

植物学报 ›› 2025, Vol. 60 ›› Issue (4): 515-532.DOI: 10.11983/CBB24112 cstr: 32102.14.CBB24112
赵蔓雅, 孙倩楠, 徐晶晶, 段恬妮, 蔡锦涛, 周婧, 范婷婷, 萧浪涛*( ), 王若仲*(
), 王若仲*( )
)
收稿日期:2024-07-23
接受日期:2025-06-04
出版日期:2025-07-10
发布日期:2025-06-04
通讯作者:
*E-mail: ltxiao@hunau.edu.cn;wangruozhong@hunau.edu.cn
基金资助:
Manya Zhao, Qiannan Sun, Jingjing Xu, Tianni Duan, Jintao Cai, Jing Zhou, Tingting Fan, Langtao Xiao*( ), Ruozhong Wang*(
), Ruozhong Wang*( )
)
Received:2024-07-23
Accepted:2025-06-04
Online:2025-07-10
Published:2025-06-04
Contact:
*E-mail: ltxiao@hunau.edu.cn;wangruozhong@hunau.edu.cn
摘要: 叶色突变体是研究光形态发生、叶绿体发育、叶绿素代谢和光合作用机制等多种生理过程的理想材料。该研究从黄瓜(Cucumis sativus) XYYH-2-1-1株系自交后代中获得1个新的黄化致死突变体ycl (yellow cotyledon lethal)。该突变体自幼苗出土后子叶一直呈黄化状态, 约2周后枯萎死亡, 其生长抑制表型为非光依赖型。与野生型相比, ycl突变体的Chl a和Chl b含量趋于零, 叶绿素生物合成途径中Mg2+螯合过程受阻。显微和超微结构分析发现, ycl叶片组织紊乱、叶绿体发育受阻。ycl的抗氧化酶活性及丙二醛含量显著升高, 说明其受到氧化胁迫, 且抗氧化能力强。ycl净光合速率极显著降低, 胞间CO2浓度上升, 推测ycl光合速率降低源于气孔导度降低、叶绿素含量减少和叶绿体发育受阻。转录组学分析表明, ycl与其野生型间存在337个差异表达基因, 光合作用、类黄酮生物合成、叶绿素代谢和活性氧代谢是导致ycl黄化致死表型形成的关键途径。通过BSA-Seq分析, ycl突变基因初步定位于3号染色体的1.48-1.9 Mb区间, 内含41个候选基因。对ycl突变体的研究为阐明黄瓜叶绿体发育的分子机制提供了参考。
赵蔓雅, 孙倩楠, 徐晶晶, 段恬妮, 蔡锦涛, 周婧, 范婷婷, 萧浪涛, 王若仲. 一个新的黄瓜叶色突变体鉴定、初定位及转录组分析. 植物学报, 2025, 60(4): 515-532.
Manya Zhao, Qiannan Sun, Jingjing Xu, Tianni Duan, Jintao Cai, Jing Zhou, Tingting Fan, Langtao Xiao, Ruozhong Wang. Identification, Mapping and Transcriptome Analysis of a New Leaf Color Mutant in Cucumber. Chinese Bulletin of Botany, 2025, 60(4): 515-532.
| Primer name | Forward primer (5′→3′) | Reverse primer (5'→3') |
|---|---|---|
| Cs-Actin | GTTACGCCCTCCCTCATGCCATTC | TCCCGTTCGGCAGTGGTGGT |
| CsaV3_3G002180 | GTCGTCCTGCCATTCGATCA | AGCACCAAGTTCACTCCAACT |
| CsaV3_5G025230 | AATTCTTCCGACCCGAACCC | AGTAGCCTTCTGCGGACCTA |
| CsaV3_1G030370 | CAGTGGCTGGATACGTCCTC | GTGAGCTCCCGCCATAAAGT |
| CsaV3_7G000620 | TGGAGCATCTCCGAAAGTGG | GGCAAGGAATTGTGATGCCA |
| CsaV3_5G028880 | TAGAACCCAGGCTCCCTCAA | CCGTGTTTTCACAAGCTTCTCT |
| CsaV3_3G041340 | AACATGTTACTGGTGGGGGC | CACATTGAAATCATTGGGTACCTG |
| CsaV3_6G037230 | CCCACTCAAGCGATGTG CTA | CCATTGACCTCAGCATTGCG |
| CsaV3_5G006200 | GACCCAGTTCAAGCTAGCCA | ACTGAGACAACAAGCGCGTA |
| CsaV3_7G008610 | GGCTCCAAGGGCCAATACAT | GTAGGCCTTGGACAGGCATT |
表1 qRT-PCR引物
Table 1 Primers used for qRT-PCR
| Primer name | Forward primer (5′→3′) | Reverse primer (5'→3') |
|---|---|---|
| Cs-Actin | GTTACGCCCTCCCTCATGCCATTC | TCCCGTTCGGCAGTGGTGGT |
| CsaV3_3G002180 | GTCGTCCTGCCATTCGATCA | AGCACCAAGTTCACTCCAACT |
| CsaV3_5G025230 | AATTCTTCCGACCCGAACCC | AGTAGCCTTCTGCGGACCTA |
| CsaV3_1G030370 | CAGTGGCTGGATACGTCCTC | GTGAGCTCCCGCCATAAAGT |
| CsaV3_7G000620 | TGGAGCATCTCCGAAAGTGG | GGCAAGGAATTGTGATGCCA |
| CsaV3_5G028880 | TAGAACCCAGGCTCCCTCAA | CCGTGTTTTCACAAGCTTCTCT |
| CsaV3_3G041340 | AACATGTTACTGGTGGGGGC | CACATTGAAATCATTGGGTACCTG |
| CsaV3_6G037230 | CCCACTCAAGCGATGTG CTA | CCATTGACCTCAGCATTGCG |
| CsaV3_5G006200 | GACCCAGTTCAAGCTAGCCA | ACTGAGACAACAAGCGCGTA |
| CsaV3_7G008610 | GGCTCCAAGGGCCAATACAT | GTAGGCCTTGGACAGGCATT |
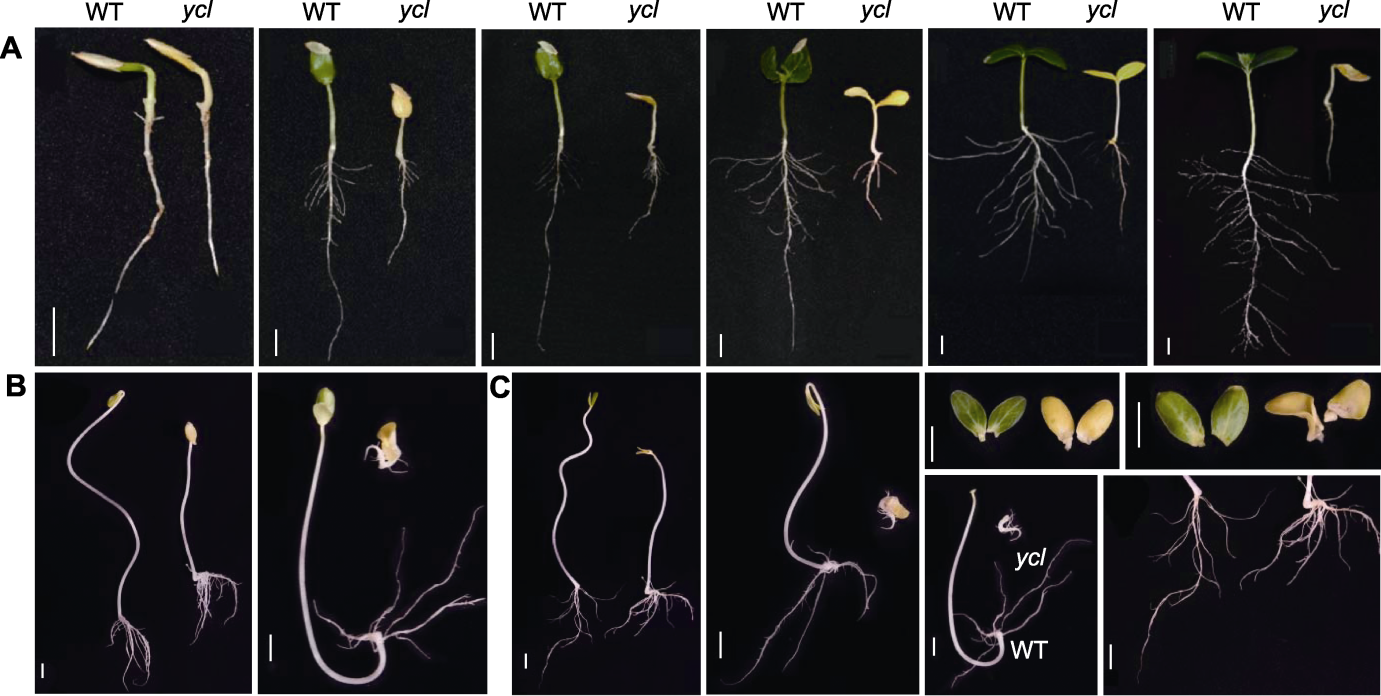
图1 ycl与XYYH-3-1黄瓜子叶期表型变化比较 (A) 4、5、6、7、8和14天野生型(WT)和ycl表型变化(自然光); (B) 第7天苗龄时2组WT和ycl表型差异(黑暗处理7天); (C) 第8天苗龄时2组WT和ycl表型差异(光照培养1天)。Bars=1 cm
Figure 1 Comparison of phenotypic changes between ycl and XYYH-3-1 at the cotyledon stage of cucumber (A) Phenotypic changes of wild type (WT) and ycl in 4, 5, 6, 7, 8 and 14 days (natural light); (B) Phenotypic differences of WT and ycl between the two groups at 7 d of age (dark treatment for 7 d); (C) Phenotypic differences of WT and ycl between the two groups at 8 d of age (light culture for 1 d). Bars=1 cm
| Material | Plant height (cm) | Root length (cm) | Stem diameter (cm) | Leaf area (cm2) |
|---|---|---|---|---|
| WT | 4.86±0.32 | 15.56±0.94 | 0.22±0.03 | 9.04±1.66 |
| ycl | 2.64±0.56** | 5.5±0.9** | 0.18±0.02* | 1.95±0.26** |
表2 ycl与XYYH-3-1黄瓜子叶期(7天)的农艺性状
Table 2 Agronomic characters of ycl and XYYH-3-1 at cotyledon stage (7 d) of cucumber
| Material | Plant height (cm) | Root length (cm) | Stem diameter (cm) | Leaf area (cm2) |
|---|---|---|---|---|
| WT | 4.86±0.32 | 15.56±0.94 | 0.22±0.03 | 9.04±1.66 |
| ycl | 2.64±0.56** | 5.5±0.9** | 0.18±0.02* | 1.95±0.26** |
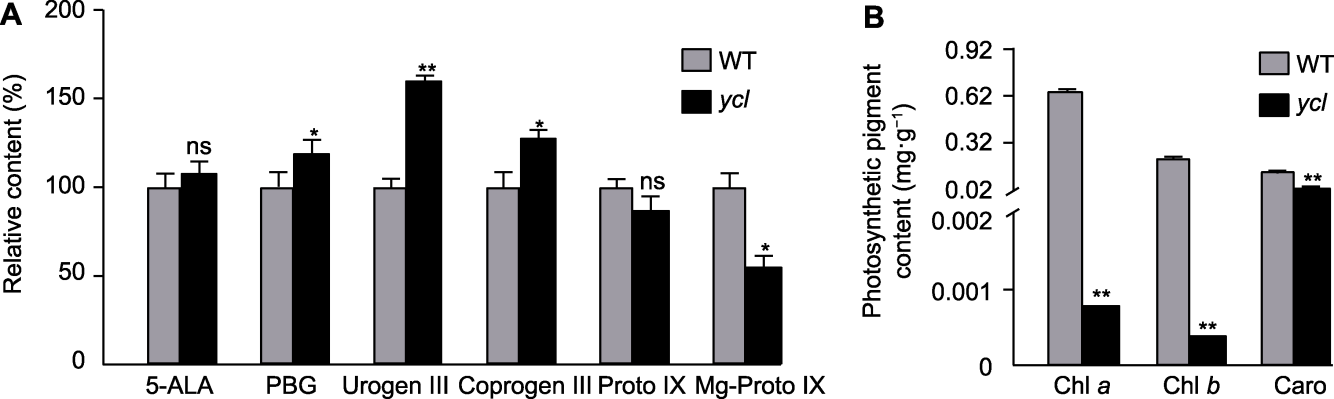
图2 ycl与XYYH-3-1叶绿素及其合成中间代谢产物的含量 (A) 叶绿素合成中间代谢产物的相对含量; (B)光合色素含量。WT: 野生型; 5-ALA: 5-氨基乙酰丙酸; PBG: 胆色素原; Urogen III: 尿卟啉原III; Coprogen III: 粪卟啉原III; Proto IX: 原卟啉IX; Mg-Proto IX: Mg-原卟啉IX。*表示差异显著(t检验, P<0.05); **表示差异极显著(t检验, P<0.01); ns表示差异不显著。
Figure 2 Content of chlorophyll and its biosynthetic intermediate metabolites in ycl and XYYH-3-1 (A) Relative content of chlorophyll biosynthetic intermediate metabolites; (B) Photosynthetic pigment content. WT: Wild type; 5-ALA: 5-aminolevulinic acid; PBG: Porphobilinogen; Urogen III: Uroporphyrinogen III; Coprogen III: Coproporphyrinogen III; Proto IX: Protoporphyrin IX; Mg-Proto IX: Mg-protoporphyrin IX. * denote significant differences (t-test, P<0.05); ** denote extremely significant differences (t-test, P<0.01); ns denote no significant difference.
| Material | Chl a (mg·g−1) | Chl b (mg·g−1) | Caro (mg·g−1) | Total Chl (mg·g−1) | Chl a/b | Total Chl/Caro |
|---|---|---|---|---|---|---|
| WT | 0.6483±0.0162 | 0.2157±0.0063 | 0.1304±0.0018 | 0.8641±0.0225 | 3.0057 | 6.6272 |
| ycl | 0.0008±0** | 0.0004±0** | 0.0265±0.0007** | 0.0012±0** | 1.9303 | 0.0443 |
表3 ycl和XYYH-3-1叶片的光合色素含量
Table 3 Photosynthetic pigment content in leaves of ycl and XYYH-3-1
| Material | Chl a (mg·g−1) | Chl b (mg·g−1) | Caro (mg·g−1) | Total Chl (mg·g−1) | Chl a/b | Total Chl/Caro |
|---|---|---|---|---|---|---|
| WT | 0.6483±0.0162 | 0.2157±0.0063 | 0.1304±0.0018 | 0.8641±0.0225 | 3.0057 | 6.6272 |
| ycl | 0.0008±0** | 0.0004±0** | 0.0265±0.0007** | 0.0012±0** | 1.9303 | 0.0443 |
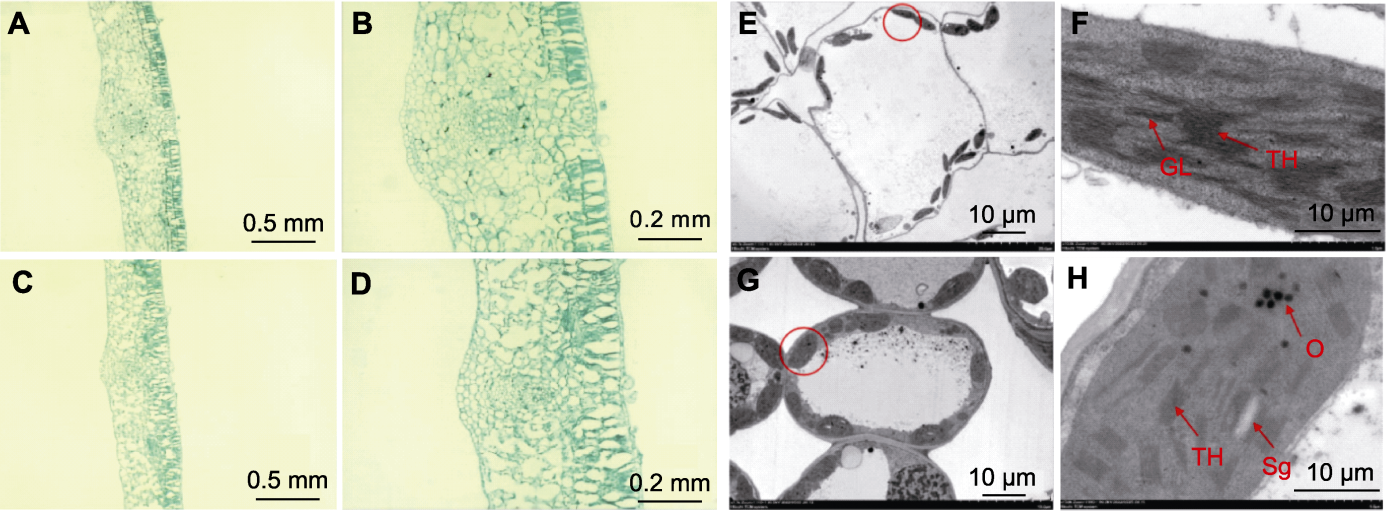
图3 ycl和XYYH-3-1叶片的显微结构及叶绿体超微结构 (A), (B) 野生型子叶显微结构; (C), (D) 突变体子叶显微结构; (E), (F) 野生型叶绿体超微结构; (G), (H) ycl叶绿体超微结构。(F)是(E)中红色圆圈中的叶绿体放大版。(H)是(G)中红色圆圈中的叶绿体放大版。Sg: 淀粉粒; TH: 类囊体; GL: 基粒片层; O: 嗜锇体
Figure 3 Microstructure and chloroplast ultrastructure of ycl and XYYH-3-1 leaves (A), (B) Wild type cotyledon microstructures; (C), (D) Mutant cotyledon microstructures; (E), (F) Wild type chloroplast ultrastructures; (G), (H) ycl chloroplast ultrastructures. (F) is an enlarged version of the chloroplast in the red circle in (E). (H) is an enlarged version of the chloroplast in the red circle in (G). Sg: Starch grain; TH: Thylakoid; GL: Grana lamella; O: Osmiophilic body
| Material | Pn (μmol·m-2·s-1) | Gs (mmol·m-2·s-1) | Ci (μmol·mol-1) | Tr (mol·m-2·s-1) |
|---|---|---|---|---|
| WT | 15.85±1.39 | 0.37±0.08 | 331±12 | 4.53±0.62 |
| ycl | -2.49±0.15** | 0.14±0.02** | 458±7** | 2.17±0.31** |
表4 ycl和XYYH-3-1植株的光合作用参数
Table 4 Photosynthesis parameters of the ycl and XYYH-3-1
| Material | Pn (μmol·m-2·s-1) | Gs (mmol·m-2·s-1) | Ci (μmol·mol-1) | Tr (mol·m-2·s-1) |
|---|---|---|---|---|
| WT | 15.85±1.39 | 0.37±0.08 | 331±12 | 4.53±0.62 |
| ycl | -2.49±0.15** | 0.14±0.02** | 458±7** | 2.17±0.31** |
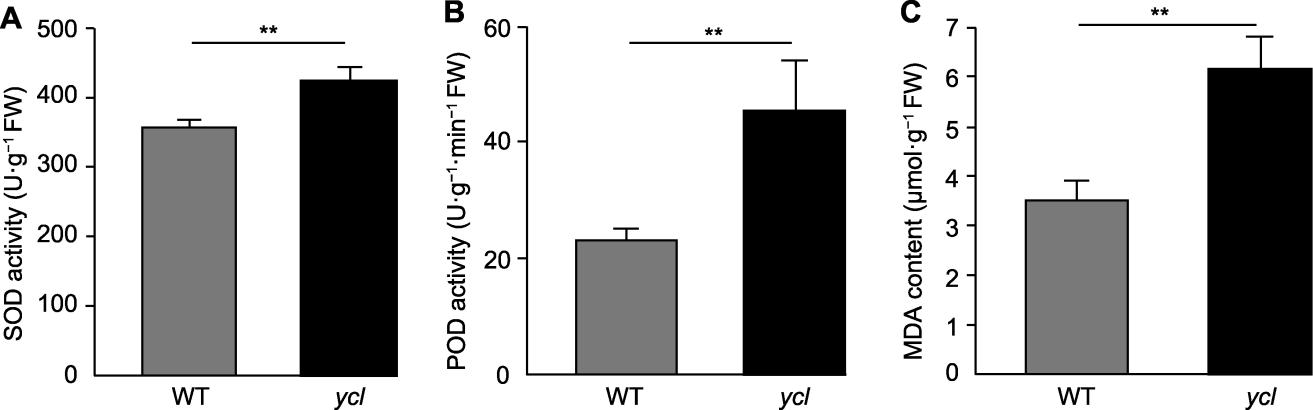
图4 ycl和XYYH-3-1植株的抗氧化物酶活性及丙二醛含量 (A) 超氧化物歧化酶(SOD)活性; (B) 过氧化物酶(POD)活性; (C) 丙二醛(MDA)含量。WT: 野生型. **表示差异极显著(t检验, P<0.01)。
Figure 4 Antioxidant enzyme activities and malondialdehyde contents of ycl and XYYH-3-1 (A) Superoxide dismutase (SOD) activity; (B) Peroxidase (POD) activity; (C) Malondialdehyde (MDA) contents. WT: Wild type. ** denote extremely significant differences (t-test, P<0.01).
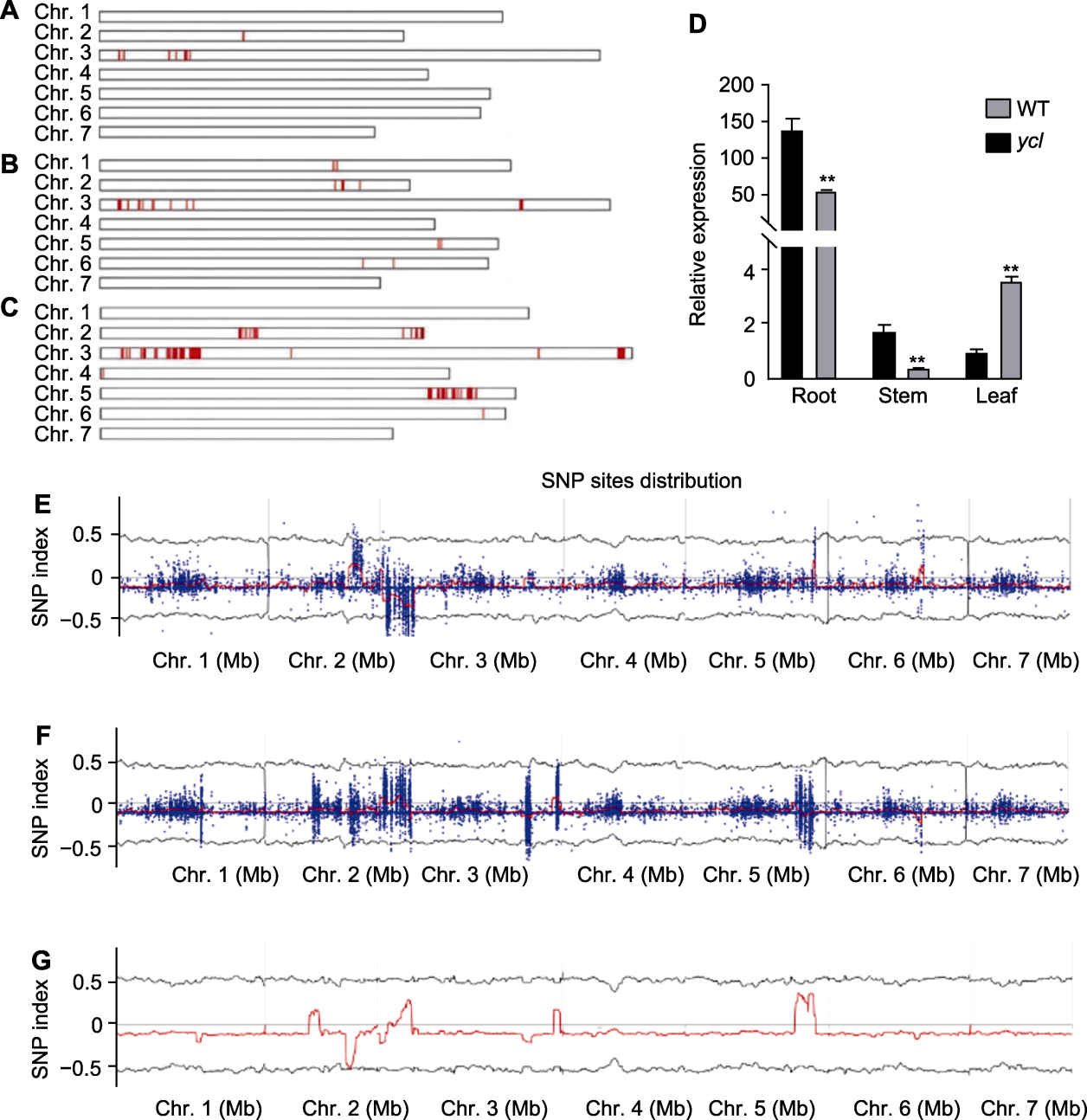
图5 突变基因的初定位及筛选 (A)-(C) 单核苷酸多态性分布((A) 第18号群体; (B) XY子叶群体; (C) XY真叶群体); (D) CsaV3_3G001980相对表达量(**表示差异极显著, t检验, P<0.01); (E)-(G) SNP index峰图((E) 第18号群体; (F) XY子叶群体; (G) XY真叶群体)。WT: 野生型; SNP: 单核苷酸多态性
Figure 5 Preliminary localisation and screening of mutant genes (A)-(C) Distribution of single nucleotide polymorphisms ((A) Population 18; (B) XY cotyledon population; (C) XY true leaf population); (D) Relative expression of CsaV3_3G001980 (** denote extremely significant differences, t-test, P<0.01); (E)-(G) SNP index plot ((E) Population 18; (F) XY cotyledon population; (G) XY true leaf population). WT: Wild type; SNP: Single nucleotide polymorphism
| Genes within the interval | Annotation |
|---|---|
| CsaV3_3G001960 | Cytochrome P450 family ent-kaurenoic acid oxidase |
| CsaV3_3G001970 | Cytochrome P450 family ent-kaurenoic acid oxidase |
| CsaV3_3G001980 | Protein NRT1/PTR FAMILY 7.3-like |
| CsaV3_3G001990 | Hsp90 co-chaperone Cdc37, N-terminally processed like |
| CsaV3_3G002000 | Ribose-phosphate pyrophosphokinase |
| CsaV3_3G002010 | Type I inositol polyphosphate 5-phosphatase, puta |
| CsaV3_3G002020 | Phenylalanine-tRNA ligase alpha subunit like |
| CsaV3_3G002030 | Two-component response regulator-like aprr2 |
| CsaV3_3G002040 | S-adenosyl-L-methionine-dependent methyltransferases superfamily protein |
| CsaV3_3G002050 | Vacuolar protein sorting-associated protein 55 homolog |
| CsaV3_3G002060 | Haloacid dehalogenase-like hydrolase |
| CsaV3_3G002070 | Haloacid dehalogenase-like hydrolase domain-containing protein |
| CsaV3_3G002080 | Regulator of nonsense transcripts 1 homolog |
| CsaV3_3G002090 | Lon protease homolog 2, peroxisomal |
| CsaV3_3G002100 | Electron carrier/iron ion-binding protein |
| CsaV3_3G002110 | BOI-related E3 ubiquitin-protein ligase 1 |
| CsaV3_3G002120 | Protein of unknown function (DUF581) |
| CsaV3_3G002130 | Poly(U)-specific endoribonuclease |
| CsaV3_3G002140 | Methyltransferase-like protein 13 |
| CsaV3_3G002150 | Beta-amylase |
| CsaV3_3G002160 | Protein kinase, putative |
| CsaV3_3G002170 | Novel plant snare, putative |
| CsaV3_3G002180 | Phosphatidylinositol 4-phosphate 5-kinase 1 |
| CsaV3_3G002190 | Coiled-coil domain-containing protein 97 |
| CsaV3_3G002200 | Calcineurin B-like protein |
| CsaV3_3G002210 | Unknown protein |
| CsaV3_3G002220 | Protein kinase |
| CsaV3_3G002230 | Unknown protein |
| CsaV3_3G002240 | Bifunctional inhibitor/plant lipid transfer protein/seed storage helical domain-containing protein |
| CsaV3_3G002250 | Peptidyl-prolyl cis-trans isomerase-like |
| CsaV3_3G002260 | DVA-1 polyprotein |
| CsaV3_3G002270 | tRNA/rRNA methyltransferase (SpoU) family protein |
| CsaV3_3G002280 | Protein lingerer like |
| CsaV3_3G002290 | Chlorophyll a/b binding family protein |
| CsaV3_3G002300 | ATP-dependent Clp protease proteolytic subunit |
| CsaV3_3G002310 | Unknown protein |
| CsaV3_3G002320 | Histone-lysine N-methyltransferase, H3 lysine-9 specific SUVH6-like |
| CsaV3_3G002330 | Kelch repeat-containing protein |
| CsaV3_3G002340 | GATA transcription factor 26-like |
| CsaV3_3G002350 | Vesicle-associated protein 2-1 |
| CsaV3_3G002360 | 50S ribosomal protein L19, chloroplastic |
表5 ycl突变位点初定位区间内基因
Table 5 The genes within the preliminarily mapped region of ycl mutant site
| Genes within the interval | Annotation |
|---|---|
| CsaV3_3G001960 | Cytochrome P450 family ent-kaurenoic acid oxidase |
| CsaV3_3G001970 | Cytochrome P450 family ent-kaurenoic acid oxidase |
| CsaV3_3G001980 | Protein NRT1/PTR FAMILY 7.3-like |
| CsaV3_3G001990 | Hsp90 co-chaperone Cdc37, N-terminally processed like |
| CsaV3_3G002000 | Ribose-phosphate pyrophosphokinase |
| CsaV3_3G002010 | Type I inositol polyphosphate 5-phosphatase, puta |
| CsaV3_3G002020 | Phenylalanine-tRNA ligase alpha subunit like |
| CsaV3_3G002030 | Two-component response regulator-like aprr2 |
| CsaV3_3G002040 | S-adenosyl-L-methionine-dependent methyltransferases superfamily protein |
| CsaV3_3G002050 | Vacuolar protein sorting-associated protein 55 homolog |
| CsaV3_3G002060 | Haloacid dehalogenase-like hydrolase |
| CsaV3_3G002070 | Haloacid dehalogenase-like hydrolase domain-containing protein |
| CsaV3_3G002080 | Regulator of nonsense transcripts 1 homolog |
| CsaV3_3G002090 | Lon protease homolog 2, peroxisomal |
| CsaV3_3G002100 | Electron carrier/iron ion-binding protein |
| CsaV3_3G002110 | BOI-related E3 ubiquitin-protein ligase 1 |
| CsaV3_3G002120 | Protein of unknown function (DUF581) |
| CsaV3_3G002130 | Poly(U)-specific endoribonuclease |
| CsaV3_3G002140 | Methyltransferase-like protein 13 |
| CsaV3_3G002150 | Beta-amylase |
| CsaV3_3G002160 | Protein kinase, putative |
| CsaV3_3G002170 | Novel plant snare, putative |
| CsaV3_3G002180 | Phosphatidylinositol 4-phosphate 5-kinase 1 |
| CsaV3_3G002190 | Coiled-coil domain-containing protein 97 |
| CsaV3_3G002200 | Calcineurin B-like protein |
| CsaV3_3G002210 | Unknown protein |
| CsaV3_3G002220 | Protein kinase |
| CsaV3_3G002230 | Unknown protein |
| CsaV3_3G002240 | Bifunctional inhibitor/plant lipid transfer protein/seed storage helical domain-containing protein |
| CsaV3_3G002250 | Peptidyl-prolyl cis-trans isomerase-like |
| CsaV3_3G002260 | DVA-1 polyprotein |
| CsaV3_3G002270 | tRNA/rRNA methyltransferase (SpoU) family protein |
| CsaV3_3G002280 | Protein lingerer like |
| CsaV3_3G002290 | Chlorophyll a/b binding family protein |
| CsaV3_3G002300 | ATP-dependent Clp protease proteolytic subunit |
| CsaV3_3G002310 | Unknown protein |
| CsaV3_3G002320 | Histone-lysine N-methyltransferase, H3 lysine-9 specific SUVH6-like |
| CsaV3_3G002330 | Kelch repeat-containing protein |
| CsaV3_3G002340 | GATA transcription factor 26-like |
| CsaV3_3G002350 | Vesicle-associated protein 2-1 |
| CsaV3_3G002360 | 50S ribosomal protein L19, chloroplastic |
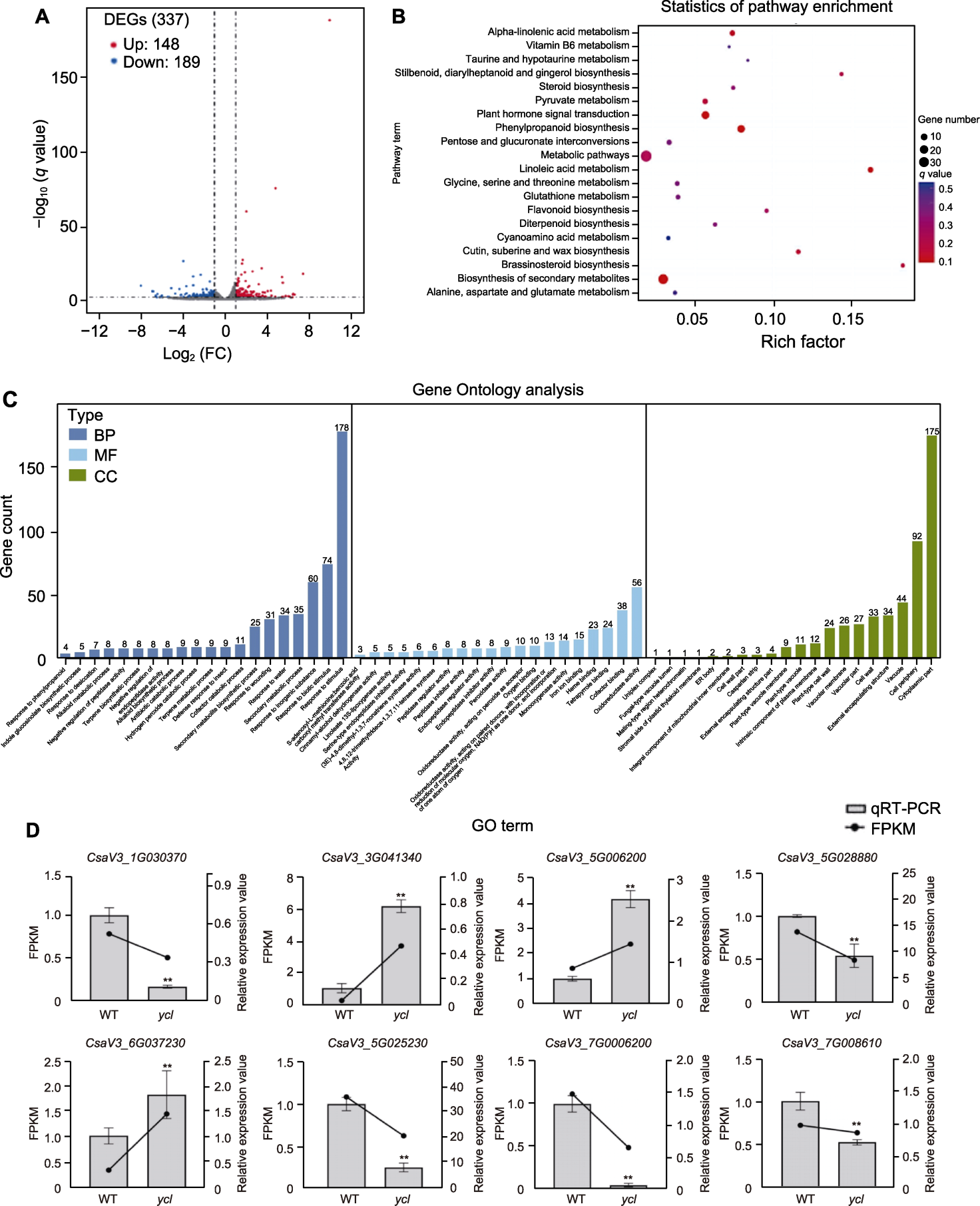
图6 ycl与XYYH-3-1间的RNA-Seq分析 (A) 差异表达基因火山图; (B) 差异表达基因KEGG途径富集散点图; (C) GO term二级分类统计结果; (D) 转录组结果的qRT-PCR验证。BP: 生物过程; MF: 分子功能; CC: 细胞组分; WT: 野生型; DEG: 差异表达基因; FPKM: 每千碱基转录本每百万映射片段数; FC: 倍性变化。**表示差异极显著(t检验, P<0.01)。
Figure 6 RNA-Seq analysis of differential expression between ycl and XYYH-3-1 (A) Differentially expressed genes volcano map; (B) Scatter plot of differentially expressed genes KEGG pathway enrichment; (C) GO term secondary classification statistics; (D) The qRT-PCR verification of transcriptome results. BP: Biological process; MF: Molecular function; CC: Cellular component; WT: Wild type; DEG: Differential expressed gene; FPKM: Fragments per kilobase per million mapped reads; FC: Fold Change. ** denote extremely significant differences (t-test, P<0.01).
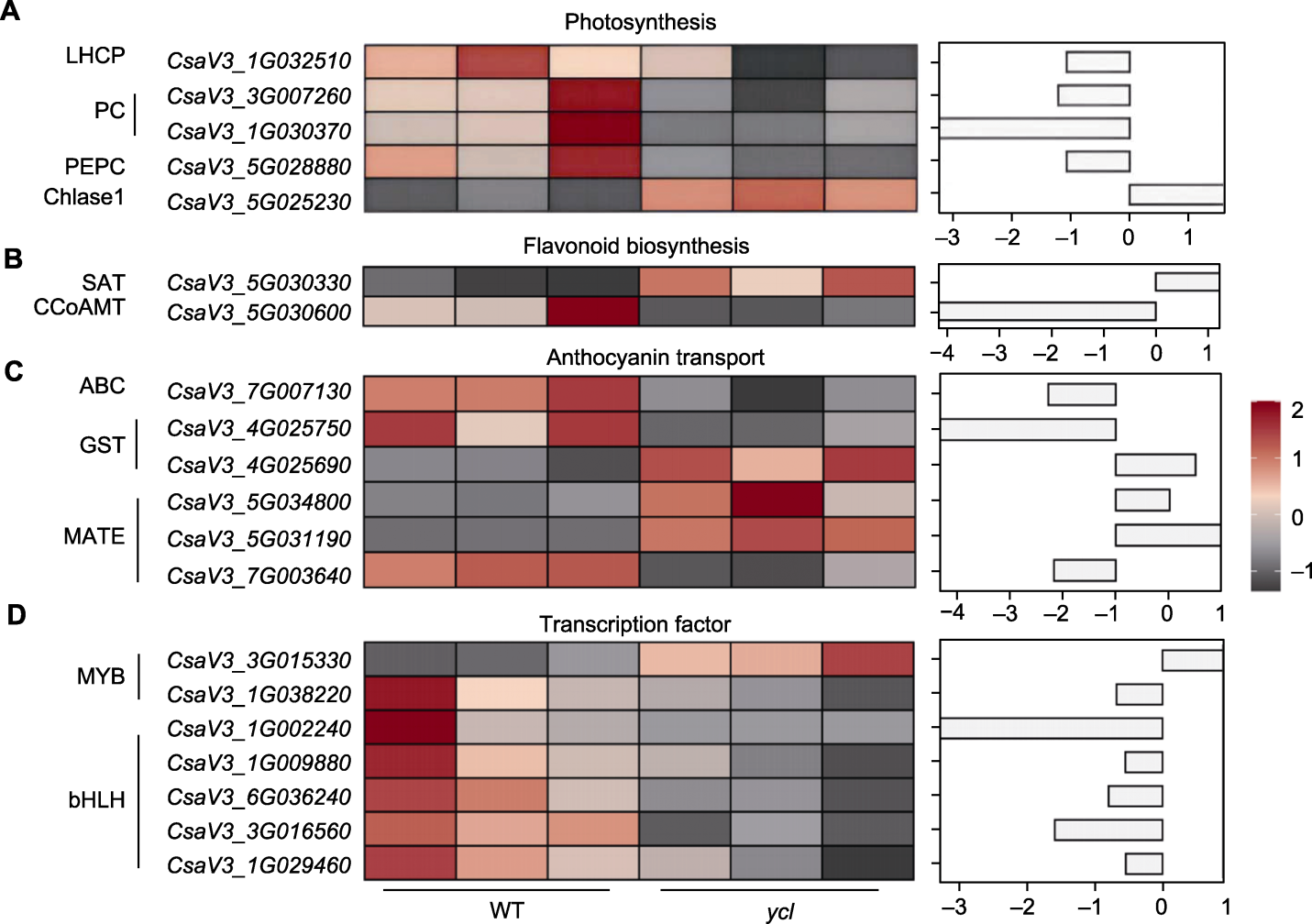
图7 与ycl表型相关的差异表达基因(DEGs) (A) 涉及光合作用的DEGs; (B) 涉及类黄酮生物合成的DEGs; (C) 涉及花青素转运的DEGs; (D) 编码转录因子的DEGs。WT: 野生型; LHCP: 叶绿素a/b结合蛋白; PC: 质体蓝素; PEPC: 磷酸烯醇式丙酮酸羧化酶; Chlase1: 叶绿素酶1; SAT: 司替木丹碱-O-乙酰转移酶; CCoAMT: 咖啡酰辅酶A-3-O甲基转移酶; GST: 谷胱甘肽S-转移酶; MATE: 多药和有毒物排出家族
Figure 7 Differentially expressed genes (DEGs) associated with the ycl phenotype (A) DEGs involved in photosynthesis; (B) DEGs involved in flavonoid biosynthesis; (C) DEGs involved in anthocyanin transport; (D) DEGs encoding transcription factors. WT: Wild type; LHCP: Chlorophyll a/b binding protein; PC: Plastocyanin; PEPC: Phosphoenolpyruvate carboxylase; Chlase1: Chlorophyllase 1; SAT: Stemmadenine O-acetyltransferase; CCoAMT: Trans- caffeoyl-CoA 3-O-methyltransferase; GST: Glutathione-S-transferase; MATE: Multidrug and toxic compound extrusion
| [1] |
Allan AC, Hellens RP, Laing WA (2008). MYB transcription factors that colour our fruit. Trends Plant Sci 13, 99-102.
DOI PMID |
| [2] | Amaresh, Krishnan SG, Vinod KK, Chinnusamy V, Sevanthi ACRMV, Dhandapani R, Ellur RK, Bhowmick PK, Bollinedi H, Umadevi P, Senapati M, Verma RK, Nagarajan M, Dhawan G, Kumar P, Singh AK (2023). Phenotypic characterization of the novel seedling stage zebra leaf mutant, Pusa Zebra 18 in rice. Plant Physiol Rep 28, 500-512. |
| [3] |
Awai K, Xu CC, Tamot B, Benning C (2006). A phosphatidic acid-binding protein of the chloroplast inner envelope membrane involved in lipid trafficking. Proc Natl Acad Sci USA 103, 10817-10822.
DOI PMID |
| [4] | Boase MR, Brendolise C, Wang L, Ngo H, Espley RV, Hellens RP, Schwinn KE, Davies KM, Albert NW (2015). Failure to launch: the self-regulating Md-MYB10R6 gene from apple is active in flowers but not leaves of Petunia. Plant Cell Rep 34, 1817-1823. |
| [5] | Bogorad L (1962). Porphyrin synthesis. Methods Enzymol 5, 885-895. |
| [6] |
Butelli E, Titta L, Giorgio M, Mock HP, Matros A, Peterek S, Schijlen EGWM, Hall RD, Bovy AG, Luo J, Martin C (2008). Enrichment of tomato fruit with health-promoting anthocyanins by expression of select transcription factors. Nat Biotechnol 26, 1301-1308.
DOI PMID |
| [7] | Chen H, Cheng ZJ, Ma XD, Wu H, Liu YL, Zhou KN, Chen YL, Ma WW, Bi JC, Zhang X, Guo XP, Wang JL, Lei CL, Wu FQ, Lin QB, Liu YQ, Liu LL, Jiang L (2013). A knockdown mutation of YELLOW-GREEN LEAF2blocks chlorophyll biosynthesis in rice. Plant Cell Rep 32, 1855-1867. |
| [8] | Chen JY, Zhao J, Liu X, Li C, Lin DZ, Dong YJ, Ye SH, Zhang XM (2010). Genetic analysis and molecular mapping of a new thermosensitive leaf-color mutant in Oryza sativa. Chin Bull Bot 45, 419-425. (in Chinese) |
|
陈佳颖, 赵剑, 刘晓, 李超, 林冬枝, 董彦君, 叶胜海, 张小明 (2010). 一个新水稻温敏感叶色突变体的遗传分析及其基因分子定位. 植物学报 45, 419-425.
DOI |
|
| [9] | Cheng MZ, Meng FY, Mo FL, Chen XL, Zhang H, Wang AX (2022). Insights into the molecular basis of a yellow leaf color mutant (ym) in tomato (Solanum lycopersicum). Sci Hortic 293, 110743. |
| [10] |
Dasgupta K, Thilmony R, Stover E, Oliveira ML, Thomson J (2017). Novel R2R3-MYB transcription factors from Prunus americana regulate differential patterns of anthocyanin accumulation in tobacco and citrus. GM Crops Food 8, 85-105.
DOI PMID |
| [11] | Dei M (1985). Benzyladenine-induced stimulation of 5-aminolevulinic acid accumulation under various light intensities in levulinic acid-treated cotyledons of etiolated cucumber. Physiol Plant 64, 153-160. |
| [12] | Deng LC, Qin P, Liu Z, Wang GL, Chen WL, Tong JH, Xiao LT, Tu B, Sun YT, Yan W, He H, Tan J, Chen XW, Wang YP, Li SG, Ma BT (2017). Characterization and fine-mapping of a novel premature leaf senescence mutant yellow leaf and dwarf 1in rice. Plant Physiol Biochem 111, 50-58. |
| [13] | Ding Y, Yang W, Su CG, Ma HH, Pan Y, Zhang XG, Li JH (2019). Tandem 13-lipoxygenase genes in a cluster confers yellow-green leaf in cucumber. Int J Mol Sci 20, 3102. |
| [14] | Du WK, Hu FR, Yuan SX, Liu C (2020). The identification of key candidate genes mediating yellow seedling lethality in a Lilium regale mutant. Mol Biol Rep 47, 2487-2499. |
| [15] | Gao ML, Hu LL, Li YH, Weng YQ (2016). The chlorophyll-deficient golden leaf mutation in cucumber is due to a single nucleotide substitution in CsChlI for magnesium chelatase I subunit. Theor Appl Genet 129, 1961-1973. |
| [16] | Gao YF, Zhao DH, Zhang JQ, Chen JS, Li JL, Weng Z, Rong LP (2021). De novo transcriptome sequencing and anthocyanin metabolite analysis reveals leaf color of Acer pseudosieboldianum in autumn. BMC Genomics 22, 383. |
| [17] |
Ghangal R, Rajkumar MS, Garg R, Jain M (2020). Genome-wide analysis of glutathione S-transferase gene family in chickpea suggests its role during seed development and abiotic stress. Mol Biol Rep 47, 2749-2761.
DOI PMID |
| [18] | Guan HY, Xu XB, He CM, Liu CX, Liu Q, Dong R, Liu TS, Wang LM (2016). Fine mapping and candidate gene analysis of the leaf-color gene ygl-1 in maize. PLoS One 11, e0153962. |
| [19] | Han HW, Zhou Y, Liu HF, Chen XJ, Wang Q, Zhuang HM, Sun XX, Ling QH, Zhang HJ, Wang BK, Wang J, Tang YP, Wang H, Liu HY (2023). Transcriptomics and metabolomics analysis provides insight into leaf color and photosynthesis variation of the yellow-green leaf mutant of hami melon (Cucumis melo L.). Plants 12, 1623. |
| [20] | Hu LL, Zhang HQ, Xie C, Wang J, Zhang JY, Wang H, Weng YQ, Chen P, Li YH (2020). A mutation in CsHD encoding a histidine and aspartic acid domain-containing protein leads to yellow young leaf-1 (yyl-1) in cucumber (Cucumis sativus L.). Plant Sci 293, 110407. |
| [21] | Huang SN, Liu ZY, Li DY, Yao RP, Hou L, Li X, Feng H (2016). Physiological characterization and comparative transcriptome analysis of a slow-growing reduced-thylakoid mutant of Chinese cabbage (Brassica campestris ssp. pekinensis). Front Plant Sci 7, 3. |
| [22] | Huang WF, Zhang Y, Shen LQ, Fang Q, Liu Q, Gong CB, Zhang C, Zhou Y, Mao C, Zhu YL, Zhang JH, Chen HP, Zhang Y, Lin YJ, Bock R, Zhou F (2020). Accumulation of the RNA polymerase subunit RpoB depends on RNA editing by OsPPR16 and affects chloroplast development during early leaf development in rice. New Phytol 228, 1401-1416. |
| [23] | Jiang SH, Chen M, He NB, Chen XL, Wang N, Sun QG, Zhang TL, Xu HF, Fang HC, Wang YC, Zhang ZY, Wu SJ, Chen XS (2019). MdGSTF6, activated by MdMYB1, plays an essential role in anthocyanin accumulation in ap- ple. Hortic Res 6, 40. |
| [24] |
Jiao FC, Zhao L, Wu XF, Song ZB, Li YP (2020). Metabolome and transcriptome analyses of the molecular mechanisms of flower color mutation in tobacco. BMC Genomics 21, 611.
DOI PMID |
| [25] | Ladygin VG (2006). Spectral features and structure of chloroplasts under an early block of chlorophyll synthesis. Bio- physics 51, 635-644. |
| [26] | Li CH, Zhu LH, Yang HJ, Song RH, Gu WH (2019). Main agronomic characters and biochemical traits of xantha mutant of vegetable soybean. Mol Plant Breed 17, 3726-3734. (in Chinese) |
| 李超汉, 朱丽华, 杨红娟, 宋荣浩, 顾卫红 (2019). 菜用大豆黄化新突变体的主要农艺性状和生理特性. 分子植物育种 17, 3726-3734. | |
| [27] | Li XF, Zhang ZL (2016). Plant Physiology Laboratory Manual (5th edn). Beijing: Science Press. pp. 30-31. (in Chinese) |
| 李小方, 张志良 (2016). 植物生理学实验指导(第5版). 北京: 科学出版社. pp. 30-31. | |
| [28] |
Lin N, Gao YM, Zhou QY, Ping XK, Li JN, Liu LZ, Yin JM (2022). Genetic mapping and physiological analysis of chlorophyll-deficient mutant in Brassica napus L. BMC Plant Biol 22, 244.
DOI PMID |
| [29] | Lin SH, Kuo HF, Canivenc G, Lin CS, Lepetit M, Hsu PK, Tillard P, Lin HL, Wang YY, Tsai CB, Gojon A, Tsay YF (2008). Mutation of the Arabidopsis NRT1.5 nitrate transporter causes defective root-to-shoot nitrate transport. Plant Cell 20, 2514-2528. |
| [30] | Liu J, Wang JY, Yao XY, Zhang Y, Li JQ, Wang XX, Xu ZJ, Chen WF (2015). Characterization and fine mapping of thermo-sensitive chlorophyll deficit mutant1 in rice (Oryza sativa L.). Breed Sci 65, 161-169. |
| [31] | Liu L, Sun TT, Liu XY, Guo Y, Huang X, Gao P, Wang XZ (2019). Genetic analysis and mapping of a striped rind gene (st3) in melon (Cucumis melo L.). Euphytica 215, 20. |
| [32] | Liu P, Li MJ (2016). Experiments in Plant Physiology (2nd edn). Beijing: Science Press. pp. 142-150. (in Chinese) |
| 刘萍, 李明军 (2016). 植物生理学实验(第2版). 北京: 科学出版社. pp. 142-150. | |
| [33] | Liu X, Huang QQ, Yang YR, Tang JY, Zhao YN, Zhang J (2021). Characterization and map-based cloning of the novel rice yellow leaf mutant yl3. J Plant Biol 64, 35-44. |
| [34] | Mao GZ, Wei HL, Hu W, Ma Q, Zhang M, Wang HT, Yu SX (2019). Fine mapping and molecular characterization of the virescent gene vsp in upland cotton (Gossypium hirsutum). Theor Appl Genet 132, 2069-2086. |
| [35] | Miao H, Zhang SP, Wang M, Wang Y, Weng YQ, Gu XF (2016). Fine mapping of virescent leaf gene v-1 in cucumber (Cucumis sativus L.). Int J Mol Sci 17, 1602. |
| [36] |
Michelmore RW, Paran I, Kesseli RV (1991). Identification of markers linked to disease-resistance genes by bulked segregant analysis: a rapid method to detect markers in specific genomic regions by using segregating populations. Proc Natl Acad Sci USA 88, 9828-9832.
DOI PMID |
| [37] | Nagata N, Tanaka R, Satoh S, Tanaka A (2005). Identification of a vinyl reductase gene for chlorophyll synthesis in Arabidopsis thaliana and implications for the evolution of prochlorococcus species. Plant Cell 17, 233-240. |
| [38] | Parks BM, Quail PH (1991). Phytochrome-deficient hy1 and hy2 long hypocotyl mutants of Arabidopsis are defective in phytochrome chromophore biosynthesis. Plant Cell 3, 1177-1186. |
| [39] | Pierce LK, Wehner TC (1990). Review of genes and linkage groups in cucumber. Hortscience 25, 605-615. |
| [40] | Qin R, Zeng DD, Liang R, Yang CC, Akhter D, Alamin M, Jin XL, Shi CH (2017). Rice gene SDL/RNRS1, encoding the small subunit of ribonucleotide reductase, is required for chlorophyll synthesis and plant growth development. Gene 627, 351-362. |
| [41] | Qiu J, Sun SQ, Luo SQ, Zhang JC, Xiao XZ, Zhang LQ, Wang F, Liu SZ (2014). Arabidopsis AtPAP1 transcription factor induces anthocyanin production in transgenic Taraxacum brevicorniculatum. Plant Cell Rep 33, 669-680. |
| [42] | Sakowska K, Alberti G, Genesio L, Peressotti A, Delle Vedove G, Gianelle D, Colombo R, Rodeghiero M, Panigada C, Juszczak R, Celesti M, Rossini M, Haworth M, Campbell BW, Mevy JP, Vescovo L, Cendrero-Mateo MP, Rascher U, Miglietta F (2018). Leaf and canopy photosynthesis of a chlorophyll deficient soybean mutant. Plant Cell Environ 41, 1427-1437. |
| [43] |
Sandhu D, Atkinson T, Noll A, Johnson C, Espinosa K, Boelter J, Abel S, Dhatt BK, Barta T, Singsaas E, Sepsenwol S, Goggi AS, Palmer RG (2016). Soybean proteins GmTic110 and GmPsbP are crucial for chloroplast development and function. Plant Sci 252, 76-87.
DOI PMID |
| [44] |
Schmittgen TD, Livak KJ (2008). Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3, 1101-1108.
DOI PMID |
| [45] | Shim KC, Kang YN, Song JH, Kim YJ, Kim JK, Kim C, Tai TH, Park I, Ahn SN (2023). A frameshift mutation in the Mg-chelatase I subunit gene OsCHLI is associated with a lethal chlorophyll-deficient, yellow seedling phenotype in rice. Plants 12, 2831. |
| [46] | Song MF, Wei QZ, Wang J, Fu WY, Qin XD, Lu XM, Cheng F, Yang K, Zhang L, Yu XQ, Li J, Chen JF, Lou QF (2018). Fine mapping of CsVYL, conferring virescent leaf through the regulation of chloroplast development in cucumber. Front Plant Sci 9, 432. |
| [47] | Stern DB, Hanson MR, Barkan A (2004). Genetics and genomics of chloroplast biogenesis: maize as a model system. Trends Plant Sci 9, 293-301. |
| [48] | Sun Y, Bai PP, Gu KJ, Yang SZ, Lin HY, Shi CG, Zhao YP (2022). Dynamic transcriptome and network-based analysis of yellow leaf mutant Ginkgo biloba. BMC Plant Biol 22, 465. |
| [49] | Tanaka R, Tanaka A (2011). Chlorophyll cycle regulates the construction and destruction of the light-harvesting complexes. Biochim Biophys Acta Bioenerg 1807, 968-976. |
| [50] |
Wu ZM, Zhang X, He B, Diao LP, Sheng SL, Wang JL, Guo XP, Su N, Wang LF, Jiang L, Wang CM, Zhai HQ, Wan JM (2007). A chlorophyll-deficient rice mutant with impaired chlorophyllide esterification in chlorophyll biosynthesis. Plant Physiol 145, 29-40.
DOI PMID |
| [51] |
Xie Y, Tan HJ, Ma ZX, Huang JR (2016). DELLA proteins promote anthocyanin biosynthesis via sequestering MYBL2 and JAZ suppressors of the MYB/bHLH/WD40 complex in Arabidopsis thaliana. Mol Plant 9, 711-721.
DOI PMID |
| [52] | Xiong LR, Du H, Zhang KY, Lv D, He HL, Pan JS, Cai R, Wang G (2021). A mutation in CsYL2.1 encoding a plastid isoform of triose phosphate isomerase leads to yellow leaf 2.1 (yl2.1) in cucumber (Cucumis sativus L.). Int J Mol Sci 22, 322. |
| [53] |
Xu BH, Zhang CY, Gu Y, Cheng R, Huang DY, Liu X, Sun YD (2023). Physiological and transcriptomic analysis of a yellow leaf mutant in watermelon. Sci Rep 13, 9647.
DOI PMID |
| [54] |
Yin JJ, Zhu XB, Yuan C, Wang J, Li WT, Wang YP, He M, Cheng QS, Ye BQ, Chen WL, Linghu QY, Wang JC, Ma BT, Qin P, Li SG, Chen XW (2015). Characterization and fine mapping of a novel vegetative senescence lethal mutant locus in rice. J Genet Genomics 42, 511-514.
DOI PMID |
| [55] | Zhang HT, Li JJ, Yoo JH, Yoo SC, Cho SH, Koh HJ, Seo HS, Paek NC (2006). Rice Chlorina-1 and Chlorina-9 encode ChlD and ChlI subunits of Mg-chelatase, a key enzyme for chlorophyll synthesis and chloroplast development. Plant Mol Biol 62, 325-337. |
| [56] | Zhang KJ, Li Y, Zhu WW, Wei YF, Njogu MK, Lou QF, Li J, Chen JF (2020b). Fine mapping and transcriptome analysis of virescent leaf gene v-2 in cucumber (Cucumis sativus L.). Front Plant Sci 11, 570817. |
| [57] | Zhang TT, Dong XY, Yuan X, Hong YY, Zhang LL, Zhang X, Chen SX (2022). Identification and characterization of CsSRP43, a major gene controlling leaf yellowing in cucumber. Hortic Res 9, uhac212. |
| [58] | Zhao CJ, Liu LJ, Safdar LB, Xie ML, Cheng XH, Liu YY, Xiang Y, Tong CB, Tu JX, Huang JY, Liu SY (2020a). Characterization and fine mapping of a yellow-virescent gene regulating chlorophyll biosynthesis and early stage chloroplast development in Brassica napus. G3(Bethesda) 10, 3201-3211. |
| [59] | Zhong XM, Sun SF, Li FH, Wang J, Shi ZS (2015). Photosynthesis of a yellow-green mutant line in maize. Photosynthetica 53, 499-505. |
| [60] | Zhou H, Lin-Wang K, Wang HL, Gu C, Dare AP, Espley RV, He HP, Allan AC, Han YP (2015). Molecular genetics of blood-fleshed peach reveals activation of anthocyanin biosynthesis by NAC transcription factors. Plant J 82, 105-121. |
| [61] |
Zhu HY, Zhang MJ, Sun SR, Yang S, Li JX, Li H, Yang HH, Zhang KG, Hu JB, Liu DM, Yang LM (2019). A single nucleotide deletion in an ABC transporter gene leads to a dwarf phenotype in watermelon. Front Plant Sci 10, 1399.
DOI PMID |
| [62] | Zhu XY, Guo S, Wang ZW, Du Q, Xing YD, Zhang TQ, Shen WQ, Sang XC, Ling YH, He GH (2016). Map-based cloning and functional analysis of YGL8, which controls leaf colour in rice (Oryza sativa). BMC Plant Biol 16, 134. |
| [1] | 李彬琪, 闫佳慧, 李豪, 辛伟, 田云鹤, 杨贞标, 唐文鑫. 黄瓜卷须缠绕过程中小G蛋白活性变化[J]. 植物学报, 2022, 57(3): 299-307. |
| [2] | 王泽义, 张恒嘉, 王玉才, 陈谢田, 巴玉春. 亏缺灌溉对板蓝根叶片光合生理特性及产量的影响[J]. 植物学报, 2020, 55(6): 705-714. |
| [3] | 刘建福, 陈育才, 王文建, 王河川, 蔡金福, 王明元, 李丹丹, 张斌, 黄昆. 航天搭载对武夷名丛相关生理及生长特性的影响[J]. 植物学报, 2020, 55(5): 564-572. |
| [4] | 曹栋栋,陈珊宇,秦叶波,吴华平,阮关海,黄玉韬. 水杨酸调控盐胁迫下羽衣甘蓝种子萌发的机理[J]. 植物学报, 2020, 55(1): 49-61. |
| [5] | 杨德卫,王莫,韩利波,唐定中,李生平. 水稻稻瘟病抗性基因的克隆、育种利用及稻瘟菌无毒基因研究进展[J]. 植物学报, 2019, 54(2): 265-276. |
| [6] | 阿曼古丽·买买提阿力, 拉扎提·努尔布拉提, 高丽丽, 张巨松, 田立文. 盐胁迫对海岛棉和陆地棉幼苗生长及 生理特性的影响[J]. 植物学报, 2017, 52(4): 465-473. |
| [7] | 胡朝芹, 刘剑宇, 王韵茜, 杨睿, 汪秉琨, 何月秋, 曾千春, 罗琼. 粳稻子预44抗LP11稻瘟病菌基因Pizy6(t)的定位[J]. 植物学报, 2017, 52(1): 61-69. |
| [8] | 王伟, 王嘉宇, 杨生龙, 刘进, 董晓雁, 王国骄, 陈温福. 水稻窄卷叶突变体nrl7的鉴定与基因定位[J]. 植物学报, 2016, 51(3): 290-295. |
| [9] | 邓磊, 杜敏敏, 李传友. 中国科学家在番茄与黄瓜的驯化及品质形成的分子机理研究中取得突破性进展[J]. 植物学报, 2015, 50(3): 275-278. |
| [10] | 刘丹, 王嘉宇, 刘进, 马殿荣, 赵明辉, 陈温福. 水稻散状穗突变体sp的遗传分析及基因初定位[J]. 植物学报, 2015, 50(2): 198-205. |
| [11] | 蒋钰东, 何沛龙, 廖红香, 张孝波, 吴国超, 何光华, 林婷婷, 桑贤春. 水稻茎秆脆性及叶尖枯死突变体fld1的鉴定与基因定位[J]. 植物学报, 2014, 49(6): 663-671. |
| [12] | 王烨, 顾兴芳, 张圣平, 苗晗, 陈国华, 谢丙炎. RNAi载体导入黄瓜的遗传转化体系[J]. 植物学报, 2014, 49(2): 183-189. |
| [13] | 孙涌栋, 罗未蓉, 李新峥, 王广印. NaHS对NaHCO3胁迫下黑籽南瓜种子萌发及生理特性的影响[J]. 植物学报, 2014, 49(1): 98-104. |
| [14] | 王环环, 蔡强, 陈明姣, 罗治靖, 张大兵, 袁政. 水稻花器官突变体apl (abnormal palea and lodicules) 的表型分析与基因初定位[J]. 植物学报, 2014, 49(1): 1-7. |
| [15] | 张现伟, 李经勇, 郑家奎, 唐永群, 雷祖燕, 程杨, 姚雄. 复粒稻种质资源及其遗传育种利用[J]. 植物学报, 2013, 48(4): 438-446. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||