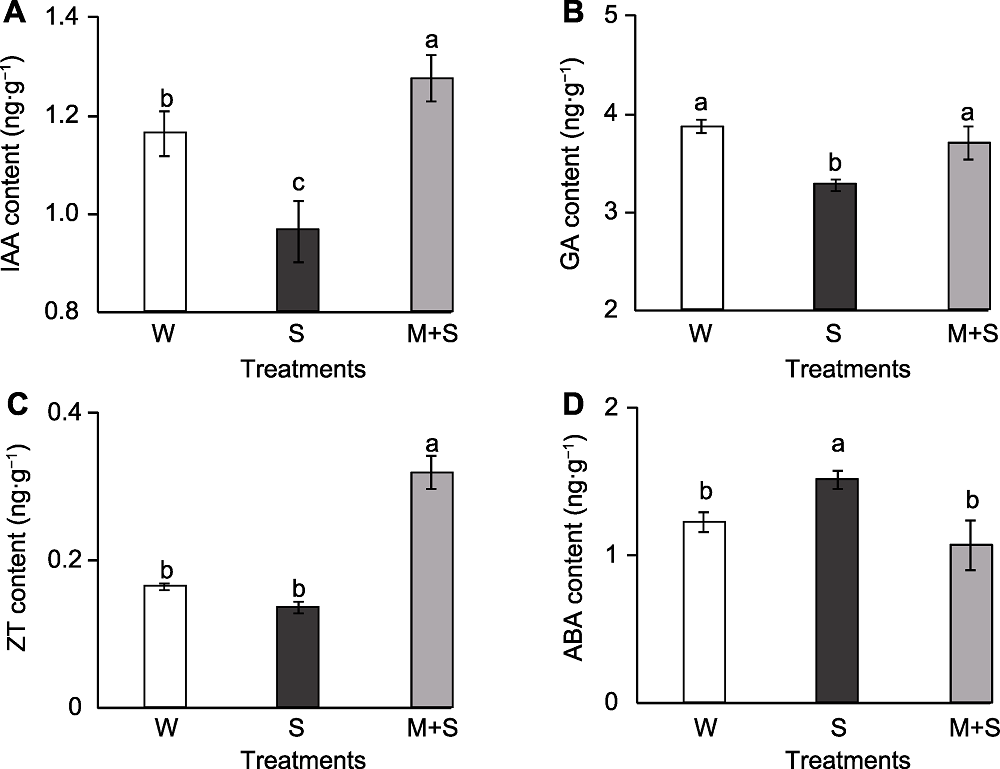
Figure 4 The endogenous hormone content of common bean sprouts (A) Auxin (IAA) content; (B) Gibberellin (GA) content; (C) Zeatin (ZT) content; (D) Abscisic acid (ABA) content. W, S, and M+S are the same as shown in Figure 1. Different lowercase letters indicate significant differences among different treatments (P<0.05).
| ID | Sample name | Raw reads | Clean reads | Error rate (%) | Q20 (%) | Q30 (%) |
|---|---|---|---|---|---|---|
| 1 | W1 | 55633866 | 53026610 | 0.01 | 96.84 | 92.16 |
| 2 | W2 | 50577884 | 48331946 | 0.02 | 96.69 | 91.84 |
| 3 | W3 | 52446224 | 50061634 | 0.02 | 96.68 | 91.86 |
| 4 | S1 | 41534300 | 40199494 | 0.02 | 94.93 | 88.25 |
| 5 | S2 | 50772136 | 49248286 | 0.02 | 95.10 | 88.47 |
| 6 | S3 | 48326882 | 46935654 | 0.02 | 95.13 | 88.53 |
| 7 | (M+S)1 | 51502046 | 49958064 | 0.02 | 95.08 | 88.43 |
| 8 | (M+S)2 | 51946416 | 50411968 | 0.02 | 94.93 | 88.21 |
| 9 | (M+S)3 | 45445652 | 44010506 | 0.02 | 95.30 | 88.83 |
Table 4 The assessment of data quality in RNA-Seq
| ID | Sample name | Raw reads | Clean reads | Error rate (%) | Q20 (%) | Q30 (%) |
|---|---|---|---|---|---|---|
| 1 | W1 | 55633866 | 53026610 | 0.01 | 96.84 | 92.16 |
| 2 | W2 | 50577884 | 48331946 | 0.02 | 96.69 | 91.84 |
| 3 | W3 | 52446224 | 50061634 | 0.02 | 96.68 | 91.86 |
| 4 | S1 | 41534300 | 40199494 | 0.02 | 94.93 | 88.25 |
| 5 | S2 | 50772136 | 49248286 | 0.02 | 95.10 | 88.47 |
| 6 | S3 | 48326882 | 46935654 | 0.02 | 95.13 | 88.53 |
| 7 | (M+S)1 | 51502046 | 49958064 | 0.02 | 95.08 | 88.43 |
| 8 | (M+S)2 | 51946416 | 50411968 | 0.02 | 94.93 | 88.21 |
| 9 | (M+S)3 | 45445652 | 44010506 | 0.02 | 95.30 | 88.83 |
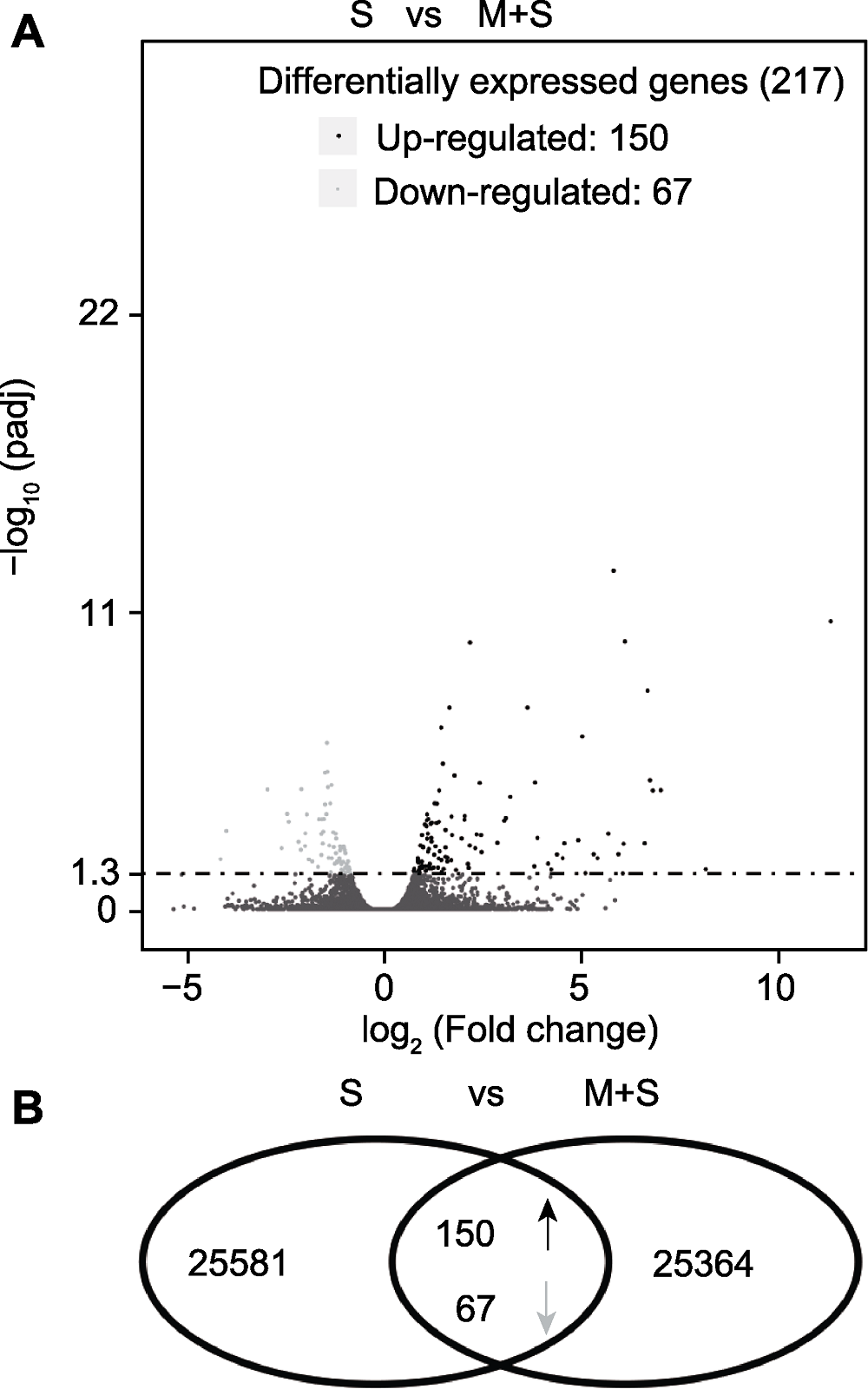
Figure 5 The analysis of differentially expressed genes (DEGs) (A) The volcano plot of DEGs in RNA-Seq; (B) The Venn diagram of DEGs in RNA-Seq. The black dots and up-arrow are up-regulated genes, the light gray dots and down-arrow are down-regulated genes while the dots under dotted line are non-differentially expressed genes. S and M+S are the same as shown in Figure 1.
| ID | GO ID | Enriched pathway | P-value |
|---|---|---|---|
| 1 | GO:0009664 | Plant-type cell wall organization | 0.0000 |
| 2 | GO:0071669 | Plant-type cell wall organization or biogenesis | 0.0000 |
| 3 | GO:0005199 | Structural constituent of cell wall | 0.0000 |
| 4 | GO:0030623 | U5 snRNA binding | 0.0000 |
| 5 | GO:0017069 | SnRNA binding | 0.0000 |
| 6 | GO:0071555 | Cell wall organization | 0.0005 |
| 7 | GO:0045229 | External encapsulating structure organization | 0.0006 |
| 8 | GO:0003910 | DNA ligase (ATP) activity | 0.0028 |
| 9 | GO:0071554 | Cell wall organization or biogenesis | 0.0037 |
| 10 | GO:0004448 | Isocitrate dehydrogenase activity | 0.0068 |
| 11 | GO:0004450 | Isocitrate dehydrogenase (NADP+) activity | 0.0068 |
| 12 | GO:0003909 | DNA ligase activity | 0.0074 |
| 13 | GO:0001539 | Cilium or flagellum-dependent cell motility | 0.0075 |
| 14 | GO:0016886 | Ligase activity, forming phosphoric ester bonds | 0.0087 |
| 15 | GO:0030193 | Regulation of blood coagulation | 0.0096 |
Table 5 GO enrichment analysis of differentially expressed genes (Top15 GO terms)
| ID | GO ID | Enriched pathway | P-value |
|---|---|---|---|
| 1 | GO:0009664 | Plant-type cell wall organization | 0.0000 |
| 2 | GO:0071669 | Plant-type cell wall organization or biogenesis | 0.0000 |
| 3 | GO:0005199 | Structural constituent of cell wall | 0.0000 |
| 4 | GO:0030623 | U5 snRNA binding | 0.0000 |
| 5 | GO:0017069 | SnRNA binding | 0.0000 |
| 6 | GO:0071555 | Cell wall organization | 0.0005 |
| 7 | GO:0045229 | External encapsulating structure organization | 0.0006 |
| 8 | GO:0003910 | DNA ligase (ATP) activity | 0.0028 |
| 9 | GO:0071554 | Cell wall organization or biogenesis | 0.0037 |
| 10 | GO:0004448 | Isocitrate dehydrogenase activity | 0.0068 |
| 11 | GO:0004450 | Isocitrate dehydrogenase (NADP+) activity | 0.0068 |
| 12 | GO:0003909 | DNA ligase activity | 0.0074 |
| 13 | GO:0001539 | Cilium or flagellum-dependent cell motility | 0.0075 |
| 14 | GO:0016886 | Ligase activity, forming phosphoric ester bonds | 0.0087 |
| 15 | GO:0030193 | Regulation of blood coagulation | 0.0096 |
| ID | KEGG ID | Enriched pathway | P-value |
|---|---|---|---|
| 1 | Pvu04626 | Plant-pathogen interaction | 0.0001 |
| 2 | Pvu03410 | Base excision repair | 0.0020 |
| 3 | Pvu03030 | DNA replication | 0.0044 |
| 4 | Pvu03430 | Mismatch repair | 0.0181 |
| 5 | Pvu04141 | Protein processing in endoplasmic re- ticulum | 0.0223 |
| 6 | Pvu00073 | Cutin, suberine and wax biosynthesis | 0.0312 |
| 7 | Pvu03420 | Nucleotide excision repair | 0.0411 |
| 8 | Pvu02010 | ABC transporters | 0.0446 |
Table 6 KEGG enrichment analysis of differentially expressed genes
| ID | KEGG ID | Enriched pathway | P-value |
|---|---|---|---|
| 1 | Pvu04626 | Plant-pathogen interaction | 0.0001 |
| 2 | Pvu03410 | Base excision repair | 0.0020 |
| 3 | Pvu03030 | DNA replication | 0.0044 |
| 4 | Pvu03430 | Mismatch repair | 0.0181 |
| 5 | Pvu04141 | Protein processing in endoplasmic re- ticulum | 0.0223 |
| 6 | Pvu00073 | Cutin, suberine and wax biosynthesis | 0.0312 |
| 7 | Pvu03420 | Nucleotide excision repair | 0.0411 |
| 8 | Pvu02010 | ABC transporters | 0.0446 |
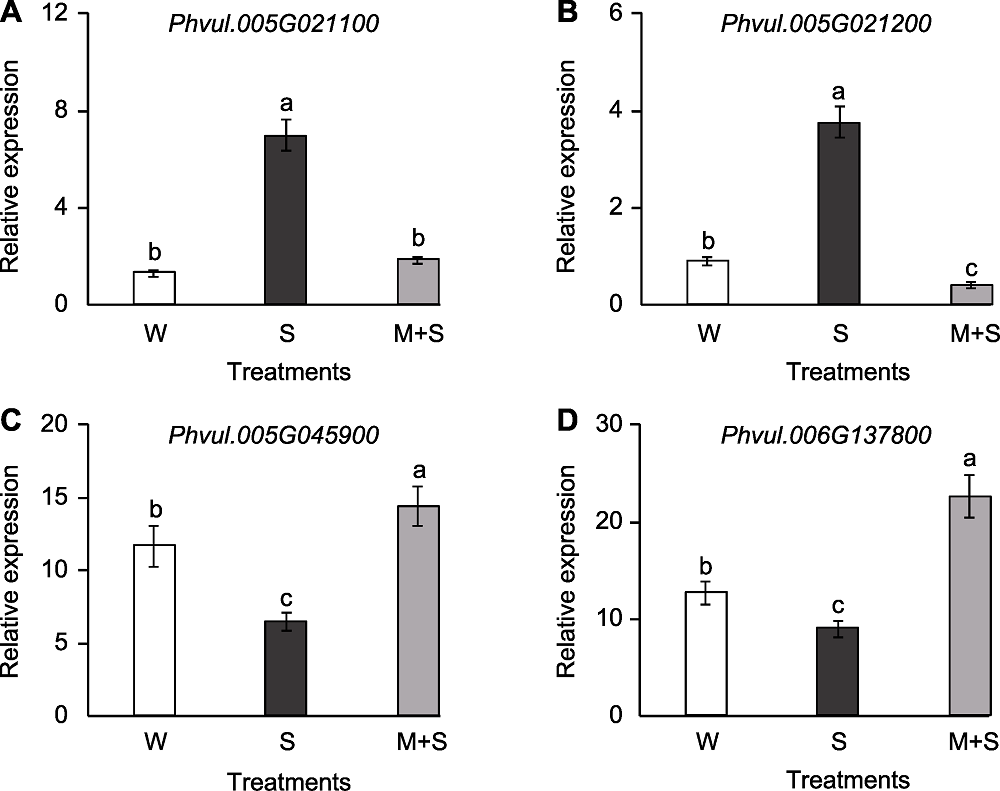
Figure 6 The gene expression analysis in KEGG enrichment pathway of common bean at sprout stage (A) The expression of Phvul.005G021100; (B) The expression of Phvul.005G021200; (C) The expression of Phvul.005G045900; (D) The expression of Phvul.006G137800. W, S, and M+S are the same as shown in Figure 1. Different lowercase letters indicate significant differences among different treatments (P<0.05).
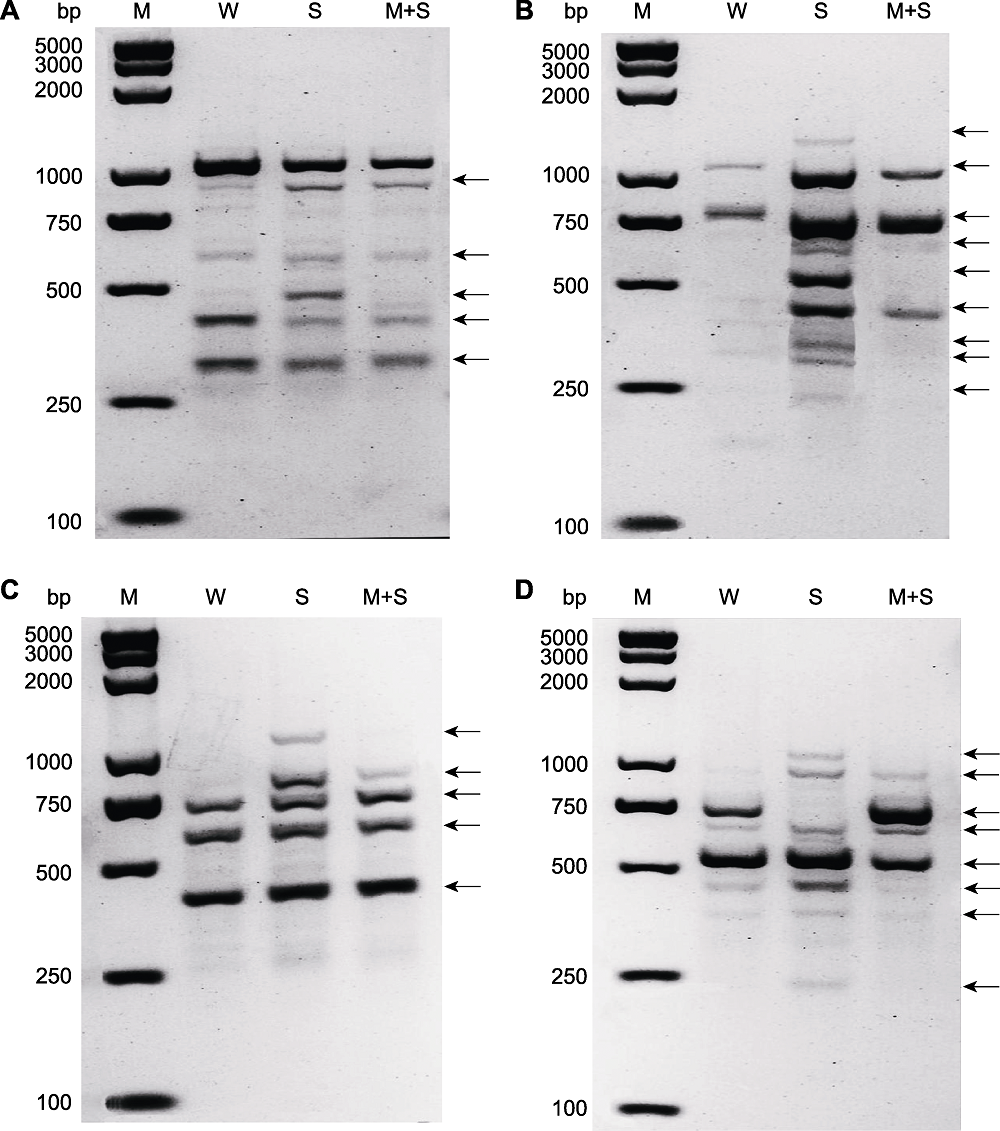
Figure 7 The random amplified polymorphic DNA (RAPD) analysis of common bean at sprout stage Random primer 1 (A), random primer 2 (B), random primer 3 (C) and random primer 4 (D) were used to amplify the RAPD polymorphisms of common bean sprouts in different treatments. W, S, and M+S are the same as shown in Figure 1. M was DL2K plusII marker, while black arrows indicate the RAPD appeared polymorphism at this site in the treatment group compared with the control.
| [1] |
艾金祥, 宋嘉怡, 严浙楠, 王志超, 陈文倩, 吴玉环, 王燕燕, 潘蕾蕾, 许俞韬, 刘鹏 (2022). 褪黑素对铅胁迫下虎舌红和朱砂根生理响应及DNA损伤的调控效应. 植物学报 57, 171-181.
DOI |
| [2] | 曹亮 (2020). 外源褪黑素对干旱胁迫下鼓粒期大豆碳氮代谢及产量品质的调控效应. 博士论文. 大庆: 黑龙江八一农垦大学. pp. 21-28. |
| [3] | 陈莉, 刘连涛, 马彤彤, 江丹, 孙红春, 张永江, 张科, 白志英, 李存东 (2019). 褪黑素对盐胁迫下棉花种子抗氧化酶活性及萌发的影响. 棉花学报 31, 438-447. |
| [4] | 陈琼, 韩瑞玺, 唐浩, 刘明月, 黄科, 周义之 (2018). 我国菜豆新品种选育研究现状及展望. 中国种业 (10), 9-14. |
| [5] | 陈瑞, 唐秀梅, 张琪, 杨蓉, 黄蕾蕾, 任晴雯, 朱森林, 刘鹏 (2020). NaCl胁迫对黑小麦根系DNA损伤的影响. 南方农业学报 51, 299-304. |
| [6] | 陈素玉, 赵强, 于高波, 任春元, 张玉先 (2022). 基于RAPD技术分析盐胁迫对大豆幼苗根系DNA的损伤. 生态学杂志 41, 1441-1447. |
| [7] | 何松榆, 秦彬, 张明聪, 金喜军, 王孟雪, 任春元, 张玉先 (2019). 水分胁迫下外源褪黑素对大豆苗期抗氧化特性和产量的影响. 大豆科学 39, 407-412. |
| [8] | 胡涛, 张鸽香, 郑福超, 曹钰 (2018). 植物盐胁迫响应的研究进展. 分子植物育种 16, 3006-3015. |
| [9] |
姜超强, 祖朝龙 (2015). 褪黑素与植物抗逆性研究进展. 生物技术通报 31(4), 47-55.
DOI |
| [10] |
雷新慧, 万晨茜, 陶金才, 冷佳俊, 吴怡欣, 王家乐, 王鹏科, 杨清华, 冯佰利, 高金锋 (2022). 褪黑素与2,4-表油菜素内酯浸种对盐胁迫下荞麦发芽与幼苗生长的促进效应. 作物学报 48, 1210-1221.
DOI |
| [11] |
刘德帅, 姚磊, 徐伟荣, 冯美, 姚文孔 (2022). 褪黑素参与植物抗逆功能研究进展. 植物学报 57, 111-126.
DOI |
| [12] | 栾非时, 祖元刚 (2002). 菜豆种质资源RAPD多样性的研究I. 植物研究 (4), 473-478. |
| [13] | 聂志刚, 王艳, 李韶山 (2009). 重金属诱导拟南芥原生质体DNA损伤的单细胞凝胶电泳检测. 植物学通报 44, 117-123. |
| [14] | 牛远, 杨修艳, 戴存凤, 王博文, 任高磊, 吴静磊, 王飞兵, 陈新红 (2018). 大豆芽期和苗期耐盐性评价指标筛选. 大豆科学 37, 215-223. |
| [15] | 孙浩月 (2021). 盐胁迫对普通菜豆(Phaseolus vulgaris L.)萌发期生长的影响及其耐盐性分子机制. 硕士论文. 大庆: 黑龙江八一农垦大学. pp. 9-10. |
| [16] |
孙莎莎, 巩彪, 温丹, 王秀峰, 魏珉, 杨凤娟, 李岩, 史庆华 (2016). 对羟基苯甲酸胁迫下褪黑素对黄瓜胚根生理生化特性的影响. 应用生态学报 27, 897-903.
DOI |
| [17] | 孙玉珺 (2019). 玉米芽期抗冷性筛选及低温胁迫下油菜素内酯对幼苗的调控效应研究. 硕士论文. 哈尔滨: 东北农业大学. pp. 15-16. |
| [18] | 王斌, 张腾霄, 刘超群, 祖余洋, 李艳芳, 孟祥才 (2022). 非生物胁迫对药用植物活性氧代谢影响的研究进展. 现代中药研究与实践 36(3), 94-98. |
| [19] | 王鹤潼, 贾春云, 张延召, 赵强, 李晓军, 巩宗强, 刘宛 (2021). 植物DNA错配修复系统响应Cd胁迫的研究进展. 农业环境科学学报 40, 700-711. |
| [20] |
杨新元 (2019). 外源褪黑素对干旱胁迫下向日葵幼苗生长、光合及抗氧化系统的影响. 华北农学报 34(4), 113-121.
DOI |
| [21] | 钟鸣, 陈琢, 刘宛, 李培军, 台培东 (2012). 逆境胁迫下植物DNA损伤和DNA错配修复研究进展. 生态学杂志 31, 2404-2411. |
| [22] | 邹京南 (2019). 外源褪黑素对干旱胁迫下大豆光合及生长的影响. 硕士论文. 大庆: 黑龙江八一农垦大学. pp. 32-35. |
| [23] |
Arnao MB, Hernández-Ruiz J (2018). Melatonin and its relationship to plant hormones. Ann Bot 121, 195-207.
DOI URL |
| [24] |
Beaver JS, Osorno JM (2009). Achievements and limitations of contemporary common bean breeding using conventional and molecular approaches. Euphytica 168, 145-175.
DOI URL |
| [25] |
Bray CM, West CE (2005). DNA repair mechanisms in plants: crucial sensors and effectors for the maintenance of genome integrity. New Phytol 168, 511-528.
PMID |
| [26] |
Cakmak I, Marschner H (1992). Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidase, and glutathione reductase in bean leaves. Plant Physiol 98, 1222-1227.
DOI PMID |
| [27] |
Chen K, Li GJ, Bressan RA, Song CP, Zhu JK, Zhao Y (2020). Abscisic acid dynamics, signaling, and functions in plants. J Integr Plant Biol 62, 25-54.
DOI |
| [28] |
Colebrook EH, Thomas SG, Phillips AL, Hedden P (2014). The role of gibberellin signaling in plant responses to abiotic stress. J Exp Biol 217, 67-75.
DOI URL |
| [29] |
Djanaguiraman M, Sheeba JA, Durga DD, Bangarusamy U (2009). Cotton leaf senescence can be delayed by nitrophenolate spray through enhanced antioxidant defence system. J Agron Crop Sci 195, 213-224.
DOI URL |
| [30] |
Elstner EF, Heupel A (1976). Inhibition of nitrite formation from hydroxylammonium chloride: a simple assay for superoxide dismutase. Anal Biochem 70, 616-620.
DOI PMID |
| [31] |
Farhangi-Abriz S, Torabian S (2017). Antioxidant enzyme and osmotic adjustment changes in bean seedlings as affected by biochar under salt stress. Ecotoxicol Environ Saf 137, 64-70.
DOI URL |
| [32] |
Ganesan K, Xu BJ (2017). Polyphenol-rich dry common beans (Phaseolus vulgaris L.) and their health benefits. Int J Mol Sci 18, 2331.
DOI URL |
| [33] |
Han YQ, Gao YM, Li M, Du YL, Zhang YX, Zhang WH, Du JD (2022). The molecular events underpinning cultivar differences in melatonin counteracting salt damage in Phaseolus vulgaris. Funct Plant Biol 49, 201-217.
DOI URL |
| [34] |
Jan JE, Reiter RJ, Wasdell MB, Bax M (2009). The role of the thalamus in sleep, pineal melatonin production, and circadian rhythm sleep disorders. J Pineal Res 46, 1-7.
DOI PMID |
| [35] |
Kaya C, Okant M, Ugurlar F, Alyemeni MN, Ashraf M, Ahmad P (2019). Melatonin-mediated nitric oxide improves tolerance to cadmium toxicity by reducing oxidative stress in wheat plants. Chemosphere 225, 627-638.
DOI PMID |
| [36] |
Lei YB, Yin CY, Li CY (2006). Differences in some morphological, physiological, and biochemical responses to drought stress in two contrasting populations of Populus przewalskii. Physiol Plant 127, 182-191.
DOI URL |
| [37] | Lerner AB, Case JD, Takahashi Y, Lee TH, Mori W (1958). Isolation of melatonin, the pineal gland factor that lightens melanocytes. Am Chem Soc 80, 2587. |
| [38] |
Lin CC, Kao CH (1999). NaCl induced changes in ionically bound peroxidase activity in roots of rice seedlings. Plant Soil 216, 147-153.
DOI URL |
| [39] |
Melis JPM, van Steeg H, Luijten M (2013). Oxidative DNA damage and nucleotide excision repair. Antioxid Redox Signal 18, 2409-2419.
DOI URL |
| [40] |
Okant M, Kaya C (2019). The role of endogenous nitric oxide in melatonin-improved tolerance to lead toxicity in maize plants. Environ Sci Pollut Res Int 26, 11864-11874.
DOI |
| [41] |
Patterson BD, MacRae EA, Ferguson IB (1984). Estimation of hydrogen peroxide in plant extracts using titanium (IV). Anal Biochem 139, 487-492.
DOI PMID |
| [42] |
Popelka JC, Terryn N, Higgins TJV (2004). Gene technology for grain legumes: can it contribute to the food challenge in developing countries? Plant Sci 167, 195-206.
DOI URL |
| [43] |
Posmyk MM, Balabusta M, Wieczorek M, Sliwinska E, Janas KM (2009). Melatonin applied to cucumber (Cucumis sativus L.) seeds improves germination during chilling stress. J Pineal Res 46, 214-223.
DOI URL |
| [44] |
Rodriguez C, Mayo JC, Sainz RM, Antolín I, Herrera F, Martín V, Reiter RJ (2004). Regulation of antioxidant enzymes: a significant role for melatonin. J Pineal Res 36, 1-9.
DOI PMID |
| [45] |
Scott TL, Rangaswamy S, Wicker CA, Izumi T (2014). Repair of oxidative DNA damage and cancer: recent progress in DNA base excision repair. Antioxid Redox Signal 20, 708-726.
DOI URL |
| [46] |
Shabala S (2013). Learning from halophytes: physiological basis and strategies to improve abiotic stress tolerance in crops. Ann Bot 112, 1209-1221.
DOI URL |
| [47] |
Sharif R, Xie C, Zhang HQ, Arnao MB, Ali M, Ali Q, Muhammad I, Shalmani A, Nawaz MA, Chen P, Li YH (2018). Melatonin and its effects on plant systems. Molecules 23, 2352.
DOI URL |
| [48] |
Singh KP, Roy D (2001). Identification of novel breast tumor- specific mutation(s) in the q11.2 region of chromosome 17 by RAPD/AP-PCR fingerprinting. Gene 269, 33-43.
PMID |
| [49] |
Singh KP, Roy D (2004). Somatic mutations in stilbene estrogen-induced Syrian hamster kidney tumors identified by DNA fingerprinting. J Carcinog 3, 4.
PMID |
| [50] |
Strother S (1988). The role of free radicals in leaf senescence. Gerontology 34, 151-156.
PMID |
| [51] |
Sun CL, Liu LJ, Wang LX, Li BH, Jin CW, Lin XY (2021). Melatonin: a master regulator of plant development and stress responses. J Integr Plant Biol 63, 126-145.
DOI |
| [52] | Sun CL, Lv T, Huang L, Liu XX, Jin CW, Lin XY (2020). Melatonin ameliorates aluminum toxicity through enhancing aluminum exclusion and reestablishing redox homeo- stasis in roots of wheat. J Pineal Res 68, e12642. |
| [53] |
Sun YL, Li F, Su N, Sun XL, Zhao SJ, Meng QW (2010). The increase in unsaturation of fatty acids of phosphatidylglycerol in thylakoid membrane enhanced salt tolerance in tomato. Photosynthetica 48, 400-408.
DOI URL |
| [54] |
Ubbens J, Cieslak M, Prusinkiewicz P, Stavness I (2018). The use of plant models in deep learning: an application to leaf counting in rosette plants. Plant Methods 14, 6.
DOI PMID |
| [55] |
Vafadar F, Amooaghaie R, Ehsanzadeh P, Ghanati F, Sajedi RH (2020). Crosstalk between melatonin and Ca2+/ CaM evokes systemic salt tolerance in Dracocephalum kotschyi. J Plant Physiol 252, 153237.
DOI URL |
| [56] |
Wang H, Takano T, Liu SK (2018). Screening and evaluation of saline-alkaline tolerant germplasm of rice (Oryza sativa L.) in soda saline-alkali soil. Agronomy 8, 205.
DOI URL |
| [57] |
Wang LQ, Li Z, Lu MZ, Wang YC (2017). ThNAC13, a NAC transcription factor from Tamarix hispida, confers salt and osmotic stress tolerance to transgenic Tamarix and Arabidopsis. Front Plant Sci 8, 635.
DOI URL |
| [58] |
Yang YR, Cao YP, Li ZX, Zhukova A, Yang ST, Wang JL, Tang ZH, Cao YH, Zhang YF, Wang DL (2020). Interactive effects of exogenous melatonin and Rhizophagus intraradices on saline-alkaline stress tolerance in Leymus chinensis. Mycorrhiza 30, 357-371.
DOI |
| [59] |
Zahedi SM, Hosseini MS, Abadía J, Marjani M (2020). Melatonin foliar sprays elicit salinity stress tolerance and enhance fruit yield and quality in strawberry (Fragaria × ananassa Duch.). Plant Physiol Biochem 149, 313-323.
DOI URL |
| [60] |
Zhan HS, Nie XJ, Zhang T, Li S, Wang XY, Du XH, Tong W, Song WN (2019). Melatonin: a small molecule but important for salt stress tolerance in plants. Int J Mol Sci 20, 709.
DOI URL |
| [61] |
Zhang JL, Wang P, Long HY, Su SS, Wu YG, Wang HR (2022). Metabolomics analysis reveals the physiological mechanism underlying growth restriction in maize roots under continuous negative pressure and stable water sup- ply. Agric Water Manag 263, 107452.
DOI URL |
| [62] |
Zhang Q, Li M, Xia CY, Zhang WJ, Yin ZG, Zhang YL (2021). Transcriptome-based analysis of salt-related genes during the sprout stage of common bean (Phaseolus vulgaris) under salt stress conditions. Biotechnol Biotechnol Equip 35, 1086-1098.
DOI URL |
| [63] |
Zhang Q, Zhang WJ, Yin ZG, Li WJ, Zhao HH, Zhang S, Zhuang L, Wang YX, Zhang WH, Du JD (2020). Genome- and transcriptome-wide identification of C3Hs in common bean (Phaseolus vulgaris L.) and structural and expression-based analyses of their functions during the sprout stage under salt-stress conditions. Front Genet 11, 564607.
DOI URL |
| [64] | Zhao Q, Chen SY, Wang GD, Du YL, Zhang ZN, Yu GB, Ren CY, Zhang YX, Du JD (2022). Exogenous melatonin enhances soybean (Glycine max (L.) Merr.) seedling tolerance to saline-alkali stress by regulating antioxidant response and DNA damage repair. Physiol Plant 174, e13731. |
| [65] |
Zhao Q, Wang HT, Du YL, Rogers HJ, Wu ZX, Jia S, Yao XD, Xie FT, Liu W (2020). MSH2 and MSH6 in mismatch repair system account for soybean (Glycine max (L.) Merr.) tolerance to cadmium toxicity by determining DNA damage response. J Agric Food Chem 68, 1974-1985.
DOI URL |
| [1] | Xu Tiantian, Yang Peijian, Zhou Xiaoxi, Cao Yi, Chen Yanhong, Liu Guoyuan, Zhang Jian, Wei Hui. Analysis of Physicochemical Characteristics and Expression Characteristics of Lagerstroemia indica GolS Family Genes [J]. Chinese Bulletin of Botany, 2025, 60(3): 393-406. |
| [2] | Jinyu Du, Zhen Sun, Yanlong Su, Heping Wang, Yaling Liu, Zhenying Wu, Feng He, Yan Zhao, Chunxiang Fu. Identification and Functional Analysis of an Agropyron mongolicum Caffeic Acid 3-O-methyltransferase Gene AmCOMT1 [J]. Chinese Bulletin of Botany, 2024, 59(3): 383-396. |
| [3] | Cai Shuyu, Liu Jianxin, Wang Guofu, Wu Liyuan, Song Jiangping. Regulatory Mechanism of Melatonin on Tomato Seed Germination Under Cd2+ Stress [J]. Chinese Bulletin of Botany, 2023, 58(5): 720-732. |
| [4] | Feifei Wang, Zhenxiang Zhou, Yi Hong, Yangyang Gu, Chao Lü, Baojian Guo, Juan Zhu, Rugen Xu. Identification of the NF-YC Genes in Hordeum vulgare and Expression Analysis Under Salt Stress [J]. Chinese Bulletin of Botany, 2023, 58(1): 140-149. |
| [5] | Xiaoming Li, Lanfen Wang, Yongsheng Tang, Yujie Chang, Juxiang Zhang, Shumin Wang, Jing Wu. Genome-wide Association Analysis of Resistance to Acanthoscelides obtectus in Common Bean [J]. Chinese Bulletin of Botany, 2023, 58(1): 77-89. |
| [6] | Yuan Li, Yujie Chang, Lanfen Wang, Shumin Wang, Jing Wu. Screening of Resistance Germplasm Resources and Genome-wide Association Study of Fusarium Wilt in Common Bean [J]. Chinese Bulletin of Botany, 2023, 58(1): 51-61. |
| [7] | Kairu Yang, Qiwei Jia, Jiayi Jin, Hanfei Ye, Sheng Wang, Qianyu Chen, Yian Guan, Chenyang Pan, Dedong Xin, Yuan Fang, Yuexing Wang, Yuchun Rao. Cloning and Functional Analysis of Rice Yellow Green Leaf Regulatory Gene YGL18 [J]. Chinese Bulletin of Botany, 2022, 57(3): 276-287. |
| [8] | Jinxiang Ai, Jiayi Song, Zhenan Yan, Zhichao Wang, Wenqian Chen, Yuhuan Wu, Yanyan Wang, Leilei Pan, Yutao Xu, Peng Liu. Effects of Exogenous Melatonin on Physiological Response and DNA Damage of Ardisia mamillata and A. crenata Under Lead Stress [J]. Chinese Bulletin of Botany, 2022, 57(2): 171-181. |
| [9] | Deshuai Liu, Lei Yao, Weirong Xu, Mei Feng, Wenkong Yao. Research Progress of Melatonin in Plant Stress Resistance [J]. Chinese Bulletin of Botany, 2022, 57(1): 111-126. |
| [10] | Yan Wang, Bowei Jia, Mingzhe Sun, Xiaoli Sun. Advances in Molecular Mechanisms of Stress Tolerance in Wild Soybean [J]. Chinese Bulletin of Botany, 2021, 56(1): 104-115. |
| [11] | Chunyan Zhang. The Measurement Methods and Principles of P700 Redox Kinetics [J]. Chinese Bulletin of Botany, 2020, 55(6): 740-748. |
| [12] | Nan Zhang,Ziguang Liu,Shichen Sun,Shengyi Liu,Jianhui Lin,Yifang Peng,Xiaoxu Zhang,He Yang,Xi Cen,Juan Wu. Response of AtR8 lncRNA to Salt Stress and Its Regulation on Seed Germination in Arabidopsis [J]. Chinese Bulletin of Botany, 2020, 55(4): 421-429. |
| [13] | Dongdong Cao,Shanyu Chen,Yebo Qin,Huaping Wu,Guanhai Ruan,Yutao Huang. Regulatory Mechanism of Salicylic Acid on Seed Germination Under Salt Stress in Kale [J]. Chinese Bulletin of Botany, 2020, 55(1): 49-61. |
| [14] | Jing Zhang,Suiwen Hou. Role of Post-translational Modification of Proteins in ABA Signaling Transduction [J]. Chinese Bulletin of Botany, 2019, 54(3): 300-315. |
| [15] | Lulu Li,Wenchao Yin,Mei Niu,Wenjing Meng,Xiaoxing Zhang,Hongning Tong. Functional Analysis of Brassinosteroids in Salt Stress Responses in Rice [J]. Chinese Bulletin of Botany, 2019, 54(2): 185-193. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||