

Chinese Bulletin of Botany ›› 2025, Vol. 60 ›› Issue (4): 597-610.DOI: 10.11983/CBB24151 cstr: 32102.14.CBB24151
• TECHNIQUES AND METHODS • Previous Articles Next Articles
Jingjing Li†, Yanfei Li†, Anqi Wang, Jiaying Wang, Chengyan Deng, Min Lu, Jianying Ma, Silan Dai*( )
)
Received:2024-10-10
Accepted:2025-01-20
Online:2025-07-10
Published:2025-01-21
Contact:
E-mail: silandai@sina.com
About author:†These authors contributed equally to this paper
Jingjing Li, Yanfei Li, Anqi Wang, Jiaying Wang, Chengyan Deng, Min Lu, Jianying Ma, Silan Dai. Establishment of Regeneration and Genetic Transformation System for Chrysanthemum × morifolium ‘Wandai Fengguang’[J]. Chinese Bulletin of Botany, 2025, 60(4): 597-610.
| No. | 6-BA (mg∙L-1) | KT (mg∙L-1) | NAA (mg∙L-1) | 2,4-D (mg∙L-1) |
|---|---|---|---|---|
| A1 | 1.5 | 0.0 | 0.2 | 0.0 |
| A2 | 1.5 | 0.0 | 0.4 | 0.0 |
| A3 | 1.5 | 0.0 | 0.6 | 0.0 |
| A4 | 1.5 | 0.0 | 0.0 | 0.2 |
| A5 | 1.5 | 0.0 | 0.0 | 0.4 |
| A6 | 1.5 | 0.0 | 0.0 | 0.6 |
| A7 | 0.0 | 1.5 | 0.2 | 0.0 |
| A8 | 0.0 | 1.5 | 0.4 | 0.0 |
| A9 | 0.0 | 1.5 | 0.6 | 0.0 |
| A10 | 0.0 | 1.5 | 0.0 | 0.2 |
| A11 | 0.0 | 1.5 | 0.0 | 0.4 |
| A12 | 0.0 | 1.5 | 0.0 | 0.6 |
Table 1 Culture media for callus induction and adventitious bud differentiation
| No. | 6-BA (mg∙L-1) | KT (mg∙L-1) | NAA (mg∙L-1) | 2,4-D (mg∙L-1) |
|---|---|---|---|---|
| A1 | 1.5 | 0.0 | 0.2 | 0.0 |
| A2 | 1.5 | 0.0 | 0.4 | 0.0 |
| A3 | 1.5 | 0.0 | 0.6 | 0.0 |
| A4 | 1.5 | 0.0 | 0.0 | 0.2 |
| A5 | 1.5 | 0.0 | 0.0 | 0.4 |
| A6 | 1.5 | 0.0 | 0.0 | 0.6 |
| A7 | 0.0 | 1.5 | 0.2 | 0.0 |
| A8 | 0.0 | 1.5 | 0.4 | 0.0 |
| A9 | 0.0 | 1.5 | 0.6 | 0.0 |
| A10 | 0.0 | 1.5 | 0.0 | 0.2 |
| A11 | 0.0 | 1.5 | 0.0 | 0.4 |
| A12 | 0.0 | 1.5 | 0.0 | 0.6 |

Figure 1 The structure of plant expression vector pBI121 RB: Right border of T-DNA region; NOS-pro: Agrobacterium tumefaciens nopaline synthase gene promoter; KanR: Kanamycin resistance screening marker gene; NOS-ter: A. tumefaciens nopaline synthase gene terminator; CaMV35S-pro: Cauliflower mosaic virus 35S promoter; CcVIT: Centaurea cyanus vacuolar iron transport protein gene; LB: Left border of T-DNA region
| Treatments | Pre-cul- ture time (d) | Concentration of Agrobacterium tumefaciens (OD600) | Infection time (min) | Co-culture time (d) |
|---|---|---|---|---|
| 1 | 0 | 0.6 | 5 | 1 |
| 2 | 0 | 0.8 | 10 | 2 |
| 3 | 0 | 1.0 | 15 | 3 |
| 4 | 1 | 0.6 | 10 | 3 |
| 5 | 1 | 0.8 | 15 | 1 |
| 6 | 1 | 1.0 | 5 | 2 |
| 7 | 2 | 0.6 | 15 | 2 |
| 8 | 2 | 0.8 | 5 | 3 |
| 9 | 2 | 1.0 | 10 | 1 |
Table 2 Orthogonal experimental table for genetic transformation screening of Chrysanthemum × morifolium ‘Wandai Fengguang’
| Treatments | Pre-cul- ture time (d) | Concentration of Agrobacterium tumefaciens (OD600) | Infection time (min) | Co-culture time (d) |
|---|---|---|---|---|
| 1 | 0 | 0.6 | 5 | 1 |
| 2 | 0 | 0.8 | 10 | 2 |
| 3 | 0 | 1.0 | 15 | 3 |
| 4 | 1 | 0.6 | 10 | 3 |
| 5 | 1 | 0.8 | 15 | 1 |
| 6 | 1 | 1.0 | 5 | 2 |
| 7 | 2 | 0.6 | 15 | 2 |
| 8 | 2 | 0.8 | 5 | 3 |
| 9 | 2 | 1.0 | 10 | 1 |
| No. | Leaf | Petioles | Transverse thin cell layers | |||
|---|---|---|---|---|---|---|
| Differentiation rate (%) | Coefficient of adventitious bud production | Differentiation rate (%) | Coefficient of adventitious bud production | Differentiation rate (%) | Coefficient of adventitious bud production | |
| A1 | 0.00±0.00 c | 0.00±0.00 c | 57.32±6.87 a | 1.85±0.29 a | 36.51±5.50 c | 3.37±0.28 a |
| A2 | 0.00±0.00 c | 0.00±0.00 c | 0.00±0.00 e | 0.00±0.00 c | 42.79±4.28 b | 2.53±0.32 b |
| A3 | 0.00±0.00 c | 0.00±0.00 c | 0.00±0.00 e | 0.00±0.00 c | 70.06±2.84 a | 3.37±0.22 a |
| A4 | 0.00±0.00 c | 0.00±0.00 c | 0.00±0.00 e | 0.00±0.00 c | 0.00±0.00 f | 0.00±0.00 g |
| A5 | 0.00±0.00 c | 0.00±0.00 c | 0.00±0.00 e | 0.00±0.00 c | 9.79±2.29 e | 1.11±0.19 ef |
| A6 | 0.00±0.00 c | 0.00±0.00 c | 0.00±0.00 e | 0.00±0.00 c | 0.00±0.00 f | 0.00±0.00 g |
| A7 | 0.00±0.00 c | 0.00±0.00 c | 36.35±3.38 b | 1.62±0.60 a | 45.93±1.28 b | 1.51±0.31 d |
| A8 | 8.59±0.44 b | 1.00±0.00 b | 0.00±0.00 e | 0.00±0.00 c | 41.95±1.88 b | 1.62±0.21 d |
| A9 | 19.44±4.81 a | 1.28±0.25 a | 22.56±4.55 c | 1.11±0.19 b | 46.30±3.21 b | 2.73±0.22 b |
| A10 | 8.33±0.00 b | 1.00±0.00 b | 0.00±0.00 e | 0.00±0.00 c | 31.31±3.50 c | 1.44±0.27 de |
| A11 | 0.00±0.00 c | 0.00±0.00 c | 0.00±0.00 e | 0.00±0.00 c | 16.67±0.00 d | 1.00±0.00 f |
| A12 | 0.00±0.00 c | 0.00±0.00 c | 8.33±0.00 d | 1.00±0.00 b | 34.98±3.90 c | 2.07±0.12 c |
Table 3 Effects of different plant growth regulators combinations on the regeneration of different explants of Chrysanthemum × morifolium ‘Wandai Fengguang’
| No. | Leaf | Petioles | Transverse thin cell layers | |||
|---|---|---|---|---|---|---|
| Differentiation rate (%) | Coefficient of adventitious bud production | Differentiation rate (%) | Coefficient of adventitious bud production | Differentiation rate (%) | Coefficient of adventitious bud production | |
| A1 | 0.00±0.00 c | 0.00±0.00 c | 57.32±6.87 a | 1.85±0.29 a | 36.51±5.50 c | 3.37±0.28 a |
| A2 | 0.00±0.00 c | 0.00±0.00 c | 0.00±0.00 e | 0.00±0.00 c | 42.79±4.28 b | 2.53±0.32 b |
| A3 | 0.00±0.00 c | 0.00±0.00 c | 0.00±0.00 e | 0.00±0.00 c | 70.06±2.84 a | 3.37±0.22 a |
| A4 | 0.00±0.00 c | 0.00±0.00 c | 0.00±0.00 e | 0.00±0.00 c | 0.00±0.00 f | 0.00±0.00 g |
| A5 | 0.00±0.00 c | 0.00±0.00 c | 0.00±0.00 e | 0.00±0.00 c | 9.79±2.29 e | 1.11±0.19 ef |
| A6 | 0.00±0.00 c | 0.00±0.00 c | 0.00±0.00 e | 0.00±0.00 c | 0.00±0.00 f | 0.00±0.00 g |
| A7 | 0.00±0.00 c | 0.00±0.00 c | 36.35±3.38 b | 1.62±0.60 a | 45.93±1.28 b | 1.51±0.31 d |
| A8 | 8.59±0.44 b | 1.00±0.00 b | 0.00±0.00 e | 0.00±0.00 c | 41.95±1.88 b | 1.62±0.21 d |
| A9 | 19.44±4.81 a | 1.28±0.25 a | 22.56±4.55 c | 1.11±0.19 b | 46.30±3.21 b | 2.73±0.22 b |
| A10 | 8.33±0.00 b | 1.00±0.00 b | 0.00±0.00 e | 0.00±0.00 c | 31.31±3.50 c | 1.44±0.27 de |
| A11 | 0.00±0.00 c | 0.00±0.00 c | 0.00±0.00 e | 0.00±0.00 c | 16.67±0.00 d | 1.00±0.00 f |
| A12 | 0.00±0.00 c | 0.00±0.00 c | 8.33±0.00 d | 1.00±0.00 b | 34.98±3.90 c | 2.07±0.12 c |
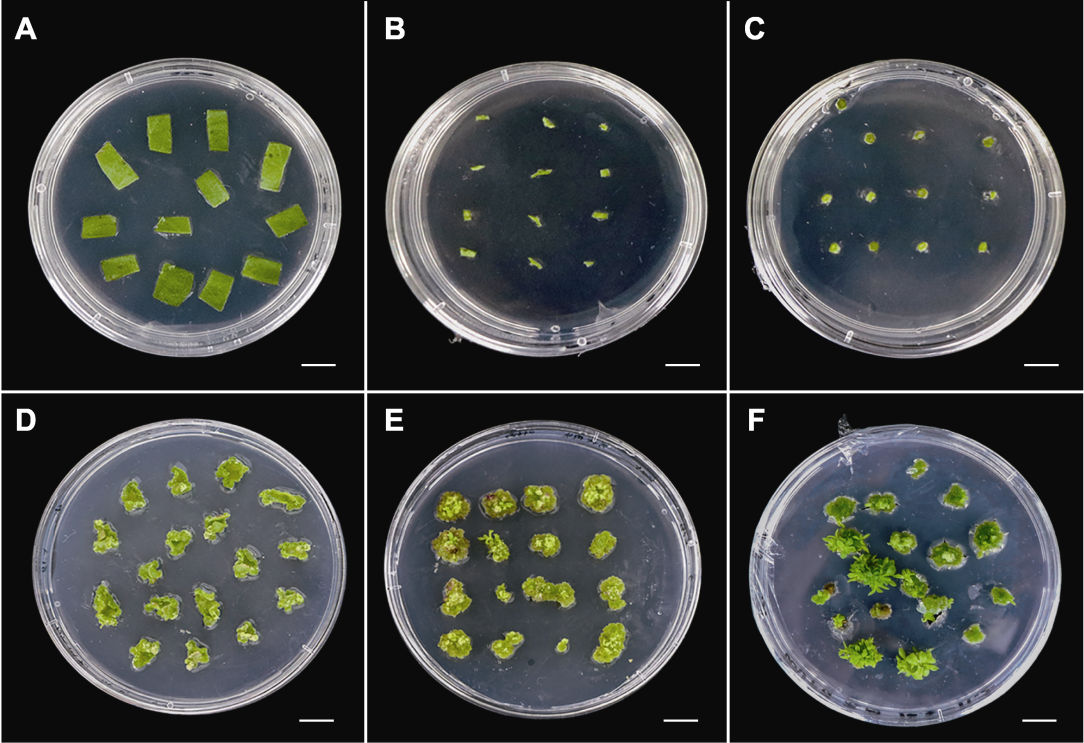
Figure 2 Regeneration of different explants of Chrysanthemum × morifolium ‘Wandai Fengguang’ (A)-(C) State of leaf, petioles, and transverse thin cell layers cultured in A7 medium (Table 1) for 0 day; (D)-(F) Regeneration of leaf, petioles, and transverse thin cell layers cultured in A7 medium for 35 days. Bars=1 cm
| Concentration of kanamycin (mg∙L-1) | Callus formation rate (%) | Differentiation rate (%) |
|---|---|---|
| 0 | 97.22±4.81 ab | 69.44±12.73 a |
| 2.5 | 100.00±0.00 a | 16.67±8.34 b |
| 5 | 80.44±4.72 bc | 5.56±4.81 bc |
| 7.5 | 63.89±12.73 cd | 0.00±0.00 c |
| 10 | 50.00±8.33 de | 0.00±0.00 c |
| 15 | 33.33±16.67 e | 0.00±0.00 c |
Table 4 Effect of kanamycin on transverse thin cell layers of Chrysanthemum × morifolium ‘Wandai Fengguang’
| Concentration of kanamycin (mg∙L-1) | Callus formation rate (%) | Differentiation rate (%) |
|---|---|---|
| 0 | 97.22±4.81 ab | 69.44±12.73 a |
| 2.5 | 100.00±0.00 a | 16.67±8.34 b |
| 5 | 80.44±4.72 bc | 5.56±4.81 bc |
| 7.5 | 63.89±12.73 cd | 0.00±0.00 c |
| 10 | 50.00±8.33 de | 0.00±0.00 c |
| 15 | 33.33±16.67 e | 0.00±0.00 c |
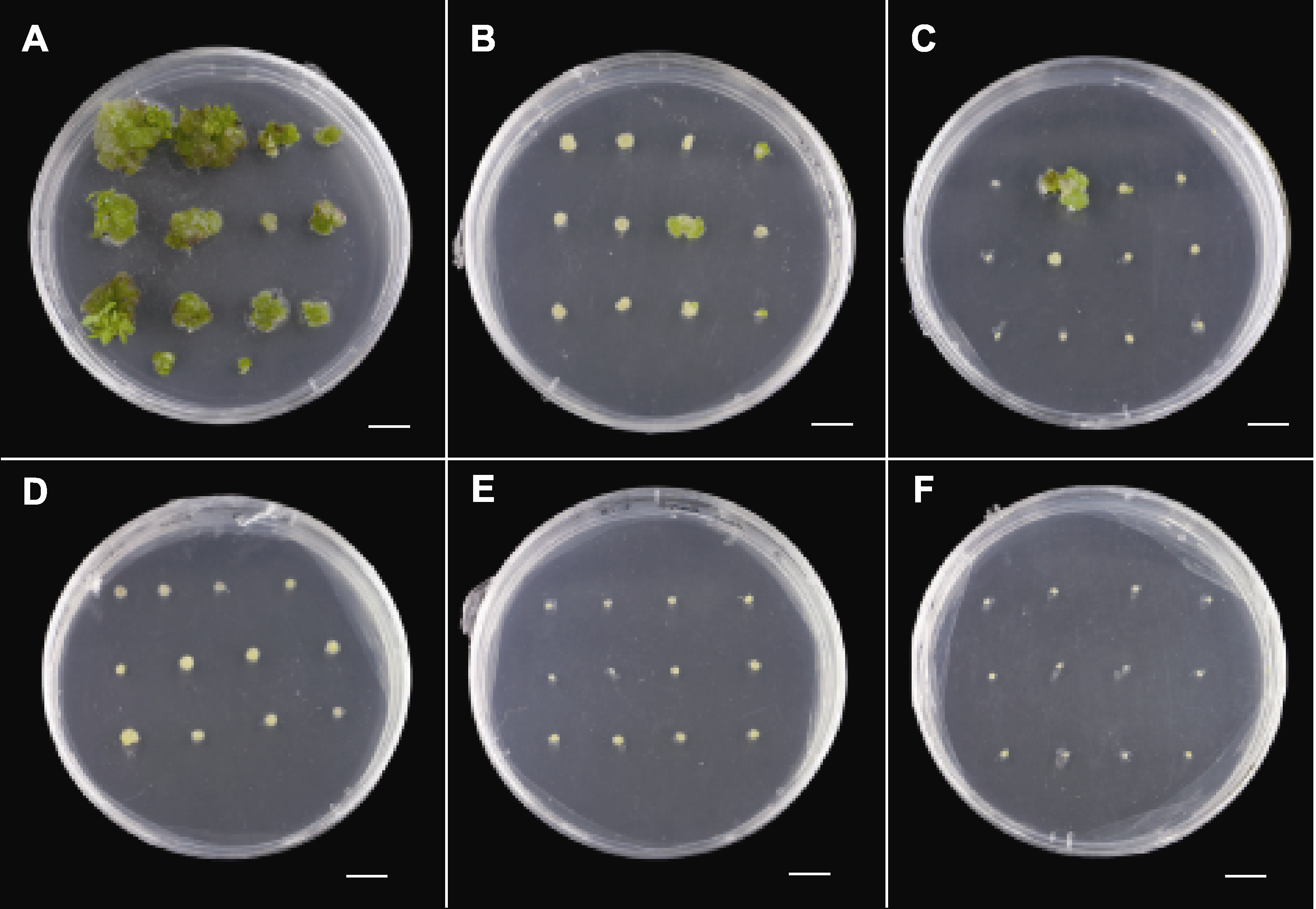
Figure 3 Effect of kanamycin on transverse thin cell layers of Chrysanthemum × morifolium ‘Wandai Fengguang’ (A)-(F) Growth of transverse thin cell layers in A3 culture medium (Table 1) containing 0, 2.5, 5, 7.5, 10, and 15 mg∙L-1 kanamycin. Bars=1 cm
| Concentration of kanamycin (mg∙L-1) | Rooting rate (%) | Average number of roots |
|---|---|---|
| 0 | 88.89±19.25 a | 3.56±1.02 a |
| 3 | 30.44±4.72 b | 0.50±0.17 b |
| 5 | 0.00±0.00 c | 0.00±0.00 b |
| 7.5 | 0.00±0.00 c | 0.00±0.00 b |
| 10 | 0.00±0.00 c | 0.00±0.00 b |
| 12 | 0.00±0.00 c | 0.00±0.00 b |
Table 5 Effect of kanamycin on rooting of adventitious bud of Chrysanthemum × morifolium ‘Wandai Fengguang’
| Concentration of kanamycin (mg∙L-1) | Rooting rate (%) | Average number of roots |
|---|---|---|
| 0 | 88.89±19.25 a | 3.56±1.02 a |
| 3 | 30.44±4.72 b | 0.50±0.17 b |
| 5 | 0.00±0.00 c | 0.00±0.00 b |
| 7.5 | 0.00±0.00 c | 0.00±0.00 b |
| 10 | 0.00±0.00 c | 0.00±0.00 b |
| 12 | 0.00±0.00 c | 0.00±0.00 b |

Figure 4 Effect of kanamycin on rooting of adventitious bud of Chrysanthemum × morifolium ‘Wandai Fengguang’ (A)-(F) Rooting of adventitious buds in MS culture media containing 0, 3, 5, 7.5, 10, and 12 mg∙L-1 kanamycin. Bars=1 cm
| Treatments | Callus formation rate (%) | Differentiation rate (%) | Coefficient of adventitious bud production | Browning rate (%) |
|---|---|---|---|---|
| 1 | 80.91±10.48 bc | 3.33±5.77 c | 0.33±0.58 b | 0.00±0.00 c |
| 2 | 94.84±4.51 a | 0.00±0.00 c | 0.00±0.00 b | 0.00±0.00 c |
| 3 | 86.96±5.54 b | 1.85±3.21 c | 0.33±0.58 b | 0.00±0.00 c |
| 4 | 71.11±7.70 cd | 0.00±0.00 c | 0.00±0.00 b | 0.00±0.00 c |
| 5 | 79.55±9.91 bc | 8.84±0.44 bc | 1.33±0.58 a | 0.00±0.00 c |
| 6 | 64.07±7.56 d | 14.44±6.76 ab | 1.83±1.04 a | 28.52±5.70 a |
| 7 | 81.85±3.61 bc | 0.00±0.00 c | 0.00±0.00 b | 18.15±3.61 b |
| 8 | 95.96±3.51 a | 21.00±9.78 a | 1.38±0.40 a | 0.00±0.00 c |
| 9 | 96.08±6.79 a | 1.96±3.40 c | 0.33±0.58 b | 0.00±0.00 c |
Table 6 Effect of different transformation conditions on the growth of resistant buds of Chrysanthemum × morifolium ‘Wandai Fengguang’
| Treatments | Callus formation rate (%) | Differentiation rate (%) | Coefficient of adventitious bud production | Browning rate (%) |
|---|---|---|---|---|
| 1 | 80.91±10.48 bc | 3.33±5.77 c | 0.33±0.58 b | 0.00±0.00 c |
| 2 | 94.84±4.51 a | 0.00±0.00 c | 0.00±0.00 b | 0.00±0.00 c |
| 3 | 86.96±5.54 b | 1.85±3.21 c | 0.33±0.58 b | 0.00±0.00 c |
| 4 | 71.11±7.70 cd | 0.00±0.00 c | 0.00±0.00 b | 0.00±0.00 c |
| 5 | 79.55±9.91 bc | 8.84±0.44 bc | 1.33±0.58 a | 0.00±0.00 c |
| 6 | 64.07±7.56 d | 14.44±6.76 ab | 1.83±1.04 a | 28.52±5.70 a |
| 7 | 81.85±3.61 bc | 0.00±0.00 c | 0.00±0.00 b | 18.15±3.61 b |
| 8 | 95.96±3.51 a | 21.00±9.78 a | 1.38±0.40 a | 0.00±0.00 c |
| 9 | 96.08±6.79 a | 1.96±3.40 c | 0.33±0.58 b | 0.00±0.00 c |

Figure 5 Genetic transformation screening of Chrysanthemum × morifolium ‘Wandai Fengguang’ (A)-(I) Growth of transverse thin cell layers in treatments 1-9 (see Table 2). Bars=1 cm
| Pre-cultivation time (d) | Callus formation rate (%) | Differentiation rate (%) | Coefficient of adventitious bud production | Brow- ning rate (%) |
|---|---|---|---|---|
| 0 | 87.57 a | 1.73 a | 0.22 b | 0.00 a |
| 1 | 71.58 b | 7.76 a | 1.06 a | 9.51 a |
| 2 | 91.29 a | 7.66 a | 0.57 ab | 6.05 a |
Table 7 Effect of pre-culture time on the growth of resistant buds in Chrysanthemum × morifolium ‘Wandai Fengguang’
| Pre-cultivation time (d) | Callus formation rate (%) | Differentiation rate (%) | Coefficient of adventitious bud production | Brow- ning rate (%) |
|---|---|---|---|---|
| 0 | 87.57 a | 1.73 a | 0.22 b | 0.00 a |
| 1 | 71.58 b | 7.76 a | 1.06 a | 9.51 a |
| 2 | 91.29 a | 7.66 a | 0.57 ab | 6.05 a |
| Concentration of Agrobacterium (OD600) | Callus formation rate (%) | Differentiation rate (%) | Coefficient of adventitious bud production | Browning rate (%) |
|---|---|---|---|---|
| 0.6 | 77.96 b | 1.11 b | 0.11 b | 6.05 a |
| 0.8 | 90.11 a | 9.95 a | 0.90 a | 0.00 a |
| 1.0 | 82.37 ab | 6.09 ab | 0.83 ab | 9.51 a |
Table 8 Effect of Agrobacterium concentration on the growth of resistant buds in Chrysanthemum × morifolium ‘Wandai Fengguang’
| Concentration of Agrobacterium (OD600) | Callus formation rate (%) | Differentiation rate (%) | Coefficient of adventitious bud production | Browning rate (%) |
|---|---|---|---|---|
| 0.6 | 77.96 b | 1.11 b | 0.11 b | 6.05 a |
| 0.8 | 90.11 a | 9.95 a | 0.90 a | 0.00 a |
| 1.0 | 82.37 ab | 6.09 ab | 0.83 ab | 9.51 a |
| Infection time (min) | Callus formation rate (%) | Differentiation rate (%) | Coefficient of adventitious bud production | Browning rate (%) |
|---|---|---|---|---|
| 5 | 80.31 a | 12.93 a | 1.18 a | 9.51 a |
| 10 | 87.34 a | 0.65 b | 0.11 b | 0.00 a |
| 15 | 82.78 a | 3.56 b | 0.56 ab | 6.05 a |
Table 9 Effect of infection time on the growth of resistant buds in Chrysanthemum × morifolium ‘Wandai Fengguang’
| Infection time (min) | Callus formation rate (%) | Differentiation rate (%) | Coefficient of adventitious bud production | Browning rate (%) |
|---|---|---|---|---|
| 5 | 80.31 a | 12.93 a | 1.18 a | 9.51 a |
| 10 | 87.34 a | 0.65 b | 0.11 b | 0.00 a |
| 15 | 82.78 a | 3.56 b | 0.56 ab | 6.05 a |
| Co-culture time (d) | Callus formation rate (%) | Differentiation rate (%) | Coefficient of adventitious bud production | Browning rate (%) |
|---|---|---|---|---|
| 1 | 85.51 a | 4.71 a | 0.67 a | 0.00 b |
| 2 | 80.25 a | 4.81 a | 0.61 a | 15.56 a |
| 3 | 84.68 a | 7.62 a | 0.57 a | 0.00 b |
Table 10 Effect of co-culture time on the growth of resistant buds in Chrysanthemum × morifolium ‘Wandai Fengguang’
| Co-culture time (d) | Callus formation rate (%) | Differentiation rate (%) | Coefficient of adventitious bud production | Browning rate (%) |
|---|---|---|---|---|
| 1 | 85.51 a | 4.71 a | 0.67 a | 0.00 b |
| 2 | 80.25 a | 4.81 a | 0.61 a | 15.56 a |
| 3 | 84.68 a | 7.62 a | 0.57 a | 0.00 b |
| Factors | Pre-cultivation time (d) | Concentration of Agrobacterium tumefaciens (OD600) | Infectation time (min) | Co-culture time (d) |
|---|---|---|---|---|
| K1 | 5.18 | 3.33 | 38.77 | 14.13 |
| K2 | 23.28 | 29.84 | 1.96 | 14.44 |
| K3 | 22.96 | 18.25 | 10.69 | 22.85 |
| k1 | 1.73 | 1.11 | 12.92 | 4.71 |
| k2 | 7.76 | 9.95 | 0.65 | 4.81 |
| k3 | 7.65 | 6.08 | 3.56 | 7.62 |
| R | 5.93 | 8.84 | 12.27 | 2.80 |
Table 11 Range analysis of resistance bud differentiation rate of Chrysanthemum × morifolium ‘Wandai Fengguang’ under different transformation conditions
| Factors | Pre-cultivation time (d) | Concentration of Agrobacterium tumefaciens (OD600) | Infectation time (min) | Co-culture time (d) |
|---|---|---|---|---|
| K1 | 5.18 | 3.33 | 38.77 | 14.13 |
| K2 | 23.28 | 29.84 | 1.96 | 14.44 |
| K3 | 22.96 | 18.25 | 10.69 | 22.85 |
| k1 | 1.73 | 1.11 | 12.92 | 4.71 |
| k2 | 7.76 | 9.95 | 0.65 | 4.81 |
| k3 | 7.65 | 6.08 | 3.56 | 7.62 |
| R | 5.93 | 8.84 | 12.27 | 2.80 |
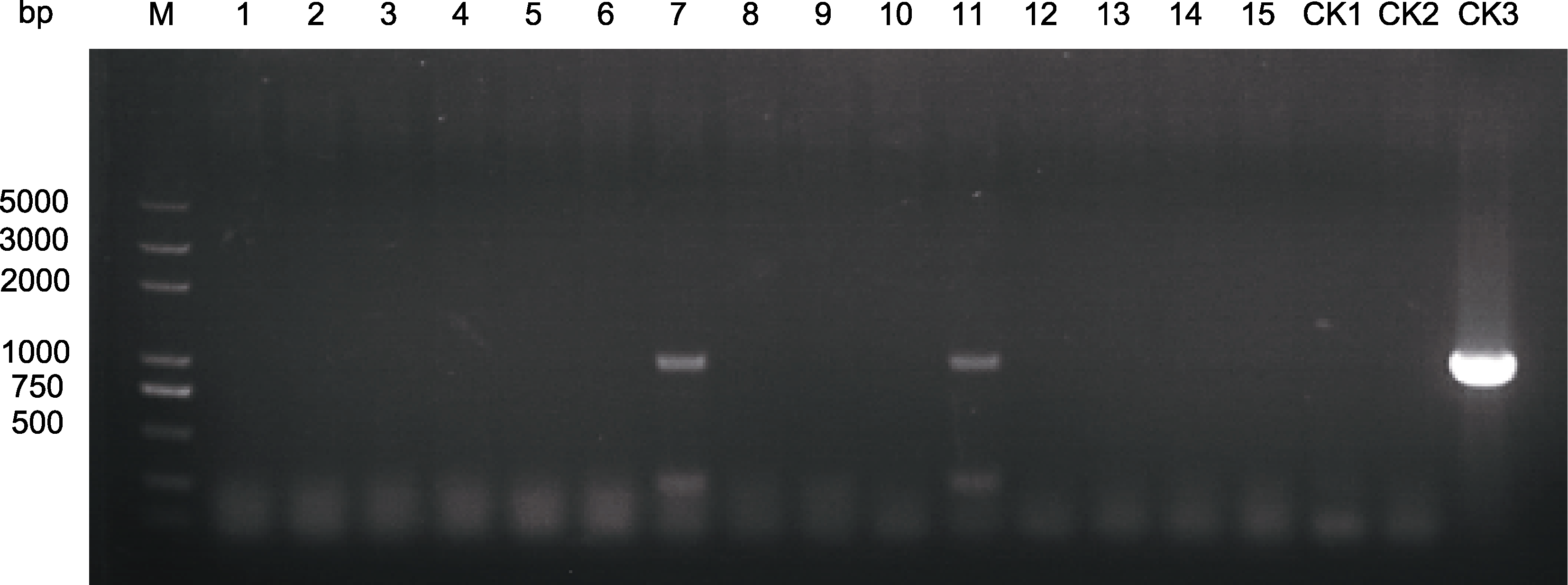
Figure 6 PCR amplification on the resistant plantlets of Chrysanthemum × morifolium ‘Wandai Fengguang’ M: DL2000 plus DNA marker; 1-15: 抗性苗; CK1: ddH2O; CK2: 野生型; CK3: 质粒 M: DL2000 plus DNA marker; 1-15: Resistant plantlets; CK1: ddH2O; CK2: Wild type; CK3: Plasmid
| [1] |
戴思兰, 洪艳 (2016). 基于花青素苷合成和呈色机理的观赏植物花色改良分子育种. 中国农业科学 49, 529-542.
DOI |
| [2] | 郭兆奎, 万秀清, 魏继承, 于艳华, 于金涛 (1999). 适于PCR分析的烤后烟叶DNA提取方法的研究. 中国烟草科学 20 (4), 5-8. |
| [3] | 韩科厅 (2010). 花青素苷合成关键结构基因导入对菊花花色的影响. 博士论文. 北京: 北京林业大学. pp. 41-47. |
| [4] | 何姗 (2020). 农杆菌介导CmWRKY15-1基因对菊花的遗传转化. 硕士论文. 沈阳: 沈阳农业大学. pp. 12-14. |
| [5] | 洪艳, 白新祥, 孙卫, 贾锋炜, 戴思兰 (2012). 菊花品种花色表型数量分类研究. 园艺学报 39, 1330-1340. |
| [6] | 贾红梅, 王碧玉, 刘迪, 毛洪玉 (2017). 农杆菌介导CBL基因对菊花品种‘C008’的转化. 西北林学院学报 32, 184-189. |
| [7] | 姜宁宁, 付建新, 戴思兰 (2012). 中国传统菊花品种‘小林静’再生及转化体系的建立. 生物技术通报 28(4), 87-92. |
| [8] | 李辛雷, 陈发棣, 王红, 房伟民, 管志勇 (2004). 菊花外植体再生体系的研究. 上海农业学报 20(2), 13-16. |
| [9] | 李亚军, 李悦, 黄河, 戴思兰 (2018). 切花菊‘粉贵人’高效再生体系的建立. 见: 中国观赏园艺研究进展2018. 哈尔滨: 中国园艺学会观赏园艺专业委员会. pp. 427-434. |
| [10] |
廖敏凌, 蒲娅, 武晓云, 马朝峰, 王文奎, 戴思兰 (2023). 平潭野菊混合瓣型株系再生体系的建立. 植物学报 58, 449-460.
DOI |
| [11] | 刘明星 (2020). 盆栽小菊‘Branfountain Pink’遗传转化体系的建立. 硕士论文. 南京: 南京农业大学. pp. 14-30. |
| [12] |
逯锦春, 曹丽娜, 佟冠杰, 王鑫颖, 张利英, 喻锌, 李荟芳, 李彦慧 (2022). 大花银莲花愈伤组织诱导及再生体系的建立. 植物学报 57, 217-226.
DOI |
| [13] |
罗虹, 温小蕙, 周圆圆, 戴思兰 (2020). 芳香堆心菊离体再生体系的建立. 植物学报 55, 318-328.
DOI |
| [14] | 马琦 (2020). 少芽切花菊分枝性及其遗传转化体系的研究. 硕士论文. 南京: 南京农业大学. pp. 23-30. |
| [15] | 亓帅, 付建新, 王翊, 杨立文, 戴思兰 (2014). 甘菊下胚轴遗传转化体系的建立. 分子植物育种 12, 356-362. |
| [16] | 曲爱爱 (2016). 菊花遗传转化体系建立及VtF3'5'H基因转化‘南农粉翠’的研究. 硕士论文. 南京: 南京农业大学. pp. 23-30. |
| [17] | 时颂, 李青, 赵霜, 戴思兰, 李娜娜 (2013). 不同切花菊品种及处理对愈伤组织诱导和分化的影响. 东北林业大学学报 41, 77-81. |
| [18] | 滕如萍, 张佳祺, 刘晓芬, 余璐, 张潮, 李方 (2025). 菊花‘神马’组培再生体系的优化. 分子植物育种 23, 1550-1557. |
| [19] | 王碧玉 (2017). 菊花再生及遗传转化体系的研究. 硕士论文. 沈阳: 沈阳农业大学. pp. 8-15. |
| [20] | 王想 (2018). 神农香菊单萜合酶基因的克隆及对野菊的遗传转化. 硕士论文. 哈尔滨: 东北林业大学. pp. 36-38. |
| [21] | 王亚琴 (2020). 万寿菊再生和遗传转化体系的建立及重要性状的遗传分析. 硕士论文. 武汉: 华中农业大学. pp. 4-7. |
| [22] | 王亚琴, 韦陆丹, 王文静, 刘宝骏, 张春玲, 张俊卫, 何燕红 (2020). 万寿菊再生体系的建立及优化. 植物学报 55, 749-759. |
| [23] | 王自布, 莫国秀, 罗会兰, 张德英 (2015). 菊花不同外植体组培快繁及其再生体系的研究. 北方园艺 (18), 106-109. |
| [24] | 魏曼曼, 王江民, Imtiaz M, 洪波 (2014). 菊花花色嵌合花瓣的离体培养及植株再生. 北京林业大学学报 36(4), 107-112. |
| [25] |
武晓云, 廖敏凌, 李雪茹, 舒梓淳, 辛佳潼, 张伯晗, 戴思兰 (2024). 毛华菊3种瓣型株系再生体系的建立. 植物学报 59, 245-256.
DOI |
| [26] | 吴志苹, 高亦珂, 范敏, 高耀辉 (2020). 菊花‘金不凋’再生及遗传转化体系的构建. 分子植物育种 18, 150-158. |
| [27] | 徐式近, 徐忠传 (2013). 不同菊花品种高效直接再生体系的构建. 江苏农业科学 41(11), 52-54, 100. |
| [28] | 许志茹, 陈智华, 姜艳东, 侯杰, 佟玲, 李玉花 (2013). 露地菊离体再生体系建立及BrDFR基因遗传转化. 园艺学报 40, 1517-1526. |
| [29] | 阳淑金, 宋爱萍, 何深颖, 朱晓晨, 孙静, 高姣姣, 王银杰, 陈发棣, 蒋甲福 (2015). CaMV 35S启动子在菊花中驱动GUS外源基因的表达分析. 南京农业大学学报 38, 554-559. |
| [30] |
余晓敏, 王亚琴, 刘雨菡, 易庆平, 程文翰, 朱钰, 段枫, 张莉雪, 何燕红 (2023). 根癌农杆菌介导万寿菊遗传转化体系的建立. 植物学报 58, 760-769.
DOI |
| [31] |
赵静雅, 徐素娟, 陈发棣, 滕年军 (2019). 匍匐型地被菊再生及遗传转化体系的建立. 核农学报 33, 1686-1697.
DOI |
| [32] | 赵伶俐, 石少川, 张启翔, 高亦珂 (2011). 农杆菌介导的地被菊遗传转化体系的优化. 分子植物育种 9, 74-80. |
| [33] | Adedeji OS, Naing AH, Kim CK (2020). Protoplast isolation and shoot regeneration from protoplast-derived calli of Chrysanthemum cv. White ND. Plant Cell Tissue organ Cult 141, 571-581. |
| [34] | Bernula D, Benkő P, Kaszler N, Domonkos I, Valkai I, Szőllősi R, Ferenc G, Ayaydin F, Fehér A, Gémes K (2020). Timely removal of exogenous cytokinin and the prevention of auxin transport from the shoot to the root affect the regeneration potential of Arabidopsis roots. Plant Cell Tissue Organ Cult 140, 327-339. |
| [35] |
Brugliera F, Tao GQ, Tems U, Kalc G, Mouradova E, Price K, Stevenson K, Nakamura N, Stacey I, Katsumoto Y, Tanaka Y, Mason JG (2013). Violet/blue chrysanthemums—metabolic engineering of the anthocyanin biosynthetic pathway results in novel petal colors. Plant Cell Physiol 54, 1696-1710.
DOI PMID |
| [36] | Han XY, Luo YT, Lin JY, Wu HY, Sun H, Zhou LJ, Chen SM, Guan ZY, Fang WM, Zhang F, Chen FD, Jiang JF (2021). Generation of purple-violet chrysanthemums via anthocyanin B-ring hydroxylation and glucosylation introduced from Osteospermum hybrid F3'5'H and Clitoria ternatea A3'5'GT. Ornamental Plant Res 1, 4. |
| [37] | Huang H, Hu K, Han KT, Xiang QY, Dai SL (2013). Flower colour modification of chrysanthemum by suppression of F3'H and overexpression of the exogenous Senecio cruentus F3'5'H gene. PLoS One 8, e74395. |
| [38] | Li YF, Wang JY, Lu CF, Wang ZM, Deng CY, Gao K, Li JJ, Fang ZJ, Liu H, Hong Y, Dai SL (2024). Flavonoid extracts from chrysanthemum with appropriate anthocyanins turn blue when exposed to iron ions. Hortic Plant J 10, 837-852. |
| [39] | Lim KB, Kwon SJ, Lee SI, Hwang YJ, Naing AH (2012). Influence of genotype, explant source, and gelling agent on in vitro shoot regeneration of chrysanthemum. Hortic Environ Biotechnol 53, 329-335. |
| [40] | Long Y, Yang Y, Pan GT, Shen YO (2022). New insights into tissue culture plant-regeneration mechanisms. Front Plant Sci 13, 926752. |
| [41] | Momonoi K, Yoshida K, Mano S, Takahashi H, Nakamori C, Shoji K, Nitta A, Nishimura M (2009). A vacuolar iron transporter in tulip, TgVit1, is responsible for blue coloration in petal cells through iron accumulation. Plant J 59, 437-447. |
| [42] | Naing AH, Park KI, Chung MY, Lim KB, Kim CK (2016). Optimization of factors affecting efficient shoot regeneration in chrysanthemum cv. Shinma. Braz J Bot 39, 975-984. |
| [43] |
Noda N, Aida R, Kishimoto S, Ishiguro K, Fukuchi-Mizutani M, Tanaka Y, Ohmiya A (2013). Genetic engineering of novel bluer-colored chrysanthemums produced by accumulation of delphinidin-based anthocyanins. Plant Cell Physiol 54, 1684-1695.
DOI PMID |
| [44] | Noda N, Yoshioka S, Kishimoto S, Nakayama M, Douzono M, Tanaka Y, Aida R (2017). Generation of blue chrysanthemums by anthocyanin B-ring hydroxylation and glucosylation and its coloration mechanism. Sci Adv 3, e1602785. |
| [45] | Renou JP, Brochard P, Jalouzot R (1993). Recovery of transgenic chrysanthemum (Dendranthema grandiflora Tzvelev) after hygromycin resistance selection. Plant Sci 89, 185-197. |
| [46] | Shiono M, Matsugaki N, Takeda K (2005). Structure of the blue cornflower pigment. Nature 436, 791. |
| [47] |
Shoji K, Miki N, Nakajima N, Momonoi K, Kato C, Yoshida K (2007). Perianth bottom-specific blue color development in tulip cv. Murasakizuisho requires ferric ions. Plant Cell Physiol 48, 243-251.
PMID |
| [48] | Shoji K, Momonoi K, Tsuji T (2010). Alternative expression of vacuolar iron transporter and ferritin genes leads to blue/purple coloration of flowers in tulip cv. ‘Murasakizuisho’. Plant Cell Physiol 51, 215-224. |
| [49] | Song JY, Mattson NS, Jeong BR (2011). Efficiency of shoot regeneration from leaf, stem, petiole and petal explants of six cultivars of Chrysanthemum morifolium. Plant Cell Tissue Organ Cult 107, 295-304. |
| [50] | Takeda K, Osakabe A, Saito S, Furuyama D, Tomita A, Kojima Y, Yamadera M, Sakuta M (2005). Components of protocyanin, a blue pigment from the blue flowers of Centaurea cyanus. Phytochemistry 66, 1607-1613. |
| [51] | Takeda K, Yamaguchi S, Iwata K, Tsujino Y, Fujimori T, Husain SZ (1996). A malonylated anthocyanin and flavonols in the blue flowers of Meconopsis. Phytochemistry 42, 863-865. |
| [52] |
Tanaka M, Fujimori T, Uchida I, Yamaguchi S, Takeda K (2001). A malonylated anthocyanin and flavonols in blue Meconopsis flowers. Phytochemistry 56, 373-376.
PMID |
| [53] |
Yoshida K, Negishi T (2013). The identification of a vacuolar iron transporter involved in the blue coloration of cornflower petals. Phytochemistry 94, 60-67.
DOI PMID |
| [1] | Ruxin Zhang, Chenrong Li, Tongxin Wang, Jie Li, Tingge Li, Huixian Xu, Meier Li, Ying Zhao, Ting Peng, Jian Wang. Establishment of a Regeneration System for Viola × wittrochiana [J]. Chinese Bulletin of Botany, 2025, 60(6): 1-0. |
| [2] | Yuze Liu, Yifei Wang, Weizhen Ren, Hao Li, Bin Lu, Bingshe Lu, Xiaoyue Yu. Establishment of Immature Embryo Rescue and Regeneration System for Pyrus calleryana cv. ‘Cleveland’ [J]. Chinese Bulletin of Botany, 2024, 59(5): 800-809. |
| [3] | Hao Zeng, Peifang Li, Zhihui Guo, Chunlin Liu, Ying Ruan. Establishment of a Regeneration System for Lunaria annua [J]. Chinese Bulletin of Botany, 2024, 59(3): 433-440. |
| [4] | Xiaoyun Wu, Minling Liao, Xueru Li, Zichun Shu, Jiatong Xin, Bohan Zhang, Silan Dai. Establishment of Regeneration System of Chrysanthemum vestitum with Three Floret Forms [J]. Chinese Bulletin of Botany, 2024, 59(2): 245-256. |
| [5] | Minling Liao, Ya Pu, Xiaoyun Wu, Chaofeng Ma, Wenkui Wang, Silan Dai. Establishment of Regeneration System of Chrysanthemum indicum in Pingtan with Various Ligulate Floret Form [J]. Chinese Bulletin of Botany, 2023, 58(3): 449-460. |
| [6] | Qian Luo, Yansha Zhang, Jing Ou. Callus Induction and Plant Regeneration of Cerasus serrulata var. lannesiana cv. ‘Grandiflora’ [J]. Chinese Bulletin of Botany, 2021, 56(4): 451-461. |
| [7] | Xuebin Song, Kang Gao, He Huang, Zhilan Liu, Silan Dai, Yu Ji. Quantitative Definition and Classification of Leaves in Large- flowered Chinese Chrysanthemum Based on the Morphological Traits [J]. Chinese Bulletin of Botany, 2021, 56(1): 10-24. |
| [8] | Dongrui Zhang, Zhigang Bu, Lingling Chen, Ying Chang. Establishment of a Tissue Culture and Rapid Propagation System of Dryopteris fragrans [J]. Chinese Bulletin of Botany, 2020, 55(6): 760-767. |
| [9] | Hong Luo, Xiaohui Wen, Yuanyuan Zhou, Silan Dai. Establishment of In Vitro Regeneration System of Helenium aromaticum [J]. Chinese Bulletin of Botany, 2020, 55(3): 318-328. |
| [10] | Yue Xu,Yingping Cao,Yu Wang,Chunxiang Fu,Shaojun Dai. Agrobacterium rhizogenes-mediated Transformation System of Spinacia oleracea [J]. Chinese Bulletin of Botany, 2019, 54(4): 515-521. |
| [11] | Zhang Xuhong, Wang Di, Liang Zhenxu, Sun Meiyu, Zhang Jinzheng, Shi Lei. Callus Induction and Establishment of a Plant Regeneration System with Lilium martagon [J]. Chinese Bulletin of Botany, 2018, 53(6): 840-847. |
| [12] | An Baiyi, Guo Cainan, Bao Wenhui, Li Fengfei, Zhao He, Chen Li, An Fengyun. Rapid Propagation of Symplocos paniculata In Vitro [J]. Chinese Bulletin of Botany, 2018, 53(5): 693-699. |
| [13] | Xiting Zhao, Liwei Jiang, Miao Wang, Yuting Zhu, Wenfang Zhang, Mingjun Li. Establishment of Transgenic Acceptor by Indirect Somatic Embryogenesis Regeneration and Transformation of CmTGA1 Gene in Chrysanthemum morifolium cv. ‘Huaihuang’ [J]. Chinese Bulletin of Botany, 2016, 51(4): 525-532. |
| [14] | Jiangmin Wang, Fadi Chen, Weimin Fang, Sumei Chen, Zhiyong Guan, Haiyan Tang. Differentiation of Cut Chrysanthemum Cultivars Based on Multiple Foliar Morphological Parameters [J]. Chinese Bulletin of Botany, 2013, 48(6): 608-615. |
| [15] | Mingli Zhu, Qianqian Liu, Silan Dai. Karyotype Analysis of 38 Large-flowered Chrysanthemum Cultivars from China [J]. Chinese Bulletin of Botany, 2011, 46(4): 447-455. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||