

植物学报 ›› 2021, Vol. 56 ›› Issue (4): 462-469.DOI: 10.11983/CBB20194 cstr: 32102.14.CBB20194
收稿日期:2020-11-30
接受日期:2021-03-01
出版日期:2021-07-01
发布日期:2021-06-30
通讯作者:
季代丽
作者简介:*E-mail: jidaili@ibcas.ac.cn基金资助:
Qiuxin Li1,2, Wei Chi1,2, Daili Ji1,*( )
)
Received:2020-11-30
Accepted:2021-03-01
Online:2021-07-01
Published:2021-06-30
Contact:
Daili Ji
摘要: 高等植物叶绿体中的基粒是由多个圆盘状类囊体堆叠在一起形成的特殊结构, 它的形成可以将光合蛋白复合体分配在类囊体膜的不同位置, 使类囊体膜具有横向异质性, 能有效进行光合作用。促进基粒形成的关键是使类囊体膜弯曲, 目前发现导致膜弯曲的关键因子是CURT1蛋白。该文对近年在拟南芥(Arabidopsis thaliana)和蓝藻(Cyanobacteria)中有关CURT1蛋白的研究进展进行综述, 并对未来类囊体膜结构与功能的动态调控研究进行展望。
李秋信, 迟伟, 季代丽. CURT1调控类囊体膜弯曲的研究进展. 植物学报, 2021, 56(4): 462-469.
Qiuxin Li, Wei Chi, Daili Ji. Research Progress of CURT1 on Regulating Thylakoid Membrane Curvature. Chinese Bulletin of Botany, 2021, 56(4): 462-469.
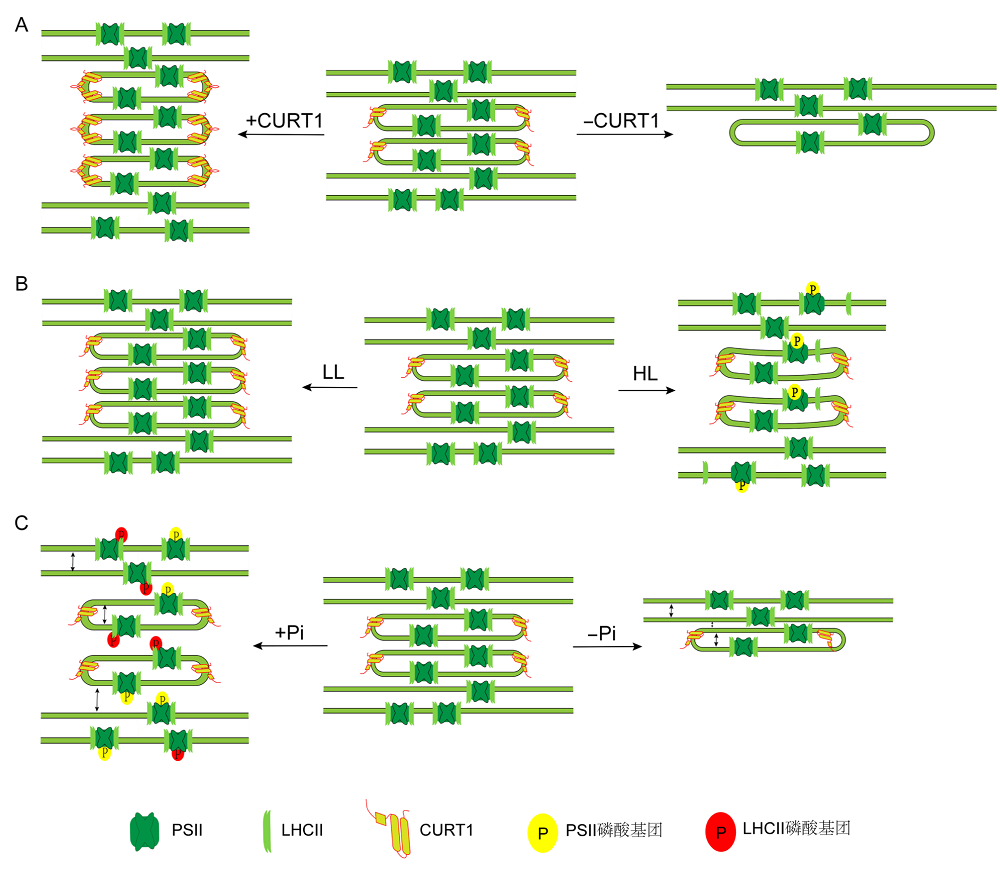
图1 类囊体超微结构的可塑性 (A) CURT1蛋白水平影响基粒结构: CURT1蛋白含量增加, 类囊体基粒片层数增加, 直径变窄; CURT1蛋白含量减少, 类囊体基粒片层数变少, 直径变宽; (B) 光强影响类囊体结构: 低光下基粒片层数增多, 高光下基粒直径变窄, 基粒高度增加; (C) PSII磷酸化水平影响类囊体膜结构: PSII磷酸化增强, 基粒直径变小、变高, 类囊体膜间隙变大, 囊腔增厚; PSII磷酸化水平下降, 基粒直径变大, 类囊体膜间隙变小, 基粒层数变少。LL: 低光; HL: 高光; PSII: 光系统II; LHCII: 捕光蛋白复合物II; CURT1: 弯曲类囊体膜蛋白1
Figure 1 The plasticity of thylakoid ultrastructure (A) Effects of CURT1 levels on granum dimensions: With the level of CURT1 proteins increased, the grana displays more layers of membrane and decreased diameter; Grana without CURT1 proteins significantly increased in diameter but contain far fewer layers of membrane; (B) Effects of changes in light conditions on granum dimensions: under low light levels, the numbers of layers in grana stacks are increased, and high light intensities lead to significant reduction in the diameter, and to partial transversal unstacking of grana discs; (C) Effects of PSII phosphorylation on granum dimensions: increased levels of PSII phosphorylation lead to significant reduction in the diameter, the enlargement of the vertical gaps between thylakoid layers, and swelling of the thylakoid lumen; Decreased levels of PSII phosphorylation lead to increased diameter, decreased gaps of adjacent layers within the granum, and fewer layers of membrane. LL: Low light; HL: High light; PSII: Photosystem II; LHC II: Light-harvesting complex II; CURT1: CURVATURE THYLAKOID 1
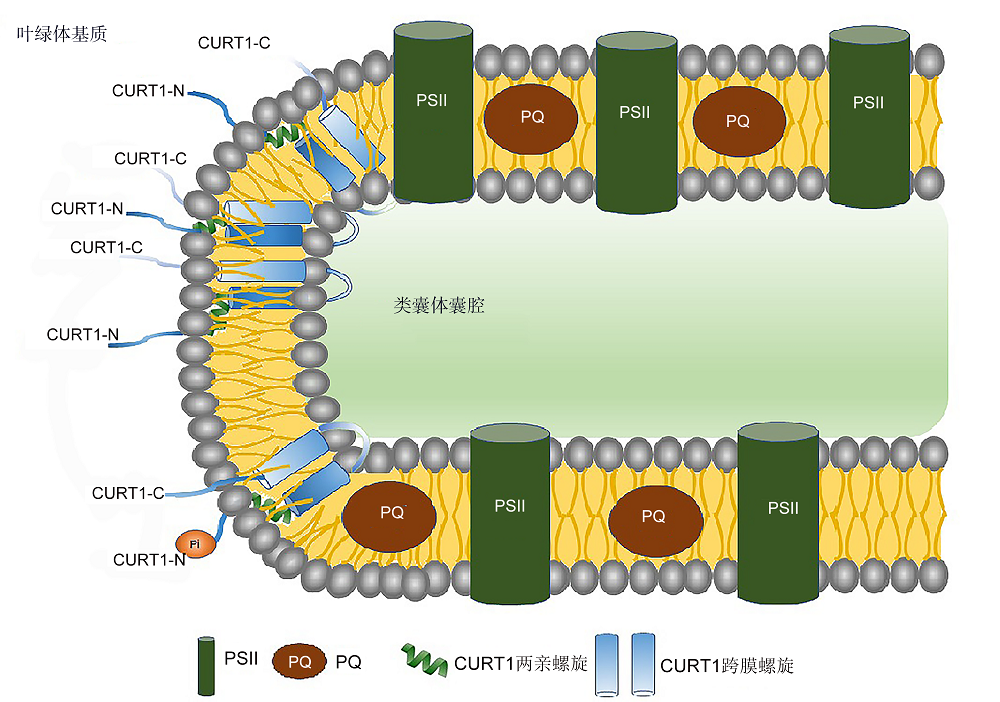
图2 CURT1蛋白插入类囊体膜导致膜弯曲模式 PQ: 质体醌。PSII和CURT1同图1。
Figure 2 The functional model for CURT1 insertion leads to thylakoid membrane curvature PQ: Plastoquinone. PSII and CURT1 see Figure 1.

图3 不同光照条件下CURT1调控基粒结构变化影响光合作用的机理 过表达以及缺失CURT1植株在高光下对PSII修复的影响以及弱光下对状态转变的影响。PSI: 光系统I。PSII、LHCII和CURT1同图1。
Figure 3 CURT1 modulate grana structure to regulate photosynthesis in response to different light conditions Overexpression or knock out of CURT1 protein influence the PSII repair process under high light condition and state transition during low light condition. PSI: Photosystem I. PSII, LHCII and CURT1 see Figure 1.
| [1] | 代玉华, 刘训言, 孟庆伟, 赵世杰 (2004). 低温胁迫对类囊体膜脂代谢的影响. 植物学通报 21, 506-511. |
| [2] | 付振书, 赵世杰, 孟庆伟 (2004). 类囊体腔的酸化与过剩激发能耗散. 植物学通报 21, 486-494. |
| [3] |
Alimohamadi H, Rangamani P (2018). Modeling membrane curvature generation due to membrane-protein interactions. Biomolecules 8, 120.
DOI URL |
| [4] |
Anderson JM (1986). Photoregulation of the composition, function, and structure of thylakoid membranes. Annu Rev Plant Physiol 37, 93-136.
DOI URL |
| [5] |
Armbruster U, Labs M, Pribil M, Viola S, Xu WT, Scharfenberg M, Hertle AP, Rojahn U, Jensen PE, Rappaport F, Joliot P, Dörmann P, Wanner G, Leister D (2013). Arabidopsis CURVATURE THYLAKOID 1 proteins modify thylakoid architecture by inducing membrane curvature. Plant Cell 25, 2661-2678.
DOI URL |
| [6] |
Austin II JR, Staehelin LA (2011). Three-dimensional architecture of grana and stroma thylakoids of higher plants as determined by electron tomography. Plant Physiol 155, 1601-1611.
DOI URL |
| [7] |
Chuartzman SG, Nevo R, Shimoni E, Charuvi D, Kiss V, Ohad I, Brumfeld V, Reich Z (2008). Thylakoid membrane remodeling during state transitions in Arabidopsis. Plant Cell 20, 1029-1039.
DOI PMID |
| [8] |
Daum B, Kühlbrandt W (2011). Electron tomography of plant thylakoid membranes. J Exp Bot 62, 2393-2402.
DOI URL |
| [9] |
Gkeka P, Sarkisov L (2010). Interactions of phospholipid bilayers with several classes of amphiphilic α-helical peptides: insights from coarse-grained molecular dynamics simulations. J Phys Chem B 114, 826-839.
DOI URL |
| [10] |
Hansson M, Vener AV (2003). Identification of three previously unknown in vivo protein phosphorylation sites in thylakoid membranes of Arabidopsis thaliana. Mol Cell Proteomics 2, 550-559.
PMID |
| [11] |
Heinz S, Rast A, Shao L, Gutu A, Gügel IL, Heyno E, Labs M, Rengstl B, Viola S, Nowaczyk MM, Leister D, Nickelsen J (2016). Thylakoid membrane architecture in Synechocystis depends on CurT, a homolog of the granal CURVATURE THYLAKOID1 proteins. Plant Cell 28, 2238-2260.
DOI URL |
| [12] |
Jarsch IK, Daste F, Gallop JL (2016). Membrane curvature in cell biology: an integration of molecular mechanisms. J Cell Biol 214, 375-387.
DOI PMID |
| [13] | Jensen PE, Leister D (2014). Chloroplast evolution, structure and functions. F1000Prime Rep 6, 40. |
| [14] |
Kirchhoff H (2013). Architectural switches in plant thylakoid membranes. Photosynth Res 116, 481-487.
DOI URL |
| [15] | Kirchhoff H (2014). Structural changes of the thylakoid membrane network induced by high light stress in plant chloroplasts. Philos Trans R Soc Lond B Biol Sci 369, 20130225. |
| [16] |
Kirchhoff H (2018). Structure-function relationships in photosynthetic membranes: challenges and emerging fields. Plant Sci 266, 76-82.
DOI PMID |
| [17] |
Kirchhoff H (2019). Chloroplast ultrastructure in plants. New Phytol 223, 565-574.
DOI PMID |
| [18] |
Könnel A, Bugaeva W, Gügel IL, Philippar K (2019). BANFF: bending of bilayer membranes by amphiphilic α-helices is necessary for form and function of organelles. Biochem Cell Biol 97, 243-256.
DOI URL |
| [19] |
Lambrev PH, Akhtar P (2019). Macroorganisation and flexibility of thylakoid membranes. Biochem J 476, 2981-3018.
DOI PMID |
| [20] |
Mareš J, Strunecký O, Bučinská L, Wiedermannová J (2019). Evolutionary patterns of thylakoid architecture in cyanobacteria. Front Microbiol 10, 277.
DOI PMID |
| [21] |
McMahon HT, Boucrot E (2015). Membrane curvature at a glance. J Cell Sci 128, 1065-1070.
DOI PMID |
| [22] |
McMahon HT, Gallop JL (2005). Membrane curvature and mechanisms of dynamic cell membrane remodelling. Nature 438, 590-596.
DOI URL |
| [23] |
Nixon PJ, Michoux F, Yu JF, Boehm M, Komenda J (2010). Recent advances in understanding the assembly and repair of photosystem II. Ann Bot 106, 1-16.
DOI URL |
| [24] |
Peter BJ, Kent HM, Mills IG, Vallis Y, Butler PJG, Evans PR, McMahon HT (2004). BAR domains as sensors of membrane curvature: the amphiphysin BAR structure. Science 303, 495-499.
DOI URL |
| [25] |
Pribil M, Labs M, Leister D (2014). Structure and dynamics of thylakoids in land plants. J Exp Bot 65, 1955-1972.
DOI URL |
| [26] |
Pribil M, Sandoval-Ibáñez O, Xu WT, Sharma A, Labs M, Liu QP, Galgenmüller C, Schneider T, Wessels M, Matsubara S, Jansson S, Wanner G, Leister D (2018). Fine-tuning of photosynthesis requires CURVATURE THYLAKOID1-mediated thylakoid plasticity. Plant Physiol 176, 2351-2364.
DOI URL |
| [27] |
Shimoni E, Rav-Hon O, Ohad I, Brumfeld V, Reich Z (2005). Three-dimensional organization of higher-plant chloroplast thylakoid membranes revealed by electron tomography. Plant Cell 17, 2580-2586.
PMID |
| [28] |
Stengel A, Gügel IL, Hilger D, Rengstl B, Jung H, Nickelsen J (2012). Initial steps of photosystem II de novo assembly and preloading with manganese take place in biogenesis centers in Synechocystis. Plant Cell 24, 660-675.
DOI URL |
| [29] |
Trotta A, Bajwa AA, Mancini I, Paakkarinen V, Pribil M, Aro EM (2019). The role of phosphorylation dynamics of CURVATURE THYLAKOID 1B in plant thylakoid membranes. Plant Physiol 181, 1615-1631.
DOI URL |
| [30] |
Wood WHJ, Barnett SFH, Flannery S, Hunter CN, Johnson MP (2019). Dynamic thylakoid stacking is regulated by LHCII phosphorylation but not its interaction with PSI. Plant Physiol 180, 2152-2166.
DOI URL |
| [1] | 刘雨函, 曹启江, 张诗晗, 李益慧, 王菁, 谭晓萌, 刘筱儒, 王显玲. 拟南芥AtFTCD-L参与根系响应土壤紧实度的机制研究[J]. 植物学报, 2025, 60(4): 1-0. |
| [2] | 陈静, 张丙昌, 刘燕晋, 武杰, 赵康, 明姣. 荒漠生物结皮细鞘丝藻类(Leptolyngbya-like)蓝藻多样性[J]. 生物多样性, 2024, 32(9): 24186-. |
| [3] | 景艳军, 林荣呈. 蓝光受体CRY2化身“暗黑舞者”[J]. 植物学报, 2024, 59(6): 878-882. |
| [4] | 罗燕, 刘奇源, 吕元兵, 吴越, 田耀宇, 安田, 李振华. 拟南芥光敏色素突变体种子萌发的光温敏感性[J]. 植物学报, 2024, 59(5): 752-762. |
| [5] | 陈艳晓, 李亚萍, 周晋军, 解丽霞, 彭永彬, 孙伟, 和亚男, 蒋聪慧, 王增兰, 郑崇珂, 谢先芝. 拟南芥光敏色素B氨基酸位点突变对其结构与功能的影响[J]. 植物学报, 2024, 59(3): 481-494. |
| [6] | 杨继轩, 王雪霏, 顾红雅. 西藏野生拟南芥开花时间变异的遗传基础[J]. 植物学报, 2024, 59(3): 373-382. |
| [7] | 吴娇娇, 郭冠廷, 陈栋, 赵鑫, 龙明忠, 王登富, 李晓娜. 苔藓-蓝藻共生体多样性及固氮潜力研究现状[J]. 生物多样性, 2023, 31(8): 23081-. |
| [8] | 王钢, 王二涛. “卫青不败由天幸”——WeiTsing的广谱抗根肿病机理被揭示[J]. 植物学报, 2023, 58(3): 356-358. |
| [9] | 杨永青, 郭岩. 植物细胞质外体pH感受机制的解析[J]. 植物学报, 2022, 57(4): 409-411. |
| [10] | 支添添, 周舟, 韩成云, 任春梅. PAD4突变加速拟南芥酪氨酸降解缺陷突变体sscd1的程序性细胞死亡[J]. 植物学报, 2022, 57(3): 288-298. |
| [11] | 李艳艳, 齐艳华. 植物Aux/IAA基因家族生物学功能研究进展[J]. 植物学报, 2022, 57(1): 30-41. |
| [12] | 车永梅, 孙艳君, 卢松冲, 侯丽霞, 范欣欣, 刘新. AtMYB77促进NO合成参与调控干旱胁迫下拟南芥侧根发育[J]. 植物学报, 2021, 56(4): 404-413. |
| [13] | 王婷, 羊欢欢, 赵弘巍, JosefVoglmeir, 刘丽. 蛋白质N-糖基化在拟南芥生长周期中的变化规律及去糖基化对根发育的影响[J]. 植物学报, 2021, 56(3): 262-274. |
| [14] | 林雨晴, 齐艳华. 生长素输出载体PIN家族研究进展[J]. 植物学报, 2021, 56(2): 151-165. |
| [15] | 杜斐, 焦雨铃. WUSCHEL介导的固有免疫: 植物干细胞抵御病毒侵害的新机制[J]. 植物学报, 2020, 55(5): 537-540. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||