

植物学报 ›› 2025, Vol. 60 ›› Issue (5): 679-692.DOI: 10.11983/CBB25149 cstr: 32102.14.CBB25149
收稿日期:2025-08-16
接受日期:2025-09-02
出版日期:2025-09-10
发布日期:2025-09-02
通讯作者:
*E-mail: xwchen88@163.com
基金资助:
Zhu Xiaobo, Wang Liyin, Chen Xuewei*( )
)
Received:2025-08-16
Accepted:2025-09-02
Online:2025-09-10
Published:2025-09-02
Contact:
*E-mail: xwchen88@163.com
摘要: 水杨酸(SA)是一种植物酚类天然合成产物, 对免疫反应具有重要的调控作用。植物主要通过异分支酸合酶(ICS)途径和苯丙氨酸解氨酶(PAL)途径合成水杨酸, 并被水杨酸受体NPR1等感知, 激活植物免疫反应。拟南芥(Arabidopsis thaliana)等十字花科植物主要通过ICS途径合成水杨酸, 而单子叶植物和非十字花科双子叶植物则主要通过PAL途径合成水杨酸。长期以来, 人们对水杨酸PAL合成途径的认识不完整, 导致水稻(Oryza sativa)等作物中水杨酸介导的植物免疫反应研究滞后, 极大地制约了作物抗病育种改良进程。近期, 我国3个研究团队独立破解了水杨酸在水稻等作物中的PAL合成途径。该文以此为契机, 综述了水杨酸介导的植物免疫反应研究进展, 着重梳理了植物体内的水杨酸合成途径, 总结了水杨酸被植物感知并激活免疫反应的机制, 展望了水杨酸调控植物免疫反应研究中存在的问题和未来的研究方向, 以期为相关理论研究和抗病育种应用提供新思路和新方向。
朱孝波, 王立印, 陈学伟. 水杨酸介导的植物免疫反应: 从代谢、感知到免疫激活. 植物学报, 2025, 60(5): 679-692.
Zhu Xiaobo, Wang Liyin, Chen Xuewei. Salicylic Acid-mediated Plant Immune Responses: From Metabolism and Perception to Immune Activation. Chinese Bulletin of Botany, 2025, 60(5): 679-692.
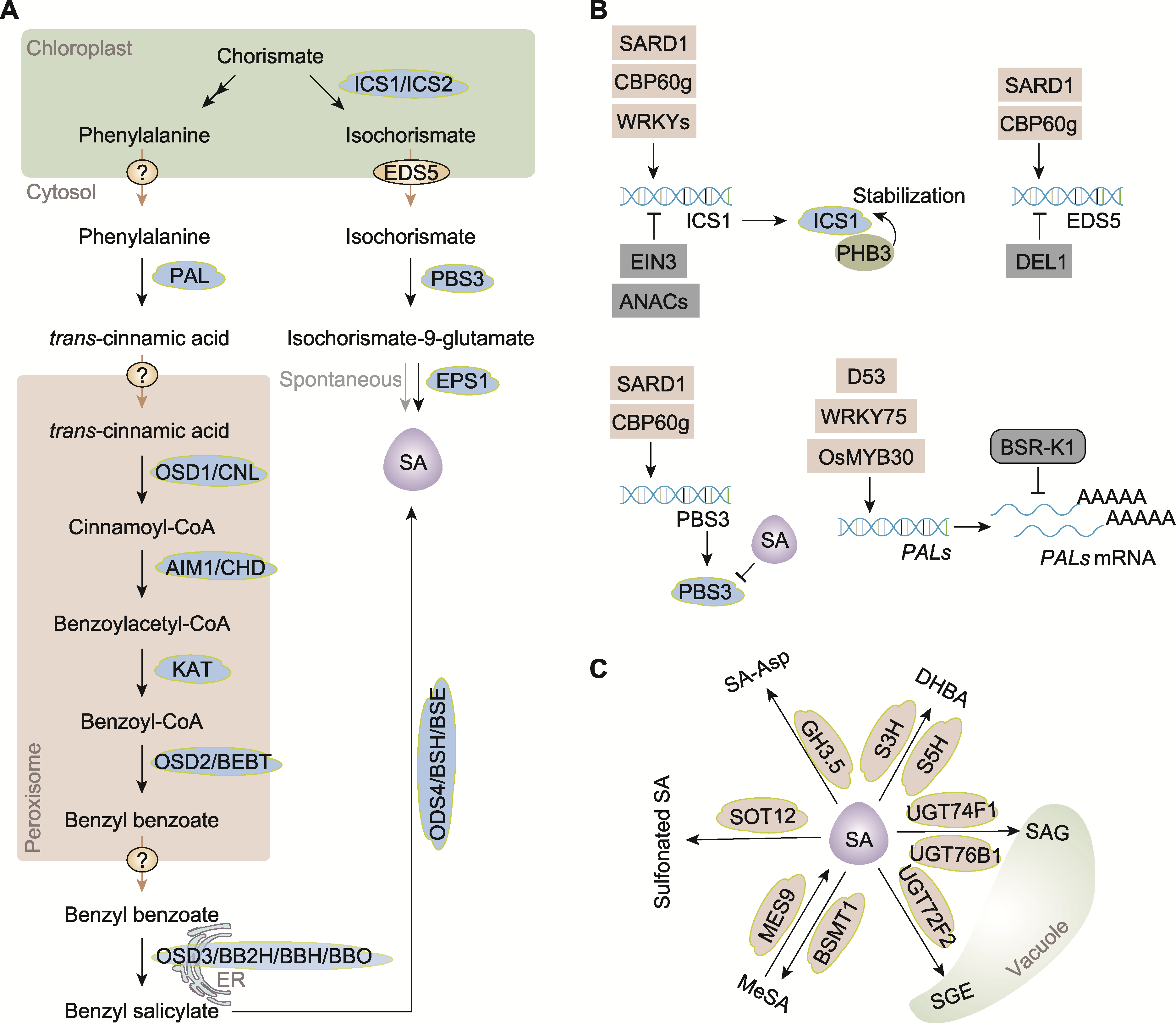
图1 水杨酸(SA)的生物合成与转录调控和代谢 (A) 水杨酸的生物合成途径(植物通过分支酸合酶(ICS)途径和苯丙氨酸解氨酶(PAL)途径合成水杨酸, 2条合成途径均起始于叶绿体, 以分支酸为前体。ICS1: 异分支酸合酶1; ICS2: 异分支酸合酶2; EDS5: 异分支酸转运蛋白; PBS3: 酰胺转移酶, EPS1: 酰基转移酶; OSD1/CNL: 肉桂酰辅酶A连接酶; AIM1/CHD: 肉桂酰辅酶A水合酶/脱氢酶; KAT: 3-酮酰辅酶A硫解酶; OSD2/BEBT: 苯甲醇苯甲酰转移酶; OSD3/BB2H/BBH/BBO: 苯甲酸苄酯2-羟化酶; OSD4/BSH/BSE: 水杨酸苄水解酶; ER: 内质网; 问号圆圈表示未知转运蛋白); (B) 水杨酸合成的转录调控(转录因子SARD1和CBP60g是ICS1、PBS3和EDS5基因表达的主要调控因子, 转录因子EIN3和多个ANAC可以抑制ICS1的表达, DEL1能够抑制EDS5的表达。PHB3可以稳定ICS1蛋白, 水杨酸可抑制PBS3的酶活性, 从而反馈调控水杨酸的合成。转录因子D53、WRKY75和OsMYB30可以调控多个PALs基因的表达。RNA结合蛋白BSR-K1可结合PAL基因的mRNA并降解, 在mRNA水平调控其表达); (C) 水杨酸的代谢(水杨酸可由水杨酸羟化酶S5H转化为2,5-二羟基苯甲酸(2,5-DHBA), 由S3H转化为2,3-二羟基苯甲酸(2,3-DHBA); 被UGT74F1和UGT76B1转化为水杨酸葡萄糖苷(SAG), 被UGT74F2转化为水杨酸葡萄糖酯(SGE), SAG和SGE可转运到液泡中进行储存; 还可被羧基甲基转移酶BSMT1甲基化, 生成甲基水杨酸(MeSA), MeSA可由甲基酯酶MES9转化为水杨酸; 水杨酸可在磺基转移酶SOT12的催化下生成磺基化的水杨酸; 还可在酰基酸氨基合成酶GH3.5的作用下, 与天门冬氨酸(Asp)结合, 生成SA-Asp)。
Figure 1 Biosynthesis, transcriptional regulation, and metabolism of salicylic acid (SA) (A) Biosynthetic pathways of SA (plants synthesize SA via two main pathways: the isochorismate synthase (ICS) pathway and the phenylalanine ammonia-lyase (PAL) pathway. Both originate in the chloroplast, using chorismate as the precursor. ICS1: Isochorismate synthase 1; ICS2: Isochorismate synthase 2; EDS5: Isochorismate transporter; PBS3: Amide transferase; EPS1: Acyl transferase; OSD1/CNL: Cinnamoyl-CoA ligase; AIM1/CHD: Cinnamoyl-CoA hydratase/dehydrogenase; KAT: 3-ketoacyl- CoA thiolase; OSD2/BEBT: Benzyl alcohol O-benzoyltransferase; OSD3/BB2H/BBH/BBO: Benzyl benzoate 2-hydroxylase; OSD4/BSH/BSE: Benzyl salicylate hydrolase; ER: Endoplasmic reticulum; question marked circles indicate unknown transporter); (B) Transcriptional regulation of SA biosynthesis (transcription factors SARD1 and CBP60g are major regulators of ICS1, PBS3, and EDS5 gene expression. EIN3 and several ANAC transcription factors repress ICS1 expression, while DEL1 suppresses EDS5 expression. PHB3 stabilizes the ICS1 protein. SA itself inhibits the enzymatic activity of PBS3, forming a feedback loop to regulate its own synthesis. Transcription factors D53, WRKY75, and OsMYB30 regulate the expression of multiple PAL genes. RNA-binding protein BSR-K1 can bind to the mRNA of the PAL genes and degrade them, thereby regulating their expression at the mRNA level); (C) Metabolism of SA (SA can be hydroxylated by salicylic acid 5-hydroxylase (S5H) to form 2,5-dihydroxybenzoic acid (2,5-DHBA), and by S3H to produce 2,3-dihydroxybenzoic acid (2,3-DHBA). It can also be glycosylated by UGT74F1 and UGT76B1 to form salicylic acid glucoside (SAG), or by UGT74F2 to form salicylic acid glucose ester (SGE). SAG and SGE are transported into vacuoles for storage. SA can also be methylated by the carboxyl methyltransferase BSMT1 to produce methyl salicylate (MeSA), which is converted back to SA by methyl esterase MES9. SA can be converted into sulfonated SA under the catalysis of sulfite transferase SOT12. Additionally, SA can conjugate with aspartic acid (Asp) via the action of acyl acid amido synthetase GH3.5 to form SA-Asp).
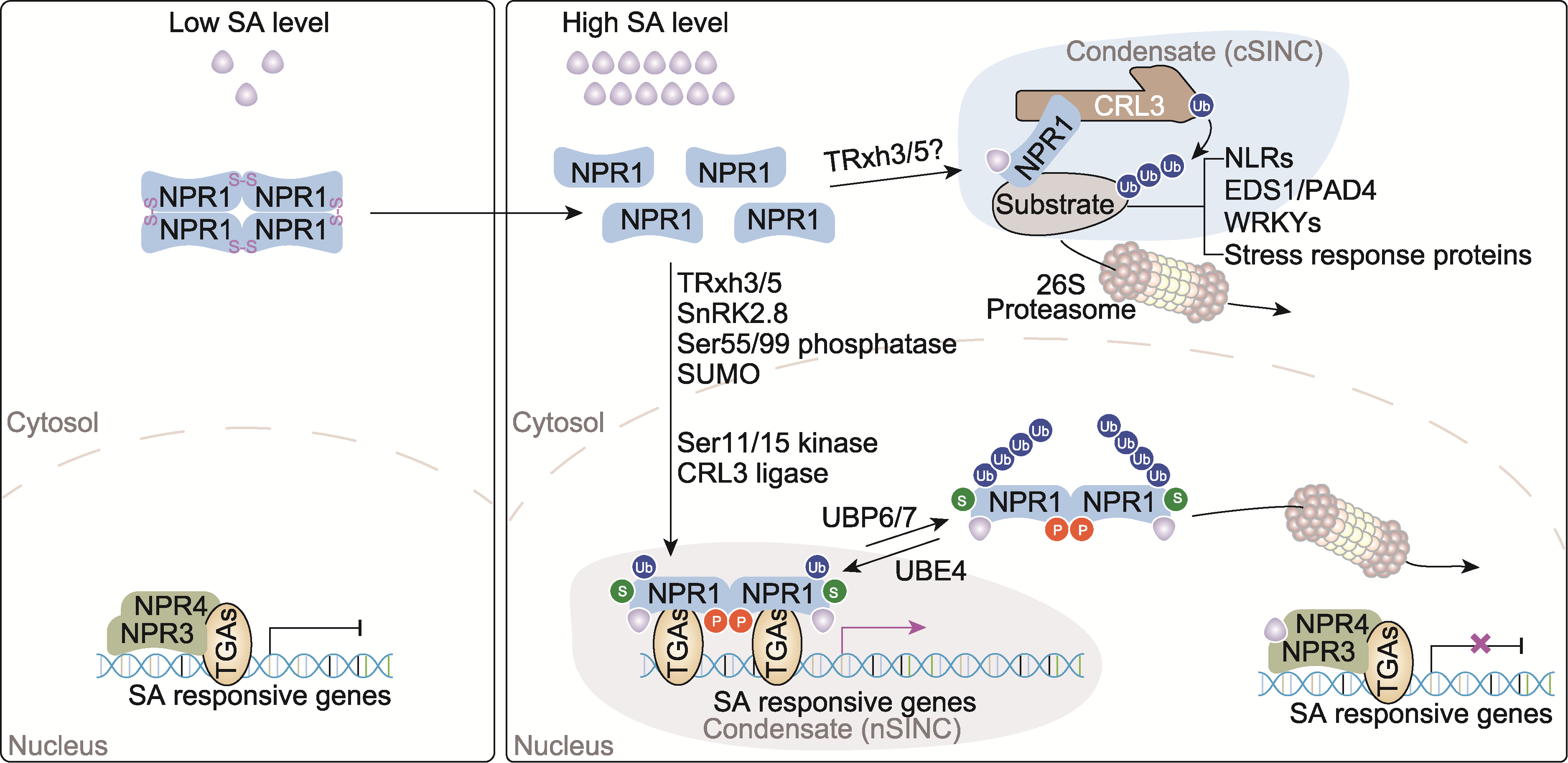
图2 水杨酸(SA)信号的感知与激活 在低浓度水杨酸条件下, NPR1蛋白通过二硫键(S-S)连接, 以寡聚体的形式存在于细胞质中; 而NPR3/4蛋白则在细胞核中与TGAs转录因子互作, 抑制水杨酸响应基因的表达。当细胞内水杨酸浓度升高时, 一方面NPR3/4可与水杨酸结合, 解除NPR3/4对下游水杨酸响应基因表达的抑制作用。另一方面, 水杨酸诱导细胞氧化还原状态改变, 在TRXh3/5的还原作用下, NPR1间的二硫键被破坏, NPR1解聚为单体; 解聚的NPR1在胞质中与水杨酸结合, 随后作为CRL3 E3泛素连接酶的底物识别蛋白, 与胞质中抗病蛋白(NLRs)及胁迫响应相关蛋白等形成水杨酸诱导的细胞质蛋白聚集体(cSINC), 调控这些蛋白的降解和稳态, 从而调控植物免疫反应。此外, 胞质中的NPR1蛋白还可经过蛋白激酶SnRK2.8的磷酸化(橘黄色圆形P)、未知蛋白磷酸酶对Ser55/99的去磷酸化及类泛素化修饰酶SUMO3的类泛素化修饰(绿色圆形S)等系列蛋白翻译后修饰作用后进入细胞核; 在细胞核中NPR1形成二聚体, Ser11/15被未知激酶磷酸化, 并在CRL3 E3泛素连接酶作用下发生单泛素化或短链泛素化修饰(单个蓝色圆形Ub); NPR1构象改变并与水杨酸结合, 与TGAs转录因子互作激活水杨酸响应基因的表达。在细胞核内, NPR1与TGAs转录因子和蛋白修饰酶及其它转录调控蛋白互作, 形成调控水杨酸响应基因表达的蛋白聚集体, 称为水杨酸诱导的细胞核蛋白聚集体(nSINC)。随后, 在泛素连接酶UBE4/MUSE3的作用下, NPR1的泛素化修饰链延长(多个蓝色圆形Ub), 促使NPR1进入26S蛋白酶体进行降解; 进入蛋白酶体的NPR1蛋白可在蛋白酶体相关去泛素化酶UBP6/7的作用下, 发生去泛素化作用, 解除NPR1的泛素蛋白酶体降解, 恢复其转录激活活性。
Figure 2 Perception and activation of salicylic acid (SA) signaling At low intracellular SA levels, NPR1 proteins are present in the cytosol as oligomers linked by disulfide bonds (S-S). Meanwhile, NPR3 and NPR4 proteins interact with TGA transcription factors in the nucleus to repress the expression of SA-responsive genes. When intracellular SA levels rise, NPR3/4 bind to SA, relieving their repression of downstream SA-responsive gene expression. SA also induces changes in the cellular redox state, and the disulfide bonds between NPR1 molecules are reduced by TRXh3/5, leading to monomerization of NPR1. Cytoplasmic NPR1 binds SA and acts as a substrate recognition protein for the CRL3 E3 ubiquitin ligase. It forms SA-induced NPR1 cytoplasmic protein condensates (cSINC) with disease resistance proteins (NLRs) and stress-related proteins, regulating their degradation and homeostasis to modulate plant immune responses. Additionally, cytoplasmic NPR1 undergoes a series of post-translational modifications before entering the nucleus, such as phosphorylation by protein kinase SnRK2.8 (orange circle labeled “P”), dephosphorylation at Ser55/99 by unknown phosphatases, and SUMOylation by SUMO3 (green circle labeled “S”). When enters in the nucleus, NPR1 forms dimers and is phosphorylated at Ser11/15 by unknown kinases, and undergoes mono- or short-chain ubiquitination (single blue circle labeled “Ub”) mediated by CRL3 E3 ligase. These modifications change NPR1’s conformation, enabling SA binding and interaction with TGA transcription factors to activate SA-responsive genes. Nuclear NPR1, together with TGAs, protein modifiers, and other transcriptional regulators, forms nuclear protein condensates known as SA-induced NPR1 nuclear condensates (nSINC), which orchestrate SA-responsive genes’ expression. Subsequently, NPR1 undergoes polyubiquitination (multiple blue circles labeled “Ub”) mediated by the ubiquitin ligase UBE4/MUSE3, targeting it for degradation via the 26S proteasome. However, NPR1 can be deubiquitinated by proteasome-associated deubiquitinases UBP6/7, preventing its degradation and restoring its transcriptional activation function.
| [1] | Baek D, Pathange P, Chung JS, Jiang JF, Gao LQ, Oikawa A, Hirai MY, Saito K, Pare PW, Shi HZ (2010). A stress-inducible sulphotransferase sulphonates salicylic acid and confers pathogen resistance in Arabidopsis. Plant Cell Environ 33, 1383-1392. |
| [2] | Canet JV, Dobón A, Fajmonová J, Tornero P (2012). The BLADE-ON-PETIOLE genes of Arabidopsis are essential for resistance induced by methyl jasmonate. BMC Plant Biol 12, 199. |
| [3] | Cao H, Bowling SA, Gordon AS, Dong XN (1994). Characterization of an Arabidopsis mutant that is nonresponsive to inducers of systemic acquired resistance. Plant Cell 6, 1583-1592. |
| [4] | Castelló MJ, Medina-Puche L, Lamilla J, Tornero P (2018). NPR1 paralogs of Arabidopsis and their role in salicylic acid perception. PLoS One 13, e0209835. |
| [5] | Chen F, D’Auria JC, Tholl D, Ross JR, Gershenzon J, Noel JP, Pichersky E (2003). An Arabidopsis thaliana gene for methylsalicylate biosynthesis, identified by a biochemical genomics approach, has a role in defense. Plant J 36, 577-588. |
| [6] | Chen Y, Shen H, Wang MY, Li Q, He ZH (2013). Salicyloyl-aspartate synthesized by the acetyl-amido synthetase GH3.5 is a potential activator of plant immunity in Arabidopsis. Acta Bioch Bioph Sin 45, 827-836. |
| [7] |
Chen ZX, Silva H, Klessig DF (1993). Active oxygen species in the induction of plant systemic acquired resistance by salicylic acid. Science 262, 1883-1886.
DOI PMID |
| [8] |
Chern M, Bai W, Ruan DL, Oh T, Chen XW, Ronald PC (2014). Interaction specificity and coexpression of rice NPR1 homologs 1 and 3 (NH1 and NH3), TGA transcription factors and negative regulator of resistance (NRR) proteins. BMC Genomics 15, 461.
DOI PMID |
| [9] |
Choi HW, Klessig DF (2016). DAMPs, MAMPs, and NAMPs in plant innate immunity. BMC Plant Biol 16, 232.
PMID |
| [10] | Dean JV, Delaney SP (2008). Metabolism of salicylic acid in wild-type, ugt74f1 and ugt74f2 glucosyltransferase mutants of Arabidopsis thaliana. Physiol Plant 132, 417-425. |
| [11] |
Delaney TP, Friedrich L, Ryals JA (1995). Arabidopsis signal transduction mutant defective in chemically and biologically induced disease resistance. Proc Natl Acad Sci USA 92, 6602-6606.
DOI PMID |
| [12] |
Ding YL, Sun TJ, Ao K, Peng YJ, Zhang YX, Li X, Zhang YL (2018). Opposite roles of salicylic acid receptors NPR1 and NPR3/NPR4 in transcriptional regulation of plant immunity. Cell 173, 1454-1467.
DOI PMID |
| [13] | Fang X, Xie Y, Yuan Y, Long Q, Zhang L, Abid G, Zhang WQ (2025). The role of salicylic acid in plant defense responses against biotic stresses. Plant Horm 1, e004. |
| [14] | Fu ZQ, Dong XN (2013). Systemic acquired resistance: turning local infection into global defense. Ann Rev Plant Biol 64, 839-863. |
| [15] |
Gaffney T, Friedrich L, Vernooij B, Negrotto D, Nye G, Uknes S, Ward E, Kessmann H, Ryals J (1993). Requirement of salicylic acid for the induction of systemic acquired resistance. Science 261, 754-756.
DOI PMID |
| [16] |
He J, Liu YQ, Yuan DY, Duan MJ, Liu YL, Shen ZJ, Yang CY, Qiu ZY, Liu DM, Wen PZ, Huang J, Fan DJ, Xiao SZ, Xin YY, Chen XN, Jiang L, Wang HY, Yuan LP, Wan JM (2020). An R2R3 MYB transcription factor confers brown planthopper resistance by regulating the phenylalanine ammonia-lyase pathway in rice. Proc Natl Acad Sci USA 117, 271-277.
DOI PMID |
| [17] | Huang S, Zhu SW, Kumar P, MacMicking JD (2021). A phase-separated nuclear GBPL circuit controls immunity in plants. Nature 594, 424-429. |
| [18] |
Huang WJ, Wang YR, Li X, Zhang YL (2020). Biosynthesis and regulation of salicylic acid and N-hydroxypipecolic acid in plant immunity. Mol Plant 13, 31-41.
DOI PMID |
| [19] | Huang XX, Zhu GQ, Liu Q, Chen L, Li YJ, Hou BK (2018). Modulation of plant salicylic acid-associated immune responses via glycosylation of dihydroxybenzoic acids. Plant Physiol 176, 3103-3119. |
| [20] | Jin HS, Choi SM, Kang MJ, Yun SH, Kwon DJ, Noh YS, Noh B (2018). Salicylic acid-induced transcriptional reprogramming by the HAC-NPR1-TGA histone acetyltransferase complex in Arabidopsis. Nucleic Acids Res 46, 11712-11725. |
| [21] | Jones JDG, Dangl JL (2006). The plant immune system. Nature 444, 323-329. |
| [22] | Kotera Y, Komori H, Tasaki K, Takagi K, Imano S, Katou S (2023). The peroxisomal β-oxidative pathway and benzyl alcohol O-benzoyltransferase HSR201 cooperatively contribute to the biosynthesis of salicylic acid. Plant Cell Physiol 64, 758-770. |
| [23] | Kumar S, Zavaliev R, Wu QL, Zhou Y, Cheng J, Dillard L, Powers J, Withers J, Zhao JS, Guan ZQ, Borgnia MJ, Bartesaghi A, Dong XN, Zhou P (2022). Structural basis of NPR1 in activating plant immunity. Nature 605, 561-566. |
| [24] | Lee HJ, Park YJ, Seo PJ, Kim JH, Sim HJ, Kim SG, Park CM (2015). Systemic immunity requires SnRK2.8-mediated nuclear import of NPR1 in Arabidopsis. Plant Cell 27, 3425-3438. |
| [25] |
León J, Shulaev V, Yalpani N, Lawton MA, Raskin I (1995). Benzoic acid 2-hydroxylase, a soluble oxygenase from tobacco, catalyzes salicylic acid biosynthesis. Proc Natl Acad Sci USA 92, 10413-10417.
DOI PMID |
| [26] |
Li Q, Zhou MX, Chhajed S, Yu FH, Chen SX, Zhang YP, Mou ZL (2023). N-hydroxypipecolic acid triggers systemic acquired resistance through extracellular NAD(P). Nat Commun 14, 6848.
DOI PMID |
| [27] | Lim GH (2023). Regulation of salicylic acid and N-hydroxy- pipecolic acid in systemic acquired resistance. Plant Pathol J 39, 21-27. |
| [28] | Liu YN, Sun TJ, Sun YL, Zhang YJ, Radojičić A, Ding YL, Tian HN, Huang XC, Lan JM, Chen SY, Orduna AR, Zhang KW, Jetter R, Li X, Zhang YL (2020). Diverse roles of the salicylic acid receptors NPR1 and NPR3/ NPR4 in plant immunity. Plant Cell 32, 4002-4016. |
| [29] | Liu YN, Xu L, Wu MS, Wang JJ, Qiu D, Lan JM, Lu JX, Zhang Y, Li X, Zhang YL (2025). Three-step biosynthesis of salicylic acid from benzoyl-CoA in plants. Nature doi: 10.1038/s41586-41025-09185-41587. |
| [30] |
Mackelprang R, Okrent RA, Wildermuth MC (2017). Preference of Arabidopsis thaliana GH3.5 acyl amido synthetase for growth versus defense hormone acyl substrates is dictated by concentration of amino acid substrate aspartate. Phytochemistry 143, 19-28.
DOI PMID |
| [31] | Manohar M, Tian MY, Moreau M, Park SW, Choi HW, Fei ZJ, Friso G, Asif M, Manosalva P, von Dahl CC, Shi K, Ma SS, Dinesh-Kumar SP, O'Doherty I, Schroeder FC, van Wijk KJ, Klessig DF (2015). Identification of multiple salicylic acid-binding proteins using two high throughput screens. Front Plant Sci 5, 777. |
| [32] |
Meng L, Yang HP, Yang JL, Wang YP, Ye TT, Xiang L, Chan Z, Wang YP (2024). Tulip transcription factor TgWRKY75 activates salicylic acid and abscisic acid biosynthesis to synergistically promote petal senescence. J Exp Bot 75, 2435-2450.
DOI PMID |
| [33] |
Mou ZL, Fan WH, Dong XN (2003). Inducers of plant systemic acquired resistance regulate NPR1 function through redox changes. Cell 113, 935-944.
DOI PMID |
| [34] | Noutoshi Y, Okazaki M, Kida T, Nishina Y, Morishita Y, Ogawa T, Suzuki H, Shibata D, Jikumaru Y, Hanada A, Kamiya Y, Shirasu K (2012). Novel plant immune-priming compounds identified via high-throughput chemical screening target salicylic acid glucosyltransferases in Arabidopsis. Plant Cell 24, 3795-3804. |
| [35] |
Pajerowska-Mukhtar KM, Wang W, Tada Y, Oka N, Tucker CL, Fonseca JP, Dong XN (2012). The HSF-like transcription factor TBF1 is a major molecular switch for plant growth-to-defense transition. Curr Biol 22, 103-112.
DOI PMID |
| [36] | Park SW, Kaimoyo E, Kumar D, Mosher S, Klessig DF (2007). Methyl salicylate is a critical mobile signal for plant systemic acquired resistance. Science 318, 113-116. |
| [37] | Peng YJ, Yang JF, Li X, Zhang YL (2021). Salicylic acid: biosynthesis and signaling. Ann Rev Plant Biol 72, 761-791. |
| [38] | Pieterse CMJ, Van der Does D, Zamioudis C, Leon-Reyes A, Van Wees SCM (2012). Hormonal modulation of plant immunity. Ann Rev Cell Dev Biol 28, 489-521. |
| [39] |
Rekhter D, Lüdke D, Ding YL, Feussner K, Zienkiewicz K, Lipka V, Wiermer M, Zhang YL, Feussner I (2019). Isochorismate-derived biosynthesis of the plant stress hormone salicylic acid. Science 365, 498-502.
DOI PMID |
| [40] |
Saleh A, Withers J, Mohan R, Marqués J, Gu YN, Yan SP, Zavaliev R, Nomoto M, Tada Y, Dong XN (2015). Posttranslational modifications of the master transcriptional regulator NPR1 enable dynamic but tight control of plant immune responses. Cell Host Microbe 18, 169-182.
DOI PMID |
| [41] |
Seguel A, Jelenska J, Herrera-Vásquez A, Marr SK, Joyce MB, Gagesch KR, Shakoor N, Jiang SC, Fonseca A, Wildermuth MC, Greenberg JT, Holuigue L (2018). PROHIBITIN3 forms complexes with ISOCHORISMATE SYNTHASE1 to regulate stress-induced salicylic acid biosynthesis in Arabidopsis. Plant Physiol 176, 2515-2531.
DOI PMID |
| [42] |
Shen MZ, Lim CJ, Park J, Kim JE, Baek D, Nam J, Lee SY, Pardo JM, Kim WY, Mackey D, Yun DJ (2020). HOS15 is a transcriptional corepressor of NPR1-mediated gene activation of plant immunity. Proc Natl Acad Sci USA 117, 30805-30815.
DOI PMID |
| [43] |
Silverman P, Seskar M, Kanter D, Schweizer P, Metraux JP, Raskin I (1995). Salicylic acid in rice (biosynthesis, conjugation, and possible role). Plant Physiol 108, 633-639.
DOI PMID |
| [44] | Skelly MJ, Furniss JJ, Grey H, Wong KW, Spoel SH (2019). Dynamic ubiquitination determines transcriptional activity of the plant immune coactivator NPR1. eLife 8, e47005. |
| [45] | Slaymaker DH, Navarre DA, Clark D, del Pozo O, Martin GB, Klessig DF (2002). The tobacco salicylic acid-binding protein 3 (SABP3) is the chloroplast carbonic anhydrase, which exhibits antioxidant activity and plays a role in the hypersensitive defense response. Proc Natl Acad Sci USA 99, 11640-11645. |
| [46] | Spoel SH, Dong XN (2024). Salicylic acid in plant immunity and beyond. Plant Cell 36, 1451-1464. |
| [47] |
Sun TJ, Zhang YX, Li Y, Zhang Q, Ding YL, Zhang YL (2015). ChIP-seq reveals broad roles of SARD1 and CBP60g in regulating plant immunity. Nat Commun 6, 10159.
DOI PMID |
| [48] | Tada Y, Spoel SH, Pajerowska-Mukhtar K, Mou ZL, Song JQ, Wang C, Zuo JR, Dong XN (2008). Plant immunity requires conformational charges of NPR1 via S-nitrosylation and thioredoxins. Science 321, 952-956. |
| [49] |
Tan ST, Abas M, Verstraeten I, Glanc M, Molnár G, Hajný J, Lasák P, Petřík I, Russinova E, Petrášek J, Novák O, Pospíšil J, Friml J (2020). Salicylic acid targets protein phosphatase 2A to attenuate growth in plants. Curr Biol 30, 381-395.
DOI PMID |
| [50] |
Torrens-Spence MP, Bobokalonova A, Carballo V, Glinkerman CM, Pluskal T, Shen A, Weng JK (2019). PBS3 and EPS1 complete salicylic acid biosynthesis from isochorismate in Arabidopsis. Mol Plant 12, 1577-1586.
DOI PMID |
| [51] | Ullah C, Chen YH, Ortega MA, Tsai CJ (2023). The diversity of salicylic acid biosynthesis and defense signaling in plants: knowledge gaps and future opportunities. Curr Opin Plant Biol 72, 102349. |
| [52] | Vlot AC, Dempsey DA, Klessig DF (2009). Salicylic acid, a multifaceted hormone to combat disease. Ann Rev Phytopathol 47, 177-206. |
| [53] |
Wang D, Weaver ND, Kesarwani M, Dong XN (2005). Induction of protein secretory pathway is required for systemic acquired resistance. Science 308, 1036-1040.
PMID |
| [54] | Wang W, Withers J, Li H, Zwack PJ, Rusnac DV, Shi H, Liu LJ, Yan SP, Hinds TR, Guttman M, Dong XN, Zheng N (2020). Structural basis of salicylic acid perception by Arabidopsis NPR proteins. Nature 586, 311-316. |
| [55] | Wang YK, Miao HY, Qiu JH, Liu MH, Jin GC, Zhang WX, Song SY, Fan PX, Xin XF, Hu JP, Li R, Pan RH (2025a). Species- and organ-specific contribution of peroxisomal cinnamate: CoA ligases to benzoic and salicylic acid biosynthesis. Plant Cell 37, koae329. |
| [56] | Wang YK, Song SY, Zhang WX, Deng QW, Feng YL, Tao M, Kang MN, Zhang Q, Yang LJ, Wang XY, Zhu CG, Wang XW, Zhu WX, Zhu YX, Cao PF, Chen J, Pan JH, Feng S, Chen XY, Dai HX, Song SY, Yang JH, Zhao TL, Cao FB, Tao Z, Shen XX, Last RL, Hu JP, Yu JQ, Fan PX, Pan RH (2025b). Deciphering phenylalanine-derived salicylic acid biosynthesis in plants. Nature doi: 10.1038/s41586-41025-09280-41589. |
| [57] | Wildermuth MC, Dewdney J, Wu G, Ausubel FM (2001). Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature 414, 562-565. |
| [58] | Wu N, Qin L, Peng ZH, Xia ST (2022). Research progress of mobile signal Pip/NHP in systemic acquired resistance. Chin Bull Bot 57, 412-421. (in Chinese) |
|
吴楠, 覃磊, 彭志红, 夏石头 (2022). 系统获得性抗性移动信号Pip/NHP研究进展. 植物学报 57, 412-421.
DOI |
|
| [59] | Wu Y, Zhang D, Chu JY, Boyle P, Wang Y, Brindle ID, De Luca V, Després C (2012). The Arabidopsis NPR1 protein is a receptor for the plant defense hormone salicylic acid. Cell Rep 1, 639-647. |
| [60] | Xu L, Zhao HY, Ruan WY, Deng MJ, Wang F, Peng JR, Luo J, Chen ZX, Yi KK (2017). ABNORMAL INFLORESCENCE MERISTEM1 functions in salicylic acid biosynthesis to maintain proper reactive oxygen species levels for root meristem activity in rice. Plant Cell 29, 560-574. |
| [61] |
Yalpani N, Leon J, Lawton MA, Raskin I (1993). Pathway of salicylic acid biosynthesis in healthy and virus-inoculated tobacco. Plant Physiol 103, 315-321.
DOI PMID |
| [62] | Yamasaki K, Motomura Y, Yagi Y, Nomura H, Kikuchi S, Nakai M, Shiina T (2013). Chloroplast envelope localization of EDS5, an essential factor for salicylic acid biosynthesis in Arabidopsis thaliana. Plant Signal Behav 8, e23603. |
| [63] |
Yang Y, Xu R, Ma CJ, Vlot AC, Klessig DF, Pichersky E (2008). Inactive methyl indole-3-acetic acid ester can be hydrolyzed and activated by several esterases belonging to the AtMES esterase family of Arabidopsis. Plant Physiol 147, 1034-1045.
DOI PMID |
| [64] |
Ye HT, Hou QQ, Lv HT, Shi H, Wang D, Chen YJ, Xu TS, Wang M, He M, Yin JJ, Lu X, Tang YY, Zhu XB, Zou LJ, Chen XW, Li JY, Wang B, Wang J (2024). D53 represses rice blast resistance by directly targeting phenylalanine ammonia lyases. J Integr Plant Biol 66, 1827-1830.
DOI |
| [65] | Yokoo S, Inoue S, Suzuki N, Amakawa N, Matsui H, Nakagami H, Takahashi A, Arai R, Katou S (2018). Comparative analysis of plant isochorismate synthases reveals structural mechanisms underlying their distinct biochemical properties. Biosci Rep 38, BSR20171457. |
| [66] |
Zavaliev R, Dong XN (2024). NPR1, a key immune regulator for plant survival under biotic and abiotic stresses. Mol Cell 84, 131-141.
DOI PMID |
| [67] |
Zavaliev R, Mohan R, Chen TY, Dong XN (2020). Formation of NPR1 condensates promotes cell survival during the plant immune response. Cell 182, 1093-1108.
DOI PMID |
| [68] | Zhang J, Dong SM, Wang W, Zhao JH, Chen XW, Guo HS, He GC, He ZH, Kang ZS, Peng YL, Wang GL, Zhou XP, Wang YC, Zhou JM (2019). Plant immunity and sustainable control of pests in China: advances, opportunities and challenges. Sci Sin Vitae 49, 1479-1507. (in Chinese) |
| 张杰, 董莎萌, 王伟, 赵建华, 陈学伟, 郭惠珊, 何光存, 何祖华, 康振生, 李毅, 彭友良, 王国梁, 周雪平, 王源超, 周俭民 (2019). 植物免疫研究与抗病虫绿色防控: 进展、机遇与挑战. 中国科学: 生命科学 49, 1479-1507. | |
| [69] | Zhang KW, Halitschke R, Yin CX, Liu CJ, Gan SS (2013). Salicylic acid 3-hydroxylase regulates Arabidopsis leaf longevity by mediating salicylic acid catabolism. Proc Natl Acad Sci USA 110, 14807-14812. |
| [70] | Zhang YJ, Zhao L, Zhao JZ, Li YJ, Wang JB, Guo R, Gan SS, Liu CJ, Zhang KW (2017). S5H/DMR6 encodes a salicylic acid 5-hydroxylase that fine-tunes salicylic acid homeostasis. Plant Physiol 175, 1082-1093. |
| [71] | Zhang YL, Tessaro MJ, Lassner M, Li X (2003). Knockout analysis of Arabidopsis transcription factors TGA2, TGA5, and TGA6 reveals their redundant and essential roles in systemic acquired resistance. Plant Cell 15, 2647-2653. |
| [72] |
Zhao Y, Zhu XB, Chen XW, Zhou JM (2022). From plant immunity to crop disease resistance. J Genet Genomics 49, 693-703.
DOI PMID |
| [73] |
Zhou XG, Liao HC, Chern M, Yin JJ, Chen YF, Wang JP, Zhu XB, Chen ZX, Yuan C, Zhao W, Wang J, Li WT, He M, Ma BT, Wang JC, Qin P, Chen WL, Wang YP, Liu JL, Qian YW, Wang WM, Wu XJ, Li P, Zhu LH, Li SG, Ronald PC, Chen XW (2018). Loss of function of a rice TPR-domain RNA-binding protein confers broad-spectrum disease resistance. Proc Natl Acad Sci USA 115, 3174-3179.
DOI PMID |
| [74] | Zhu B, Zhang YJ, Gao R, Wu ZH, Zhang W, Zhang C, Zhang PH, Ye C, Yao LB, Jin Y, Mao H, Tou PY, Huang P, Zhao JZ, Zhao Q, Liu CJ, Zhang KW (2025). Complete biosynthesis of salicylic acid from phenylalanine in plants. Nature doi: org/10.1038/s41586-41025-09175-41589. |
| [75] | Zhu XB, Li WT, He M, Wang J, Yu ZL, Chen XW (2020). Broad spectrum resistance in main crops: recent advances and future directions. Bull Nat Nat Sci Found China 34, 401-410. (in Chinese) |
| 朱孝波, 李伟滔, 贺闽, 王静, 于振良, 陈学伟 (2020). 作物广谱抗病研究现状与关键科学问题. 中国科学基金 34, 401-410. | |
| [76] | Zhu XB, Yin JJ, Liang SH, Liang RH, Zhou XG, Chen ZX, Zhao W, Wang J, Li WT, He M, Yuan C, Miyamoto K, Ma BT, Wang JC, Qin P, Chen WL, Wang YP, Wang WM, Wu XJ, Yamane H, Zhu LH, Li SG, Chen XW (2016). The multivesicular bodies (MVBs)-localized AAA ATPase LRD6-6 inhibits immunity and cell death likely through regulating MVBs-mediated vesicular trafficking in rice. PLoS Genet 12, e1006311. |
| [1] | 史世肸, 严顺平. 高效液相色谱法检测水杨酸的优化[J]. 植物学报, 2025, 60(5): 846-853. |
| [2] | 粟思琳, 唐先宇, 陈祎, 王婷, 夏石头. 植物系统获得性抗性的转录调控[J]. 植物学报, 2025, 60(5): 722-733. |
| [3] | 叶灿, 姚林波, 金莹, 高蓉, 谭琪, 李旭映, 张艳军, 陈析丰, 马伯军, 章薇, 张可伟. 水稻水杨酸代谢突变体高通量筛选方法的建立与应用[J]. 植物学报, 2025, 60(4): 586-596. |
| [4] | 杨莉, 曲茜彤, 陈子航, 邹婷婷, 王全华, 王小丽. 菠菜AT-hook基因家族鉴定与表达谱分析[J]. 植物学报, 2025, 60(3): 377-392. |
| [5] | 袁民航, 辛秀芳. 烽火狼烟: 水杨酸甲酯介导的植物间通讯和气传性免疫的机制解析[J]. 植物学报, 2023, 58(5): 682-686. |
| [6] | 园园, 恩和巴雅尔, 齐艳华. 植物GH3基因家族生物学功能研究进展[J]. 植物学报, 2023, 58(5): 770-782. |
| [7] | 王伟, 唐定中. 两类免疫受体强强联手筑牢植物免疫防线[J]. 植物学报, 2021, 56(2): 142-146. |
| [8] | 曹栋栋,陈珊宇,秦叶波,吴华平,阮关海,黄玉韬. 水杨酸调控盐胁迫下羽衣甘蓝种子萌发的机理[J]. 植物学报, 2020, 55(1): 49-61. |
| [9] | 代宇佳,罗晓峰,周文冠,陈锋,帅海威,杨文钰,舒凯. 生物和非生物逆境胁迫下的植物系统信号[J]. 植物学报, 2019, 54(2): 255-264. |
| [10] | 闫佳, 刘雅琼, 侯岁稳. 植物抗病蛋白研究进展[J]. 植物学报, 2018, 53(2): 250-263. |
| [11] | 李冬梅, 王路雅, 张澜玥, 帖子阳, 毛惠平. 拟南芥短肽激素PROPEP基因家族在根生长中的作用机理[J]. 植物学报, 2016, 51(2): 202-209. |
| [12] | 雷珍珍, 叶晶龙, 程海丽, 陈云, 望彗星, 许克静, 乐超银. 花魔芋抗软腐病植株的鉴定及其抗性机理的初步研究[J]. 植物学报, 2013, 48(3): 295-302. |
| [13] | 李星, 郝鹤, 池剑亭, 王红, 叶和春. 利用异源生物生产青蒿素及其前体的研究进展[J]. 植物学报, 2012, 47(6): 571-580. |
| [14] | 王家利, 刘冬成, 郭小丽, 张爱民. 生长素合成途径的研究进展[J]. 植物学报, 2012, 47(3): 292-301. |
| [15] | 陈璇, 杨明, 郭鸿彦. 大麻植物中大麻素成分研究进展[J]. 植物学报, 2011, 46(2): 197-205. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||