

Chinese Bulletin of Botany ›› 2020, Vol. 55 ›› Issue (5): 577-587.DOI: 10.11983/CBB20100 cstr: 32102.14.CBB20100
Special Issue: 粮食安全
• INVITED PROTOCOLS • Previous Articles Next Articles
Min He1, Junjie Yin1, Zhiming Feng2, Xiaobo Zhu1, Jianhua Zhao2, Shimin Zuo2,*( ), Xuewei Chen1,*(
), Xuewei Chen1,*( )
)
Received:2020-05-28
Accepted:2020-07-21
Online:2020-09-01
Published:2020-09-03
Contact:
Shimin Zuo,Xuewei Chen
Min He, Junjie Yin, Zhiming Feng, Xiaobo Zhu, Jianhua Zhao, Shimin Zuo, Xuewei Chen. Methods for Evaluation of Rice Resistance to Blast and Sheath Blight Diseases[J]. Chinese Bulletin of Botany, 2020, 55(5): 577-587.
| Solution | Composition | Amount |
|---|---|---|
| 20× Nitrate salts (1 L) | NaNO3 | 120 g |
| KCl | 10.4 g | |
| MgSO4.7H2O | 10.4 g | |
| KH2PO4 | 30.4 g | |
| Vitamin solution (1 L) | Biotin | 0.1 g |
| Pyridoxin | 0.1 g | |
| Thiamine | 0.1 g | |
| Riboflavin | 0.1 g | |
| p-aminobenzoic acid | 0.1 g | |
| Nicotinic acid | 0.1 g | |
| Trace elements (100 mL) | ZnSO4.7H2O | 2.2 g |
| H3BO3 | 1.1 g | |
| MnCl2.4H2O | 0.5 g | |
| FeSO4.7H2O | 0.5 g | |
| CoCl2.6H2O | 0.17 g | |
| CuSO4.5H2O | 0.16 g | |
| Na2MoO4.2H2O | 0.15 g | |
| CM agar medium (1 L, pH6.5) | Glucose | 10 g |
| Peptone | 2 g | |
| Yeast extract | 1 g | |
| Casamino acids | 1 g | |
| 20× Nitrate salts | 50 mL | |
| Vitamin solution | 1 mL | |
| Trace elements | 1 mL | |
| Agar | 15 g | |
| Oat tomato agar (1 L) | Oatmeal | 40 g, collect the liquid filter after boiling |
| Fresh tomato juice | 150 mL | |
| Agar | 15 g | |
| PDA medium (1 L) | Potato dextrose broth | 24 g |
| Agar | 20 g |
Table 1 Reagent formulation used for culturing Magnaporthe oryzae and Rhizoctonia solani
| Solution | Composition | Amount |
|---|---|---|
| 20× Nitrate salts (1 L) | NaNO3 | 120 g |
| KCl | 10.4 g | |
| MgSO4.7H2O | 10.4 g | |
| KH2PO4 | 30.4 g | |
| Vitamin solution (1 L) | Biotin | 0.1 g |
| Pyridoxin | 0.1 g | |
| Thiamine | 0.1 g | |
| Riboflavin | 0.1 g | |
| p-aminobenzoic acid | 0.1 g | |
| Nicotinic acid | 0.1 g | |
| Trace elements (100 mL) | ZnSO4.7H2O | 2.2 g |
| H3BO3 | 1.1 g | |
| MnCl2.4H2O | 0.5 g | |
| FeSO4.7H2O | 0.5 g | |
| CoCl2.6H2O | 0.17 g | |
| CuSO4.5H2O | 0.16 g | |
| Na2MoO4.2H2O | 0.15 g | |
| CM agar medium (1 L, pH6.5) | Glucose | 10 g |
| Peptone | 2 g | |
| Yeast extract | 1 g | |
| Casamino acids | 1 g | |
| 20× Nitrate salts | 50 mL | |
| Vitamin solution | 1 mL | |
| Trace elements | 1 mL | |
| Agar | 15 g | |
| Oat tomato agar (1 L) | Oatmeal | 40 g, collect the liquid filter after boiling |
| Fresh tomato juice | 150 mL | |
| Agar | 15 g | |
| PDA medium (1 L) | Potato dextrose broth | 24 g |
| Agar | 20 g |
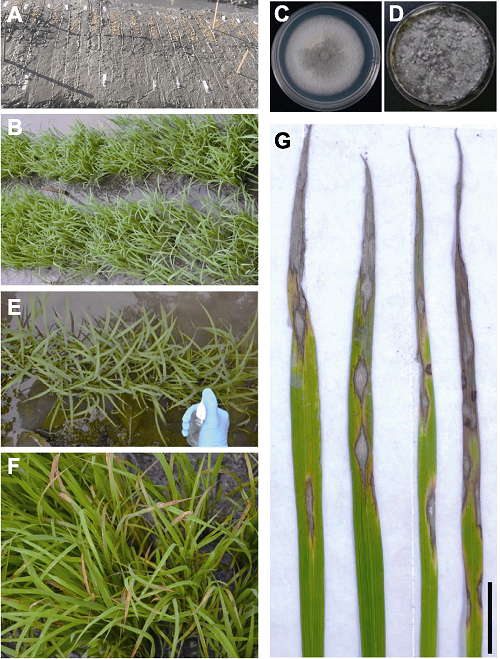
Figure 1 Spraying inoculation at rice seedling stage for evaluation of blast disease resistance and typical rice blast symptom (A) Sowing rice seeds on seedbeds; (B) Rice seedlings grown for two weeks; (C) Magnaporthe oryzae colony grown on CM agar medium; (D) M. oryzae sporulation on oat tomato agar medium; (E) Inoculation of rice seedlings by spraying spore suspension; (F) Development of blast lesions six-day post inoculation; (G) Typical blast lesions formed on rice leaves (Bar=1 cm).
| Disease score | Resistance level | State of disease |
|---|---|---|
| 0 | High resistance (HR) | No blast lesion |
| 1 | Resistance (R) | Only needle-shaped brown lesion s- pots |
| 2 | R | Slightly large brown spots with diameter less than 1 mm |
| 3 | Medium re- sistance (MR) | Round or oval gray lesion which shows brown edge and appear diameter between 1-2 mm |
| 4 | Medium su- sceptibility (MS) | Typical spindle lesion which has leng- th between 1-2 cm. The lesion is us- ually confined between two veins of a leaf and the lesion area occupies less than 2.0% of a leaf |
| 5 | MS | Typical spindle lesion whose area occupies the leaf area between 2.1%- 10.0% |
| 6 | Susceptibility (S) | Typical spindle lesion whose area occupies the leaf area between 10.1%- 25.0% |
| 7 | S | Typical spindle lesion whose area occupies the leaf area between 25.1%- 50.0% |
| 8 | High sus- ceptibility (HS) | Typical spindle lesion whose area occupies the leaf area between 50.1%- 75.0% |
| 9 | HS | Typical spindle lesion whose area oc- cupies more than 75.1% of the leaf area |
Table 2 Grades of blast severity of rice seedlings
| Disease score | Resistance level | State of disease |
|---|---|---|
| 0 | High resistance (HR) | No blast lesion |
| 1 | Resistance (R) | Only needle-shaped brown lesion s- pots |
| 2 | R | Slightly large brown spots with diameter less than 1 mm |
| 3 | Medium re- sistance (MR) | Round or oval gray lesion which shows brown edge and appear diameter between 1-2 mm |
| 4 | Medium su- sceptibility (MS) | Typical spindle lesion which has leng- th between 1-2 cm. The lesion is us- ually confined between two veins of a leaf and the lesion area occupies less than 2.0% of a leaf |
| 5 | MS | Typical spindle lesion whose area occupies the leaf area between 2.1%- 10.0% |
| 6 | Susceptibility (S) | Typical spindle lesion whose area occupies the leaf area between 10.1%- 25.0% |
| 7 | S | Typical spindle lesion whose area occupies the leaf area between 25.1%- 50.0% |
| 8 | High sus- ceptibility (HS) | Typical spindle lesion whose area occupies the leaf area between 50.1%- 75.0% |
| 9 | HS | Typical spindle lesion whose area oc- cupies more than 75.1% of the leaf area |

Figure 2 Evaluation of leaf blast disease resistance by injection inoculation at rice tillering stage (A) Inoculation of rice sheath by injection of Magnaporthe oryzae spore suspension at the stage of tillering; (B) Blast disease symptoms in the rice leaf.

Figure 3 Evaluation of neck blast disease resistance by injection inoculation at rice booting stage (A) Inoculation by injection of Magnaporthe oryzae spore suspension at the stage of booting; (B) Blast disease symptoms in the rice spike.
| Disease score | State of disease |
|---|---|
| 0 | No disease symptom |
| 1 | Blast disease symptom appears in primary or secondary branch of panicle and leads to less than 5.0% yield loss in each spike |
| 3 | Blast disease symptom appears in rice rachis or spike neck which leads to 5.1%-20.0% yield loss in each spike |
| 5 | Blast disease symptom appears in rice rachis or spike neck which leads to half-shriveled grain and 20.0%-50.0% yield loss in each spike |
| 7 | Blast disease symptom appears in rice spike neck which leads to shriveled kernel and 50.0%-70.0% yield loss in each spike |
| 9 | Blast disease symptom appears in rice spike neck which leads to more than 70.0% yield loss in each spike |
Table 3 Grades for evaluation of single panicle loss by rice blast disease
| Disease score | State of disease |
|---|---|
| 0 | No disease symptom |
| 1 | Blast disease symptom appears in primary or secondary branch of panicle and leads to less than 5.0% yield loss in each spike |
| 3 | Blast disease symptom appears in rice rachis or spike neck which leads to 5.1%-20.0% yield loss in each spike |
| 5 | Blast disease symptom appears in rice rachis or spike neck which leads to half-shriveled grain and 20.0%-50.0% yield loss in each spike |
| 7 | Blast disease symptom appears in rice spike neck which leads to shriveled kernel and 50.0%-70.0% yield loss in each spike |
| 9 | Blast disease symptom appears in rice spike neck which leads to more than 70.0% yield loss in each spike |
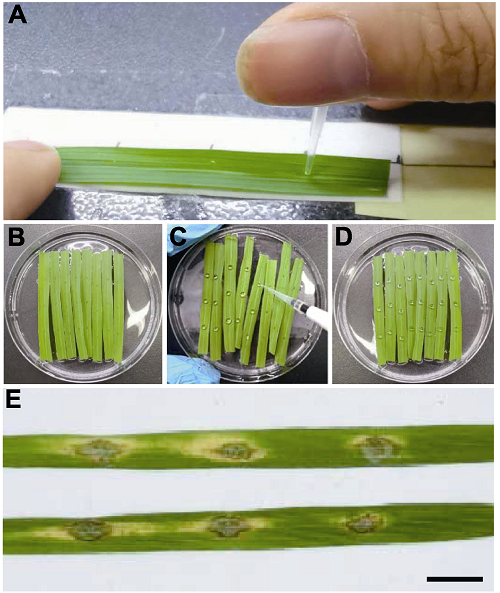
Figure 4 Evaluation of blast disease resistance by inoculation of detached rice leaves (A) Punching detached rice leaves using 10 μL pipette tips with 1.5 cm scale; (B) Rice leaves put on the surface of 6-BA solution after punching; (C) Inoculation of punched leaves by spotting spore suspension; (D) Rice leaves inoculated with spore suspension of Magnaporthe oryzae; (E) Blast lesions formed on detached rice leaves (Bar=1 cm).

Figure 5 Evaluation of sheath blight disease resistance in field by using wood veneer inoculation method at rice tillering stage (A) Wood veneer with a thickness of 0.8 mm; (B) Wood veneer colonized with mycelia of Rhizoctonia solani (hereafter named wood inoculum); (C) Mycelia of R. solani on wood inoculum observed by micrograph; (D) Wood inoculum was put into inner side of the third leaf sheath from up to down by tweezer; (E) Rice plants in the field at tillering stage used for inoculation; (F) Sheath blight development in the highly susceptible rice variety Lemont at 11 days after inoculation in the field; (G) Disease symptoms of Lemont at 30 days after heading in the field.
| Disease sore | Sheath blight severity |
|---|---|
| 0 | Plant healthy, no symptoms |
| 1 | Lesions mainly restricted to the emerged portion of sixth sheath from top on the culm |
| 1.5 | Lesions extending to lower 1/2 of the emerged portion of fifth sheath from top on the culm |
| 2 | Lesions extending to upper 1/2 of the emerged portion of fifth sheath from top on the culm |
| 2.5 | Lesions extending to lower 1/2 of the emerged portion of fourth sheath from top on the culm |
| 3 | Lesions extending to upper 1/2 of the emerged portion of fourth sheath from top on the culm |
| 3.5 | Lesions extending to lower 1/2 of the emerged portion of third sheath from top on the culm |
| 4 | Lesions extending to upper 1/2 of the emerged portion of third sheath from top on the culm |
| 4.5 | Lesions extending to lower 1/2 of the emerged portion of second sheath from top on the culm |
| 5 | Lesions extending to upper 1/2 of the emerged portion of second sheath from top on the culm |
| 5.5 | Lesions extending to lower 1/4 of the emerged portion of flag leaf sheath from top on the culm |
| 6 | Lesions usually coalescing and reaching to lower 1/4 to 1/2 of the emerged portion of flag leaf sheath on the culm |
| 6.5 | Lesions usually coalescing and reaching to lower 1/2 to 3/4 of the emerged portion of flag leaf sheath on the culm |
| 7 | Lesions usually coalescing and reaching to upper 1/4 of the emerged portion of flag leaf sheath on the culm |
| 7.5 | Lesions usually coalescing and reaching to lower 1/2 of flag leaf on the culm, the flag leaf presenting semi-rolling or <50% flag leaf tissues affected |
| 8 | Lesions usually coalescing and reaching to upper 1/2 of flag leaf on the culm, more than 50% flag leaf tissues affected |
| 8.5 | Panicle rachis and culm with brown streak and becoming light brown, all sheath and leaf tissues dead and drying on the culms, florets in lower 1/3 of panicle often not filling on the culm |
| 9 | Panicle rachis and culm dead and becoming dry, severely affected culms lodging, florets in lower 1/3 to 1/2 or more of panicle not filling on the culm |
Table 4 Rating scales for determining sheath blight disease severity on rice using field inoculation method
| Disease sore | Sheath blight severity |
|---|---|
| 0 | Plant healthy, no symptoms |
| 1 | Lesions mainly restricted to the emerged portion of sixth sheath from top on the culm |
| 1.5 | Lesions extending to lower 1/2 of the emerged portion of fifth sheath from top on the culm |
| 2 | Lesions extending to upper 1/2 of the emerged portion of fifth sheath from top on the culm |
| 2.5 | Lesions extending to lower 1/2 of the emerged portion of fourth sheath from top on the culm |
| 3 | Lesions extending to upper 1/2 of the emerged portion of fourth sheath from top on the culm |
| 3.5 | Lesions extending to lower 1/2 of the emerged portion of third sheath from top on the culm |
| 4 | Lesions extending to upper 1/2 of the emerged portion of third sheath from top on the culm |
| 4.5 | Lesions extending to lower 1/2 of the emerged portion of second sheath from top on the culm |
| 5 | Lesions extending to upper 1/2 of the emerged portion of second sheath from top on the culm |
| 5.5 | Lesions extending to lower 1/4 of the emerged portion of flag leaf sheath from top on the culm |
| 6 | Lesions usually coalescing and reaching to lower 1/4 to 1/2 of the emerged portion of flag leaf sheath on the culm |
| 6.5 | Lesions usually coalescing and reaching to lower 1/2 to 3/4 of the emerged portion of flag leaf sheath on the culm |
| 7 | Lesions usually coalescing and reaching to upper 1/4 of the emerged portion of flag leaf sheath on the culm |
| 7.5 | Lesions usually coalescing and reaching to lower 1/2 of flag leaf on the culm, the flag leaf presenting semi-rolling or <50% flag leaf tissues affected |
| 8 | Lesions usually coalescing and reaching to upper 1/2 of flag leaf on the culm, more than 50% flag leaf tissues affected |
| 8.5 | Panicle rachis and culm with brown streak and becoming light brown, all sheath and leaf tissues dead and drying on the culms, florets in lower 1/3 of panicle often not filling on the culm |
| 9 | Panicle rachis and culm dead and becoming dry, severely affected culms lodging, florets in lower 1/3 to 1/2 or more of panicle not filling on the culm |

Figure 6 Evaluation of sheath blight disease resistance in greenhouse by using wood veneer inoculation method at rice booting stage (A) Rice plants at booting stage growing in flowerpots; (B) Inoculation of rice sheath with Rhizoctonia solani wood inoculum at booting stage; (C) Rice plants growth in greenhouse after inoculation; (D) Disease symptoms of susceptible rice variety at 21 days after inoculation.
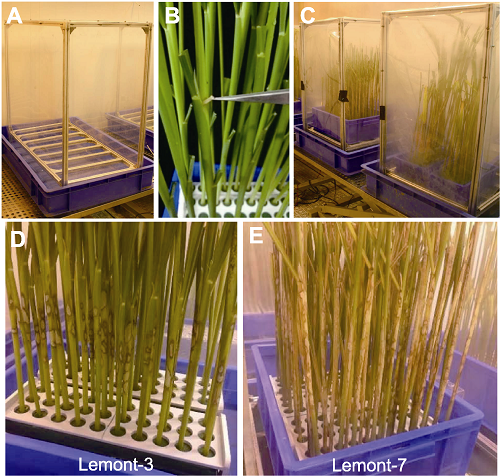
Figure 7 Methods for evaluation of sheath blight disease resistance by using detached stems of rice (A) Inoculation shelves; (B) Inoculation of detached rice stem by using wood inoculum; (C) Growth chamber containing inoculated plants; (D) Sheath blight disease symptoms of the highly susceptible cultivar Lemont at three days after inoculation; (E) Sheath blight disease symptoms of the highly susceptible cultivar Lemont at seven days after inoculation.
| Relative lesion length (%) | 0-0.15 | 0.16-0.3 | 0.31-0.45 | 0.46-0.6 | 0.61-0.75 | 0.76-1.0 |
|---|---|---|---|---|---|---|
| Resistant level | HR | R | MR | MS | S | HS |
Table 5 Grades of sheath blight resistance levels of rice varieties using detached-stem inoculation method
| Relative lesion length (%) | 0-0.15 | 0.16-0.3 | 0.31-0.45 | 0.46-0.6 | 0.61-0.75 | 0.76-1.0 |
|---|---|---|---|---|---|---|
| Resistant level | HR | R | MR | MS | S | HS |
| [1] | 曹妮, 陈渊, 季芝娟, 曾宇翔, 杨长登, 梁燕 ( 2019). 水稻抗稻瘟病分子机制研究进展. 中国水稻科学 33, 489-498. |
| [2] | 陈锦文, 谢旺有, 王天生, 陈惠清, 黄荣裕, 谢少和 ( 2015). 人工注射接种及田间病圃鉴定水稻对稻瘟病的抗病性. 广西植保 28(4), 6-10. |
| [3] | 高杜娟, 唐善军, 陈友德, 周斌 ( 2019). 水稻主要病害生物防治的研究进展. 中国农学通报 35(26), 140-147. |
| [4] | 许有嫔, 廖海澄, 陈金华, 罗櫞, 宿加, 邹成东, 李伟滔, 王静, 马炳田, 贺闽, 陈学伟 ( 2017). 利用绿色荧光蛋白GFP研究稻瘟病菌与水稻的互作. 植物保护 43(6), 53-61. |
| [5] | 杨德卫, 王莫, 韩利波, 唐定中, 李生平 ( 2019). 水稻稻瘟病抗性基因的克隆、育种利用及稻瘟菌无毒基因研究进展. 植物学报 54, 265-276. |
| [6] | 左示敏, 张亚芳, 陈宗祥, 陈夕军, 潘学彪 ( 2010). 水稻抗纹枯病遗传育种研究进展. 中国科学: 生命科学 40, 1014-1023. |
| [7] |
Dean R, van Kan JAL, Pretorius ZA, Hammond-Kosack KE, Di Pietro A, Spanu PD, Rudd JJ, Dickman M, Kahmann R, Ellis J, Foster GD ( 2012). The top 10 fungal pathogens in molecular plant pathology. Mol Plant Pathol 13, 804.
DOI URL |
| [8] |
Li WT, Chern M, Yin JJ, Wang J, Chen XW ( 2019). Recent advances in broad-spectrum resistance to the rice blast disease. Curr Opin Plant Biol 50, 114-120.
DOI URL PMID |
| [9] |
Wang L, Liu LM, Hou YX, Li L, Huang SW ( 2015). Pathotypic and genetic diversity in the population of Rhizoctonia solani AG1-IA causing rice sheath blight in China. Plant Pathol 64, 718-728.
DOI URL |
| [10] |
Wilson RA, Talbot NJ ( 2009). Under pressure: investigating the biology of plant infection by Magnaporthe oryzae. Nat Rev Microbiol 7, 185-195.
DOI URL PMID |
| [11] | Yoshida S, Fonao DA, Cock JH, Gomez KA (1976). Laboratory manual for physiological studies of rice. In: International Rice Research Institute, 3rd edn. Manila: The International Rice Research Institute. pp. 61-66. |
| [12] |
Zheng AP, Lin RM, Zhang DH, Qin PG, Xu LZ, Ai P, Ding L, Wang YR, Chen Y, Liu Y, Sun ZG, Feng HT, Liang XX, Fu RT, Tang CQ, Li Q, Zhang J, Xie ZL, Deng QM, Li SC, Wang SQ, Zhu J, Wang LX, Liu HN, Li P ( 2013). The evolution and pathogenic mechanisms of the rice sheath blight pathogen. Nat Commun 4, 1424.
DOI URL PMID |
| [1] |
Juan Cui, Xiaoyu Yu, Yuejiao Yu, Chengwei Liang, Jian Sun, Wenfu Chen.
Analysis of Texture Factors and Genetic Basis Influencing the Differences in Eating Quality between Northeast China and Japanese Japonica Rice [J]. Chinese Bulletin of Botany, 2025, 60(4): 1-0. |
| [2] | Zhao Ling, Guan Ju, Liang Wenhua, Zhang Yong, Lu Kai, Zhao Chunfang, Li Yusheng, Zhang Yadong. Mapping of QTLs for Heat Tolerance at the Seedling Stage in Rice Based on a High-density Bin Map [J]. Chinese Bulletin of Botany, 2025, 60(3): 342-353. |
| [3] | Xinyu Li, Yue Gu, Feifei Xu, Jinsong Bao. Research Progress on Post-translational Modifications of Starch Biosynthesis-related Proteins in Rice Endosperm [J]. Chinese Bulletin of Botany, 2025, 60(2): 256-270. |
| [4] | Jianguo Li, Yi Zhang, Wenjun Zhang. Iron Plaque Formation and Its Effects on Phosphorus Absorption in Rice Roots [J]. Chinese Bulletin of Botany, 2025, 60(1): 132-143. |
| [5] | Ruifeng Yao, Daoxin Xie. Activation and Termination of Strigolactone Signal Perception in Rice [J]. Chinese Bulletin of Botany, 2024, 59(6): 873-877. |
| [6] | Jinjin Lian, Luyao Tang, Yinuo Zhang, Jiaxing Zheng, Chaoyu Zhu, Yuhan Ye, Yuexing Wang, Wennan Shang, Zhenghao Fu, Xinxuan Xu, Richeng Wu, Mei Lu, Changchun Wang, Yuchun Rao. Genetic Locus Mining and Candidate Gene Analysis of Antioxidant Traits in Rice [J]. Chinese Bulletin of Botany, 2024, 59(5): 738-751. |
| [7] | Jiahui Huang, Huimin Yang, Xinyu Chen, Chaoyu Zhu, Yanan Jiang, Chengxiang Hu, Jinjin Lian, Tao Lu, Mei Lu, Weilin Zhang, Yuchun Rao. Response Mechanism of Rice Mutant pe-1 to Low Light Stress [J]. Chinese Bulletin of Botany, 2024, 59(4): 574-584. |
| [8] | Jianmin Zhou. A Combat Vehicle with a Smart Brake [J]. Chinese Bulletin of Botany, 2024, 59(3): 343-346. |
| [9] | Chaoyu Zhu, Chengxiang Hu, Zhenan Zhu, Zhining Zhang, Lihai Wang, Jun Chen, Sanfeng Li, Jinjin Lian, Luyao Tang, Qianqian Zhong, Wenjing Yin, Yuexing Wang, Yuchun Rao. Mapping of QTLs Associated with Rice Panicle Traits and Candidate Gene Analysis [J]. Chinese Bulletin of Botany, 2024, 59(2): 217-230. |
| [10] | Bao Zhu, Jiangzhe Zhao, Kewei Zhang, Peng Huang. OsCKX9 is Involved in Regulating the Rice Lamina Joint Development and Leaf Angle [J]. Chinese Bulletin of Botany, 2024, 59(1): 10-21. |
| [11] | Yanli Fang, Chuanyu Tian, Ruyi Su, Yapei Liu, Chunlian Wang, Xifeng Chen, Wei Guo, Zhiyuan Ji. Mining and Preliminary Mapping of Rice Resistance Genes Against Bacterial Leaf Streak [J]. Chinese Bulletin of Botany, 2024, 59(1): 1-9. |
| [12] | Tian Chuanyu, Fang Yanli, Shen Qing, Wang Hongjie, Chen Xifeng, Guo Wei, Zhao Kaijun, Wang Chunlian, Ji Zhiyuan. Genotypic Diversity and Pathogenisity of Xanthomonas oryzae pv. oryzae Isolated from Southern China in 2019-2021 [J]. Chinese Bulletin of Botany, 2023, 58(5): 743-749. |
| [13] | Dai Ruohui, Qian Xinyu, Sun Jinglei, Lu Tao, Jia Qiwei, Lu Tianqi, Lu Mei, Rao Yuchun. Research Progress on the Mechanisms of Leaf Color Regulation and Related Genes in Rice [J]. Chinese Bulletin of Botany, 2023, 58(5): 799-812. |
| [14] | Yuping Yan, Xiaoqi Yu, Deyong Ren, Qian Qian. Genetic Mechanisms and Breeding Utilization of Grain Number Per Panicle in Rice [J]. Chinese Bulletin of Botany, 2023, 58(3): 359-372. |
| [15] | Shang Sun, Yingying Hu, Yangshuo Han, Chao Xue, Zhiyun Gong. Double-stranded Labelled Oligo-FISH in Rice Chromosomes [J]. Chinese Bulletin of Botany, 2023, 58(3): 433-439. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||