

Chinese Bulletin of Botany ›› 2020, Vol. 55 ›› Issue (3): 279-286.DOI: 10.11983/CBB19239 cstr: 32102.14.CBB19239
• EXPERIMENTAL COMMUNICATIONS • Previous Articles Next Articles
Liurong Guan1,2,Zupei Liu2,3,Ran Xu2,Penggen Duan2,Guozheng Zhang2,3,Haiyue Yu2,3,Jing Li2,3,Yuehua Luo1,*( ),Yunhai Li2,*(
),Yunhai Li2,*( )
)
Received:2019-12-25
Accepted:2020-03-23
Online:2020-05-01
Published:2020-07-06
Contact:
Yuehua Luo,Yunhai Li
Liurong Guan, Zupei Liu, Ran Xu, Penggen Duan, Guozheng Zhang, Haiyue Yu, Jing Li, Yuehua Luo, Yunhai Li. Identification of a New OsBRI1 Weak Allele and Analysis of its Function in Grain Size Control[J]. Chinese Bulletin of Botany, 2020, 55(3): 279-286.
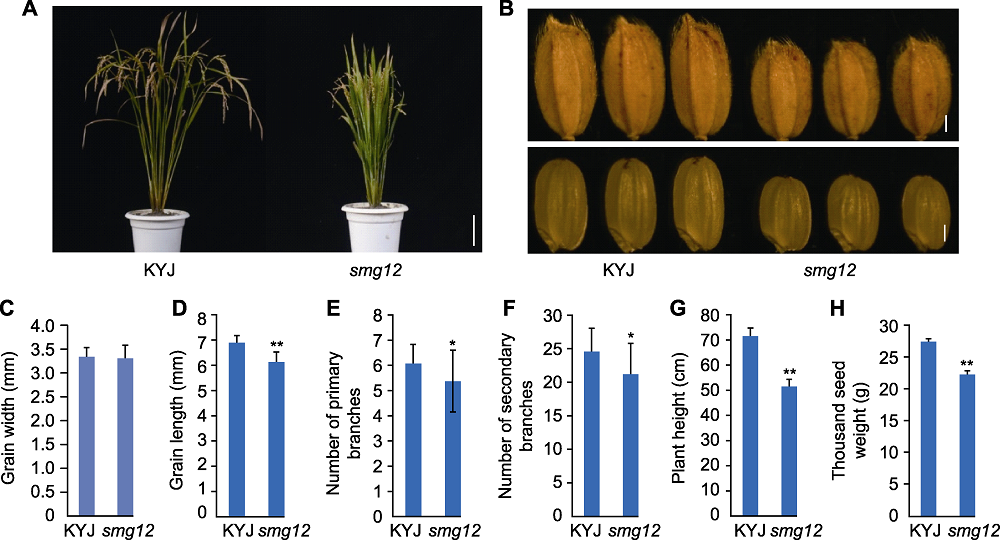
Figure 1 Analysis of rice smg12 mutant phenotypes (A) KYJ (wild type) and smg12 mutant phenotype (Bar=10 cm); (B) Grains of KYJ and smg12 (Bars=1 cm); (C) Grain width; (D) Grain length; (E) Number of primary branches; (F) Number of secondary branches; (G) Plant height; (H) Thousand seed weight. Significance is determined using t-test, * indicates significant differences at P<0.05, ** indicates significant differences at P<0.01.
| Primer name | Primer sequence (5'→3') | DNA fragment length (bp) | Enzyme |
|---|---|---|---|
| smg12-1 | CTTTCTCGGCACTTTCCTTG | 154 | HphI |
| CTATGGTCACATGGTGGCGGTG |
Table 1 Primer and enzyme for dCAPS analysis
| Primer name | Primer sequence (5'→3') | DNA fragment length (bp) | Enzyme |
|---|---|---|---|
| smg12-1 | CTTTCTCGGCACTTTCCTTG | 154 | HphI |
| CTATGGTCACATGGTGGCGGTG |
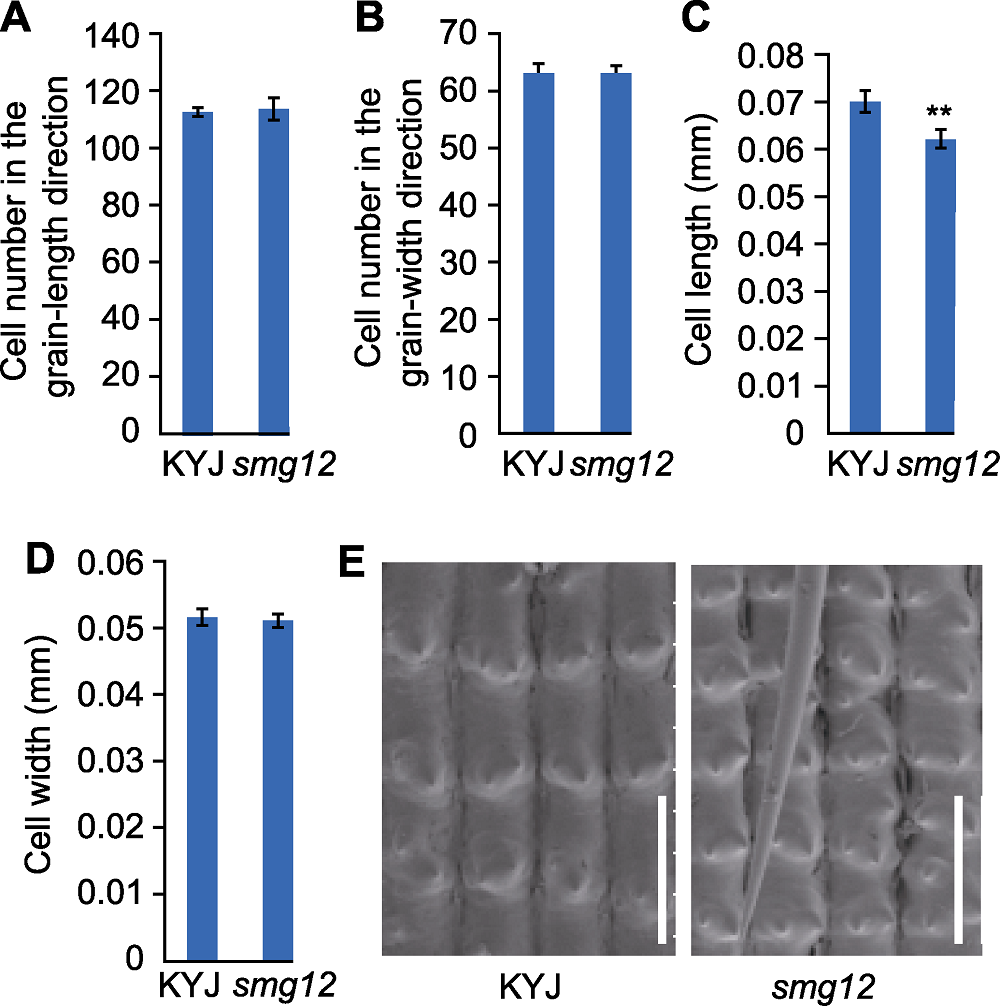
Figure 2 SMG12 regulates cell size in grain hulls of rice (A) Outer epidermal cell number in the grain-length direction of the glume; (B) Outer epidermal cell number in the grain-width direction of the glume; (C) The longitudinal length of a single cell in the glume; (D) Transverse width of a single cell in the glume; (E) Cytological analysis diagram (Bars= 0.1 mm). ** indicates extremely significant difference between the mutant and the wild type (P<0.01).
| Hybrid combinations | Phenotype of F1 | F2 generation | χ23:1 | ||
|---|---|---|---|---|---|
| Wild-phenotypic plant number | Mutant-phenotypic plant number | Total number | |||
| smg12/KYJ | Wild type | 168 | 52 | 220 | 0.151 |
Table 2 Genetic analysis of the rice mutant smg12
| Hybrid combinations | Phenotype of F1 | F2 generation | χ23:1 | ||
|---|---|---|---|---|---|
| Wild-phenotypic plant number | Mutant-phenotypic plant number | Total number | |||
| smg12/KYJ | Wild type | 168 | 52 | 220 | 0.151 |
| Chromosomal position | Physical location (bp) | Genotype (KYJ/smg12) | Gene location | Gene locus name | Frequency of sequencing (KYJ/smg12) | Mutation type |
|---|---|---|---|---|---|---|
| Chr. 1 | 15686971 | G/A | Upstream of the gene | LOC_Os01g28040 | 0/6 | / |
| Chr. 1 | 27750517 | G/A | In the gene compartment | LOC_Os01g48420; LOC_Os01g48430 | 0/12 | / |
| Chr. 1 | 28534819 | G/A | Upstream and downstream of the gene | LOC_Os01g49630; LOC_Os01g49614 | 0/14 | / |
| Chr. 1 | 29397508 | G/A | In the gene compartment | LOC_Os01g51140; LOC_Os01g51154 | 0/12 | / |
| Chr. 1 | 29788896 | G/A | Upstream of the gene | LOC_Os01g51810 | 0/17 | / |
| Chr. 1 | 29929259 | G/A | Exon | LOC_Os01g52050 | 0/19 | Changes in amino acids |
| Chr. 1 | 33892012 | T/A | Upstream of the gene | LOC_Os01g58620 | 0/1 | / |
| Chr. 1 | 39503252 | G/A | Downstream of the gene | LOC_Os01g67980 | 0/11 | / |
| Chr. 1 | 30392222 | G/A | Upstream of the gene | LOC_Os01g52840; LOC_Os01g52851 | 0/5 | / |
| Chr. 1 | 33745383 | G/A | Intron | LOC_Os01g58400 | 0/8 | / |
| Chr. 3 | 19627588 | C/A | Upstream of the gene | LOC_Os03g35390 | 0/6 | / |
| Chr. 6 | 17710551 | G/A | Intron | LOC_Os06g30610 | 0/8 | / |
Table 3 Analysis of the candidate SNPs for the smg12 mutant
| Chromosomal position | Physical location (bp) | Genotype (KYJ/smg12) | Gene location | Gene locus name | Frequency of sequencing (KYJ/smg12) | Mutation type |
|---|---|---|---|---|---|---|
| Chr. 1 | 15686971 | G/A | Upstream of the gene | LOC_Os01g28040 | 0/6 | / |
| Chr. 1 | 27750517 | G/A | In the gene compartment | LOC_Os01g48420; LOC_Os01g48430 | 0/12 | / |
| Chr. 1 | 28534819 | G/A | Upstream and downstream of the gene | LOC_Os01g49630; LOC_Os01g49614 | 0/14 | / |
| Chr. 1 | 29397508 | G/A | In the gene compartment | LOC_Os01g51140; LOC_Os01g51154 | 0/12 | / |
| Chr. 1 | 29788896 | G/A | Upstream of the gene | LOC_Os01g51810 | 0/17 | / |
| Chr. 1 | 29929259 | G/A | Exon | LOC_Os01g52050 | 0/19 | Changes in amino acids |
| Chr. 1 | 33892012 | T/A | Upstream of the gene | LOC_Os01g58620 | 0/1 | / |
| Chr. 1 | 39503252 | G/A | Downstream of the gene | LOC_Os01g67980 | 0/11 | / |
| Chr. 1 | 30392222 | G/A | Upstream of the gene | LOC_Os01g52840; LOC_Os01g52851 | 0/5 | / |
| Chr. 1 | 33745383 | G/A | Intron | LOC_Os01g58400 | 0/8 | / |
| Chr. 3 | 19627588 | C/A | Upstream of the gene | LOC_Os03g35390 | 0/6 | / |
| Chr. 6 | 17710551 | G/A | Intron | LOC_Os06g30610 | 0/8 | / |
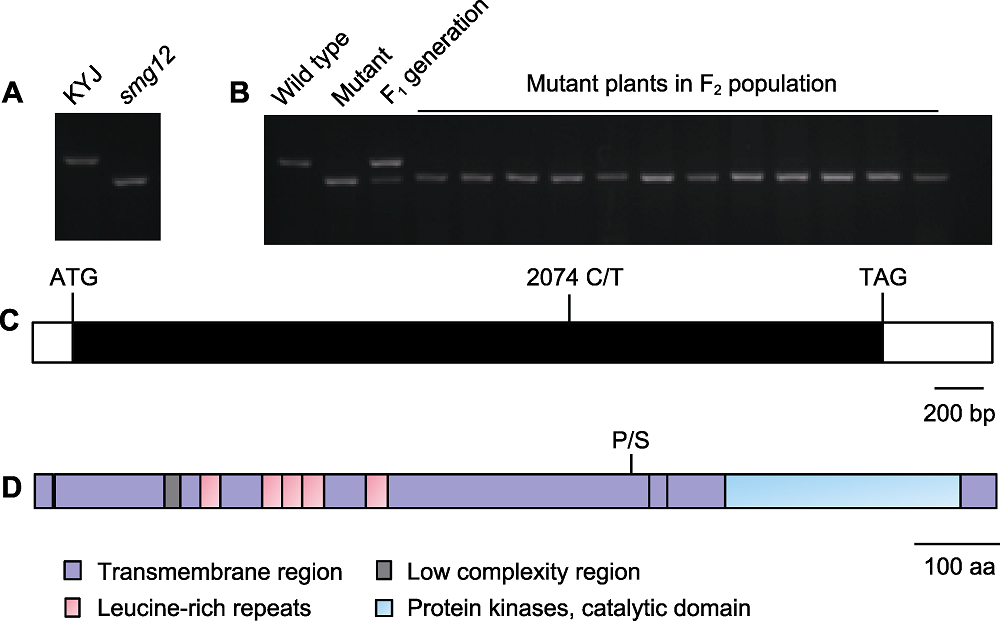
Figure 3 Identification of candidate genes (A) The dCAPS marker was developed to detect the smg12 mutation; (B) Population linkage analysis of the candidate gene LOC_Os01g52050; (C) The LOC_Os01g52050 gene structure, open boxes show the 5' and 3' untranslated regions, the closed box shows the coding sequence, and the start codon (ATG), the stop codon (TAG) and the LOC_Os01g52050 mutation site (C/T) are indicated; (D) Schematic of the LOC_Os01g52050 protein. P/S indicated the LOC_Os01g52050 mutation site.
| [1] | 宫李辉, 高振宇, 马伯军, 钱前 (2011). 水稻粒形遗传的研究进展. 植物学报 46, 597-605. |
| [2] | 侯雷平, 李梅兰 (2001). 油菜素内酯(BR)促进植物生长机理研究进展. 植物学通报 18, 560-566. |
| [3] |
Abe A, Kosugi S, Yoshida K, Natsume S, Takagi H, Kanzaki H, Matsumura H, Yoshida K, Mitsuoka C, Tamiru M, Innan H, Canno L, Kamoun S, Terauchi R (2012). Genome sequencing reveals agronomically important loci in rice using Mutmap. Nat Biotechnol 30, 174-178.
URL PMID |
| [4] |
Fan CC, Xing YZ, Mao HL, Lu TT, Han B, Xu CG, Li XH, Zhang QF (2006). GS3, a major QTL for grain length and weight and minor QTL for grain width and thickness in rice, encodes a putative transmembrane protein. Theor Appl Genet 112, 1164-1171.
URL PMID |
| [5] | James MG, Denyer K, Myers AM (2003). Starch synthesis in the cereal endosperm. Curr Opin Plant Biol 6, 215-222. |
| [6] | Li N, Xu R, Duan PG, Li YH (2018). Control of grain size in rice. Plant Reprod 31, 237-251. |
| [7] |
Li N, Xu R, Li YH (2019). Molecular networks of seed size control in plants. Annu Rev Plant Biol 70, 435-463.
URL PMID |
| [8] |
Liu LC, Tong HN, Xiao YH, Che RH, Xu F, Hu B, Liang CZ, Chu JF, Li JY, Chu CC (2015a). Activation of Big Grain1 significantly improves grain size by regulating auxin transport in rice. Proc Natl Acad Sci USA 112, 11102-11107.
URL PMID |
| [9] |
Liu SY, Hua L, Dong SJ, Chen HQ, Zhu XD, Jiang JE, Zhang F, Li YH, Fang XH, Chen F (2015b). OsMAPK6, a mitogen-activated protein kinase, influences rice grain size and biomass production. Plant J 84, 672-681.
DOI URL PMID |
| [10] | Mao HL, Sun SY, Yao JL, Wang CR, Yu SB, Xu CG, Li XH, Zhang QF (2010). Linking differential domain functions of the GS3 protein to natural variation of grain size in rice. Proc Natl Acad Sci USA 107, 19579-19584. |
| [11] |
Meng XZ, Zhang SQ (2013). MAPK cascades in plant disease resistance signaling. Annu Rev Phytopathol 51, 245-266.
URL PMID |
| [12] |
Miura K, Ashikari M, Matsuoka M (2011). The role of QTLs in the breeding of high-yielding rice. Trends Plant Sci 16, 319-326.
URL PMID |
| [13] | Morinaka Y, Sakamoto T, Inukai Y, Agetsuma M, Kitano H, Ashikari M, Matsuoka M (2006). Morphological alteration caused by brassinosteroid insensitivity increases the biomass and grain production of rice. Plant Physiol 141, 924-931. |
| [14] |
Nakamura A, Fujioka S, Sunohara H, Kamiya N, Hong Z, Inukai Y, Miura K, Takatsuto S, Yoshida S, Ueguchi- Tanaka M, Hasegawa Y, Kitano H, Matsuoka M (2006). The role of OsBRI1 and its homologous genes, OsBRL1 and OsBRL3, in rice. Plant Physiol 140, 580-590.
DOI URL PMID |
| [15] |
Sakamoto T, Matsuoka M (2008). Identifying and exploiting grain yield genes in rice. Curr Opin Plant Biol 11, 209-214.
DOI URL PMID |
| [16] |
Shomura A, Izawa T, Ebana K, Ebitani T, Kanegae H, Konishi S, Yano M (2008). Deletion in a gene associated with grain size increased yields during rice domestication. Nat Genet 40, 1023-1028.
URL PMID |
| [17] | Si LZ, Chen JY, Huang XH, Gong H, Luo JH, Hou QQ, Zhou TY, Lu TT, Zhu JJ, Shangguan YY, Chen EW, Gong CX, Zhao Q, Jing YF, Zhao Y, Li Y, Cui LL, Fan DL, Lu YQ, Weng QJ, Wang YC, Zhan QL, Liu KY, Wei XH, An K, An G, Han B (2016). OsSPL13 controls grain size in cultivated rice. Nat Genet 48, 447-456. |
| [18] |
Song XJ, Huang W, Shi M, Zhu MZ, Lin HX (2007). A QTL for rice grain width and weight encodes a previously unknown RING-type E3 ubiquitin ligase. Nat Genet 39, 623-630.
DOI URL PMID |
| [19] |
Takagi H, Tamiru M, Abe A, Yoshida K, Uemura A, Yaegashi H, Obara T, Oikawa K, Utsushi H, Kanzaki E, Mitsuoka C, Natsume S, Kosugi S, Kanzaki H, Matsumura H, Urasaki N, Kamoun S, Terauchi R (2015). Mutmap accelerates breeding of a salt-tolerant rice cultivar. Nat Biotechnol 33, 445-449.
URL PMID |
| [20] |
Tanabe S, Ashikari M, Fujioka S, Takatsuto S, Yoshida S, Yano M, Yoshimura A, Kotano H, Matsuoka M, Fujisawa Y, Kato H, Lwasaki Y (2005). A novel cytochrome P450 is implicated in brassinosteroid biosynthesis via the characterization of a rice dwarf mutant, dwarf11, with reduced seed length. Plant Cell 17, 776-790.
DOI URL PMID |
| [21] |
Tanaka A, Nakagawa H, Tomita C, Shimatani Z, Ohtake M, Nomura T, Jiang CJ, Dubozet JG, Kikuchi S, Sekimoto H, Yokota T, Asami T, Kamakura T, Mori M (2009). BRASSINOSTEROID UPREGULATED1, encoding a helix-loop-helix protein, is a novel gene involved in brassinosteroid signaling and controls bending of the lamina joint in rice. Plant Physiol 151, 669-680.
DOI URL PMID |
| [22] |
Tong HN, Liu LC, Jin Y, Du L, Yin YH, Qian Q, Zhu LH, Chu CC (2012). DWARF AND LOW-TILLERING acts as a direct downstream target of a GSK3/SHAGGY-like kinase to mediate brassinosteroid responses in rice. Plant Cell 24, 2562-2577.
URL PMID |
| [23] |
Wang SK, Wu K, Yuan QB, Liu XY, Liu ZB, Lin XY, Zeng RZ, Zhu HT, Dong GJ, Qian Q, Zhang GQ, Fu XD (2012). Control of grain size, shape and quality by OsSPL16 in rice. Nat Genet 44, 950-954.
DOI URL PMID |
| [24] | Xing YZ, Zhang QF (2010). Genetic and molecular bases of rice yield. Annu Rev Plant Biol 61, 421-442. |
| [25] | Xu R, Duan PG, Yu HY, Zhou ZK, Zhang BL, Wang RC, Li J, Zhang GZ, Zhuang SS, Lyu J, Li N, Chai TY, Tian ZX, Yao SG, Li YH (2018a). Control of grain size and weight by the OsMKKK10-OsMKK4-OsMAPK6 signaling pathway in rice. Mol Plant 11, 860-873. |
| [26] |
Xu R, Yu HY, Wang JM, Duan PG, Zhang BL, Li J, Li Y, Xu JS, Lyu J, Li N, Chai TY, Li YH (2018b). A mitogen-activated protein kinase phosphatase influences grain size and weight in rice. Plant J 95, 937-946.
DOI URL PMID |
| [27] | Zhang C, Bai MY, Chong K (2014). Brassinosteroid-mediated regulation of agronomic traits in rice. Plant Cell Rep 33, 683-696. |
| [28] |
Zhao JF, Wu CX, Yuan SJ, Yin L, Sun W, Zhao QL, Zhao BH, Li XY (2013). Kinase activity of OsBRI1 is essential for brassinosteroids to regulate rice growth and development. Plant Sci 199-200, 113-120.
DOI URL PMID |
| [29] |
Zhou SR, Yin LL, Xue HW (2013). Functional genomics based understanding of rice endosperm development. Curr Opin Plant Biol 16, 236-246.
URL PMID |
| [30] | Zuo JR, Li JY (2014). Molecular genetic dissection of quantitative trait loci regulating rice grain size. Annu Rev Genet 48, 99-118. |
| [1] |
Juan Cui, Xiaoyu Yu, Yuejiao Yu, Chengwei Liang, Jian Sun, Wenfu Chen.
Analysis of Texture Factors and Genetic Basis Influencing the Differences in Eating Quality between Northeast China and Japanese Japonica Rice [J]. Chinese Bulletin of Botany, 2025, 60(4): 1-0. |
| [2] | Zhao Ling, Guan Ju, Liang Wenhua, Zhang Yong, Lu Kai, Zhao Chunfang, Li Yusheng, Zhang Yadong. Mapping of QTLs for Heat Tolerance at the Seedling Stage in Rice Based on a High-density Bin Map [J]. Chinese Bulletin of Botany, 2025, 60(3): 342-353. |
| [3] | Xinyu Li, Yue Gu, Feifei Xu, Jinsong Bao. Research Progress on Post-translational Modifications of Starch Biosynthesis-related Proteins in Rice Endosperm [J]. Chinese Bulletin of Botany, 2025, 60(2): 256-270. |
| [4] | Jianguo Li, Yi Zhang, Wenjun Zhang. Iron Plaque Formation and Its Effects on Phosphorus Absorption in Rice Roots [J]. Chinese Bulletin of Botany, 2025, 60(1): 132-143. |
| [5] | Ruifeng Yao, Daoxin Xie. Activation and Termination of Strigolactone Signal Perception in Rice [J]. Chinese Bulletin of Botany, 2024, 59(6): 873-877. |
| [6] | Jinjin Lian, Luyao Tang, Yinuo Zhang, Jiaxing Zheng, Chaoyu Zhu, Yuhan Ye, Yuexing Wang, Wennan Shang, Zhenghao Fu, Xinxuan Xu, Richeng Wu, Mei Lu, Changchun Wang, Yuchun Rao. Genetic Locus Mining and Candidate Gene Analysis of Antioxidant Traits in Rice [J]. Chinese Bulletin of Botany, 2024, 59(5): 738-751. |
| [7] | Jiahui Huang, Huimin Yang, Xinyu Chen, Chaoyu Zhu, Yanan Jiang, Chengxiang Hu, Jinjin Lian, Tao Lu, Mei Lu, Weilin Zhang, Yuchun Rao. Response Mechanism of Rice Mutant pe-1 to Low Light Stress [J]. Chinese Bulletin of Botany, 2024, 59(4): 574-584. |
| [8] | Jianmin Zhou. A Combat Vehicle with a Smart Brake [J]. Chinese Bulletin of Botany, 2024, 59(3): 343-346. |
| [9] | Chaoyu Zhu, Chengxiang Hu, Zhenan Zhu, Zhining Zhang, Lihai Wang, Jun Chen, Sanfeng Li, Jinjin Lian, Luyao Tang, Qianqian Zhong, Wenjing Yin, Yuexing Wang, Yuchun Rao. Mapping of QTLs Associated with Rice Panicle Traits and Candidate Gene Analysis [J]. Chinese Bulletin of Botany, 2024, 59(2): 217-230. |
| [10] | Bao Zhu, Jiangzhe Zhao, Kewei Zhang, Peng Huang. OsCKX9 is Involved in Regulating the Rice Lamina Joint Development and Leaf Angle [J]. Chinese Bulletin of Botany, 2024, 59(1): 10-21. |
| [11] | Yanli Fang, Chuanyu Tian, Ruyi Su, Yapei Liu, Chunlian Wang, Xifeng Chen, Wei Guo, Zhiyuan Ji. Mining and Preliminary Mapping of Rice Resistance Genes Against Bacterial Leaf Streak [J]. Chinese Bulletin of Botany, 2024, 59(1): 1-9. |
| [12] | Tian Chuanyu, Fang Yanli, Shen Qing, Wang Hongjie, Chen Xifeng, Guo Wei, Zhao Kaijun, Wang Chunlian, Ji Zhiyuan. Genotypic Diversity and Pathogenisity of Xanthomonas oryzae pv. oryzae Isolated from Southern China in 2019-2021 [J]. Chinese Bulletin of Botany, 2023, 58(5): 743-749. |
| [13] | Dai Ruohui, Qian Xinyu, Sun Jinglei, Lu Tao, Jia Qiwei, Lu Tianqi, Lu Mei, Rao Yuchun. Research Progress on the Mechanisms of Leaf Color Regulation and Related Genes in Rice [J]. Chinese Bulletin of Botany, 2023, 58(5): 799-812. |
| [14] | Jingjing Zhao, Haibin Jia, Tien Ming Lee. Market status and the sustainable utilization strategy of wild earthworm (earth dragon) for medicinal use [J]. Biodiv Sci, 2023, 31(3): 22478-. |
| [15] | Shang Sun, Yingying Hu, Yangshuo Han, Chao Xue, Zhiyun Gong. Double-stranded Labelled Oligo-FISH in Rice Chromosomes [J]. Chinese Bulletin of Botany, 2023, 58(3): 433-439. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||