

植物学报 ›› 2021, Vol. 56 ›› Issue (3): 339-346.DOI: 10.11983/CBB20180 cstr: 32102.14.CBB20180
崔晓敏1,2, 季东超1,2, 陈彤1,*( ), 田世平1,2
), 田世平1,2
收稿日期:2020-11-11
接受日期:2021-02-25
出版日期:2021-05-01
发布日期:2021-04-30
通讯作者:
陈彤
作者简介:*E-mail: chentong@ibcas.ac.cn基金资助:
Xiaomin Cui1,2, Dongchao Ji1,2, Tong Chen1,*( ), Shiping Tian1,2
), Shiping Tian1,2
Received:2020-11-11
Accepted:2021-02-25
Online:2021-05-01
Published:2021-04-30
Contact:
Tong Chen
摘要: 植物细胞依赖细胞质膜上的受体感知并传递环境信号, 而受体通过与配体特异结合启动一系列下游信号转导途径, 维持植物正常的生命活动及其对外界环境变化的适应。类受体激酶是其中一类重要受体, 通常由胞外结合结构域、跨膜结构域和胞内激酶结构域3部分组成, 是植物适应外界环境变化的重要调节枢纽。FER属于CrRLK1L类受体蛋白激酶家族, 最早被发现在高等植物雌雄配子体识别过程中发挥作用。随后, 众多研究表明, FER在植物生长发育、激素间交互作用、植物与病原菌互作和逆境响应等多种生物学过程中扮演重要角色, 是近年来植物信号通路研究领域的“明星蛋白”。随着植物病理学研究的不断深入, FER在植物与病原菌互作过程中的功能备受关注。该文主要综述FER调节植物与病原菌互作的研究进展, 旨在为进一步解析类受体蛋白激酶在植物细胞响应病原菌侵染过程中的信号转导机制提供参考。
崔晓敏, 季东超, 陈彤, 田世平. 类受体激酶FER调节植物与病原菌相互作用的分子机制. 植物学报, 2021, 56(3): 339-346.
Xiaomin Cui, Dongchao Ji, Tong Chen, Shiping Tian. Advances in the Studies on Molecular Mechanism of Receptor-like Protein Kinase FER Regulating Host Plant-pathogen Interaction. Chinese Bulletin of Botany, 2021, 56(3): 339-346.
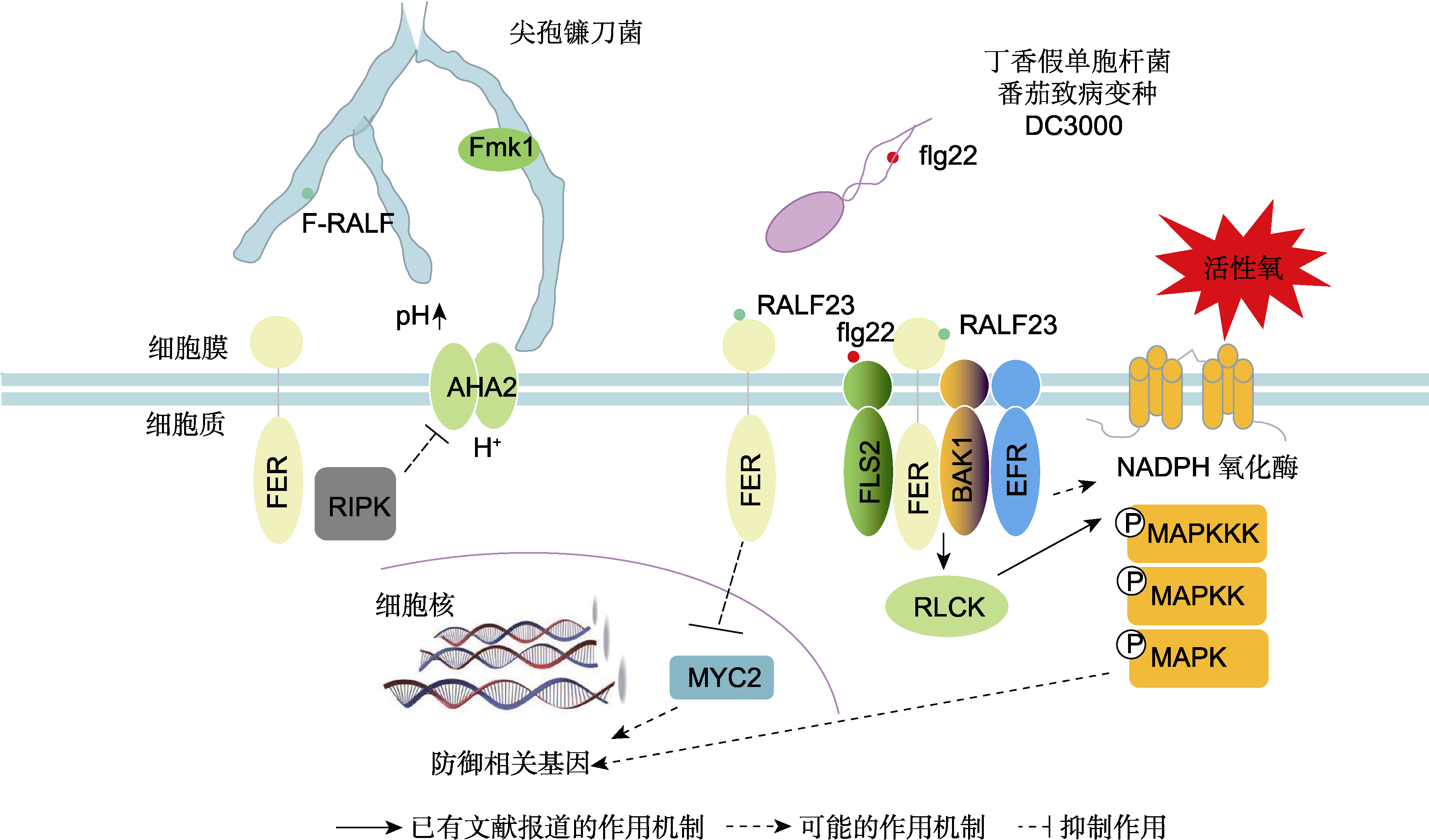
图1 FER介导的抗病相关途径示意图 FER定位于质膜微区, 能够识别尖孢镰刀菌(Fusarium oxysporium)分泌的F-RALF, 阻断AHA2介导的H+外流, 从而诱导根系胞外环境碱化, 增强真菌对植物的致病力。在此过程中, 尖孢镰刀菌细胞中丝裂原活化蛋白激酶(MAPK)途径中的Fmk1对其侵染至关重要。FER与RALF23相互作用, 负向调控茉莉酸和冠菌素信号, 正向促进植物免疫; 拟南芥(Arabidopsis thaliana) SITE-1蛋白酶(S1P)剪切内源快速碱化因子(RALF)前体肽, 而RALF与拟南芥FER相互作用抑制FLS2、BAK1和EFR免疫复合物的形成, 抑制植物免疫。FER可能调节细胞内活性氧的积累和MAPK活性。F-RALF: 尖孢镰刀菌分泌的快速碱化因子; AHA2: H+-ATPase 2; RIPK: RESISTANCE TO Pseudomonas syringae pv. maculicola 1-INDUCED PROTEIN KINASE; Fmk1: 一种保守的真菌丝裂原活化蛋白激酶(MAPK); flg22: 鞭毛蛋白抗原表位22; RALF23: 内源性肽快速碱化因子23; MYC2: MYELOCYTOMATOSIS PRO- TEINS 2; EFR: ELONGATION FACTOR THERMO UNSTABLE RECEPTOR; FLS2: FLAGELLIN-SENSING 2; BAK1: BRAS- SINOSTEROID INSENSITIVE 1-ASSOCIATED KINASE 1; RLCK: 类受体胞质激酶; MAPK: 丝裂原活化蛋白激酶; NADPH: 烟酰胺腺嘌呤二核苷酸磷酸
Figure 1 A schematic diagram of the disease resistance pathway mediated by FER The microdomain-localized FER in the plasma membrane can recognize F-RALF secreted by Fusarium oxysporium and further block AHA2-mediated H+ outflow, thus inducing the alkalinization of extracellular environment and increasing the pathogenicity of F. oxysporium. During this process, Fmk1 in the mitogen-activated protein kinase (MAPK) pathway in F. oxysporium cells is critical for infection. FER interacts with RALF23, negatively regulating JA and COR signals and positively promoting plant immunity; the SITE-1 protease (S1P) can digest endogenous fast alkalization factor (RALF) precursor peptide and the mature RALF further interacts with FER to inhibit the formation of immune complexes (mainly FLS2, BAK1, and EFR), inhibits plant immunity. FER may regulate intracellular ROS accumulation and MAPK signaling. F-RALF: F. oxysporium RALF; AHA2: H+-ATPase 2; RIPK: RESISTANCE TO Pseudomonas syringae pv. maculicola 1-INDUCED PROTEIN KINASE; Fmk1: A con- served fungal MAPK; flg22: Flagellin epitope 22; RALF23: The endogenous peptide RALF 23; MYC2: MYELOCYTOMATOSIS PROTEINS 2; EFR: ELONGATION FACTOR THERMO UNSTABLE RECEPTOR; FLS2: FLAGELLIN-SENSING 2; BAK1: BRASSINOSTEROID INSENSITIVE 1-ASSOCIATED KINASE 1; RLCK: Receptor-like cytoplasmic kinase; MAPK: Mitogenactivated protein kinase; NADPH: Nicotinamide adenine dinucleotide phosphate
| 互作蛋白 | 涉及物种 | 配体 | 主要功能/结论 | 参考文献 |
|---|---|---|---|---|
| FER与病原菌分泌的 F-RALF互作 | 拟南芥(Arabidopsis thaliana)和尖孢镰刀菌(Fusarium oxysporium) | F-RALF | FER识别尖孢镰刀菌分泌的F- RALF, 阻断质膜H+-ATPase2诱导胞外环境碱化, 从而负调控拟南芥对尖孢镰刀菌的抗性 | |
| FER与转录因子MYC2 互作 | 拟南芥和丁香假单胞杆菌番茄致病变种(Pseudomonas syrin- gae pv. tomato DC3000) | RALF23 | FER正调控拟南芥对Pst DC3000 的抗性 | |
| FER与FLS2、EFR和BAK1形成免疫复合体 | 拟南芥 | RALF23 | 拟南芥SITE-1蛋白酶剪切内源性快速碱化因子(RALF)前肽, 抑制植物免疫 | |
| FER与MLO家族成员NORTIA协同作用 | 拟南芥和高氏白粉病(Golovinomyces orontii) | FER负调节拟南芥对高氏白粉病的抗性 | ||
| FER与FLS2和BAK1互作 | 拟南芥和丁香假单胞杆菌番茄致病变种 | RALF23 | fer突变体出现氧化爆发、MAPK活性、气孔开合和细胞死亡等方面的功能缺陷 |
表1 FER调节植物细胞与病原菌间的相互作用
Table 1 FER regulates the interaction between host plant cells and pathogens
| 互作蛋白 | 涉及物种 | 配体 | 主要功能/结论 | 参考文献 |
|---|---|---|---|---|
| FER与病原菌分泌的 F-RALF互作 | 拟南芥(Arabidopsis thaliana)和尖孢镰刀菌(Fusarium oxysporium) | F-RALF | FER识别尖孢镰刀菌分泌的F- RALF, 阻断质膜H+-ATPase2诱导胞外环境碱化, 从而负调控拟南芥对尖孢镰刀菌的抗性 | |
| FER与转录因子MYC2 互作 | 拟南芥和丁香假单胞杆菌番茄致病变种(Pseudomonas syrin- gae pv. tomato DC3000) | RALF23 | FER正调控拟南芥对Pst DC3000 的抗性 | |
| FER与FLS2、EFR和BAK1形成免疫复合体 | 拟南芥 | RALF23 | 拟南芥SITE-1蛋白酶剪切内源性快速碱化因子(RALF)前肽, 抑制植物免疫 | |
| FER与MLO家族成员NORTIA协同作用 | 拟南芥和高氏白粉病(Golovinomyces orontii) | FER负调节拟南芥对高氏白粉病的抗性 | ||
| FER与FLS2和BAK1互作 | 拟南芥和丁香假单胞杆菌番茄致病变种 | RALF23 | fer突变体出现氧化爆发、MAPK活性、气孔开合和细胞死亡等方面的功能缺陷 |
| 1 | 季东超, 宋凯, 邢晶晶, 陈彤, 田世平 (2015). LysM蛋白介导植物免疫防卫反应及其信号激发的研究进展. 植物学报 50, 628-636. |
| 2 | 朱巍巍, 马天意, 张梅娟, 沙伟 (2018). 类受体蛋白激酶在植物中的研究进展. 基因组学与应用生物学 37, 451-458. |
| 3 | Chen J, Liu SR, Ming ZH, Liu XM, Yu F (2020). FERONIA cytoplasmic domain: node of varied signal outputs. aBIO- TECH 1, 135-146. |
| 4 |
Cheung AY, Wu HM (2011). Theseus 1, FERONIA and relatives: a family of cell wall-sensing receptor kinases? Curr Opin Plant Biol 14, 632-641.
DOI URL |
| 5 |
Deslauriers SD, Larsen PB (2010). FERONIA is a key modulator of brassinosteroid and ethylene responsiveness in Arabidopsis hypocotyls. Mol Plant 3, 626-640.
DOI PMID |
| 6 |
Duan QH, Kita D, Li C, Cheung AY, Wu HM (2010). FERONIA receptor-like kinase regulates RHO GTPase signaling of root hair development. Proc Natl Acad Sci USA 107, 17821-17826.
DOI URL |
| 7 |
Duan QL, Liu MCJ, Kita D, Jordan SS, Yeh FLJ, Yvon R, Carpenter H, Federico AN, Garcia-Valencia LE, Eyles SJ, Wang CS, Wu HM, Cheung AY (2020). FERONIA controls pectin- and nitric oxide-mediated male-female interaction. Nature 579, 561-566.
DOI URL |
| 8 |
Escobar-Restrepo JM, Huck N, Kessler S, Gagliardini V, Gheyselinck J, Yang WC, Grossniklaus U (2007). The FERONIA receptor-like kinase mediates male-female interactions during pollen tube reception. Science 317, 656-660.
DOI URL |
| 9 |
Gjetting SK, Mahmood K, Shabala L, Kristensen A, Shabala S, Palmgren M, Fuglsang AT (2020). Evidence for multiple receptors mediating RALF-triggered Ca 2+ sig- naling and proton pump inhibition. Plant J 104, 433-446.
DOI URL |
| 10 |
Guo HQ, Li L, Ye HX, Yu XF, Algreen A, Yin YH (2009). Three related receptor-like kinases are required for optimal cell elongation in Arabidopsis thaliana. Proc Natl Acad Sci USA 106, 7648-7653.
DOI URL |
| 11 |
Guo HQ, Nolan TM, Song GY, Liu SZ, Xie ZL, Chen JN, Schnable PS, Walley JW, Yin YH (2018). FERONIA receptor kinase contributes to plant immunity by suppres- sing jasmonic acid signaling in Arabidopsis thaliana. Curr Biol 28, 3316-3324.
DOI URL |
| 12 |
Huang GQ, Li E, Ge FR, Li S, Wang Q, Zhang CQ, Zhang Y (2013). Arabidopsis RopGEF4 and RopGEF10 are important for FERONIA-mediated developmental but not environmental regulation of root hair growth. New Phytol 200, 1089-1101.
DOI URL |
| 13 |
Huang YY, Liu XX, Xie Y, Lin XY, Hu ZJ, Wang H, Wang LF, Dang WQ, Zhang LL, Zhu Y, Feng H, Pu M, Zhao JQ, Zhang JW, Li Y, Fan J, Wang WM (2020 a). Identification of FERONIA-like receptor genes involved in rice- Magnaporthe oryzae interaction. Phytopathol Res 2, 14.
DOI URL |
| 14 |
Huang YY, Yin CC, Liu J, Feng BM, Ge DD, Kong L, Ortiz-Morea FA, Richter J, Hauser MT, Wang WM, Shan LB, He P (2020b). A trimeric CrRLK1L-LLG1 complex genetically modulates SUMM2-mediated autoimmunity. Nat Commun 11, 4859.
DOI URL |
| 15 |
Huck N, Moore JM, Federer M, Grossniklaus U (2003). The Arabidopsis mutant feronia disrupts the female gametophytic control of pollen tube reception. Development 130, 2149-2159.
DOI URL |
| 16 |
Ji DC, Chen T, Zhang ZQ, Li BQ, Tian SP (2020b). Versatile roles of the receptor-like kinase feronia in plant growth, development and host-pathogen interaction. Int J Mol Sci 21, 7881.
DOI URL |
| 17 |
Ji DC, Cui XM, Qin GZ, Chen T, Tian SP (2020a). SlFERL interacts with S-adenosylmethionine synthetase to regulate fruit ripening. Plant Physiol 184, 2168-2181.
DOI URL |
| 18 |
Jia MR, Ding N, Zhang Q, Xing SN, Wei LZ, Zhao YY, Du P, Mao WW, Li JZ, Li BB, Jia WS (2017a). A FERONIA-like receptor kinase regulates strawberry (Fragaria × ananassa) fruit ripening and quality formation. Front Plant Sci 8, 1099.
DOI URL |
| 19 |
Jia MR, Du P, Ding N, Zhang Q, Xing SN, Wei LZ, Zhao YY, Mao WW, Li JZ, Li BB, Jia WS (2017b). Two FERONIA-like receptor kinases regulate apple fruit ripe- ning by modulating ethylene production. Front Plant Sci 8, 1406.
DOI URL |
| 20 |
Jose J, Ghantasala S, Choudhury SR (2020). Arabidopsis transmembrane receptor-like kinases (RLKs): a bridge between extracellular signal and intracellular regulatory machinery. Int J Mol Sci 21, 4000.
DOI URL |
| 21 |
Keinath NF, Kierszniowska S, Lorek J, Bourdais G, Kessler SA, Shimosato-Asano H, Grossniklaus U, Schulze WX, Robatzek S, Panstruga R (2010). PAMP (pathogen-associated molecular pattern)-induced changes in plasma membrane compartmentalization reveal novel components of plant immunity. J Biol Chem 285, 39140-39149.
DOI URL |
| 22 |
Kessler SA, Shimosato-Asano H, Keinath NF, Wuest SE, Ingram G, Panstruga R, Grossniklaus U (2010). Conserved molecular components for pollen tube reception and fungal invasion. Science 330, 968-971.
DOI URL |
| 23 |
Li S, Zhang Y (2014). To grow or not to grow: FERONIA has her say. Mol Plant 7, 1261-1263.
DOI URL |
| 24 |
Liu J, Huang YY, Kong L, Yu X, Feng BM, Liu DR, Zhao BY, Mendes GC, Yuan PG, Ge DD, Wang WM, Fontes EPB, Li PW, Shan LB, He P (2020). The malectin-like receptor-like kinase LETUM1 modulates NLR protein SUMM2 activation via MEKK2 scaffolding. Nat Plants 6, 1106-1115.
DOI URL |
| 25 |
Mao DD, Yu F, Li J, Van de Poel B, Tan D, Li JL, Liu YQ, Li XS, Dong MQ, Chen LB, Li DP, Luan S (2015). FERONIA receptor kinase interacts with S-adenosylme- thionine synthetase and suppresses S-adenosylme-thio- nine production and ethylene biosynthesis in Arabidopsis. Plant Cell Environ 38, 2566-2574.
DOI URL |
| 26 |
Masachis S, Segorbe D, Turrà D, Leon-Ruiz M, Fürst U, El Ghalid M, Leonard G, López-Berges MS, Richards TA, Felix G, Di Pietro A (2016). A fungal pathogen secretes plant alkalinizing peptides to increase infection. Nat Microbiol 1, 16043.
DOI PMID |
| 27 |
Ngo QA, Vogler H, Lituiev DS, Nestorova A, Grossniklaus U (2014). A calcium dialog mediated by the FERONIA signal transduction pathway controls plant sperm delivery. Dev Cell 29, 491-500.
DOI URL |
| 28 |
Shih HW, Miller ND, Dai C, Spalding EP, Monshausen GB (2014). The receptor-like kinase FERONIA is required for mechanical signal transduction in Arabidopsis seedlings. Curr Biol 24, 1887-1892.
DOI URL |
| 29 |
Shiu SH, Karlowski WM, Pan RS, Tzeng YH, Mayer KFX, Li WH (2004). Comparative analysis of the receptor-like kinase family in Arabidopsis and rice. Plant Cell 16, 1220-1234.
DOI URL |
| 30 |
Stegmann M, Monaghan J, Smakowska-Luzan E, Rove- nich H, Lehner A, Holton N, Bellkhadir Y, Zipfel C (2017). The receptor kinase FER is a RALF-regulated scaffold controlling plant immune signaling. Science 355, 287-289.
DOI URL |
| 31 |
Tang DZ, Wang GX, Zhou JM (2017). Receptor kinases in plant-pathogen interactions: more than pattern recognition. Plant Cell 29, 618-637.
DOI URL |
| 32 |
Xin XF, He SY (2013). Pseudomonas syringae pv. tomato DC3000: a model pathogen for probing disease susceptibility and hormone signaling in plants. Annu Rev Phytopathol 51, 473-498.
DOI URL |
| 33 |
Yu F, Qian LC, Nibau C, Duan QH, Kita D, Levasseur K, Li XQ, Lu CQ, Li H, Hou CC, Li LG, Buchanan BB, Chen LB, Cheung AY, Li DP, Luan S (2012). FERONIA receptor kinase pathway suppresses abscisic acid signaling in Arabidopsis by activating ABI2 phosphatase. Proc Natl Acad Sci USA 109, 14693-14698.
DOI URL |
| 34 | Yu M, Li RL, Cui YN, Chen WJ, Li B, Zhang X, Bu YF, Cao YY, Xing JJ, Jewaria PK, Li XJ, Bhalerao RP, Yu F, Lin JX (2020). The RALF1-FERONIA interaction modulates endocytosis to mediate control of root growth in Arabidopsis. Development 147, dev189902. |
| 35 |
Zhang X, Peng H, Zhu SR, Xing JJ, Li X, Zhu ZZ, Zheng JY, Wang L, Wang BQ, Chen J, Ming ZH, Yao K, Jian JZ, Luan S, Coleman-Derr D, Liao HD, Peng YS, Peng DL, Yu F (2020a). Nematode-encoded RALF peptide mi- mics facilitate parasitism of plants through the FERONIA receptor kinase. Mol Plant 13, 1434-1454.
DOI URL |
| 36 |
Zhang X, Yang ZH, Wu DS, Yu F (2020b). RALF-FERONIA signaling: linking plant immune response with cell growth. Plant Commun 1, 100084.
DOI URL |
| 37 |
Zhao CZ, Zayed O, Yu ZP, Jiang W, Zhu PP, Hsu CC, Zhang LR, Tao WA, Lozano-Durán R, Zhu JK (2018). Leucine-rich repeat extensin proteins regulate plant salt tolerance in Arabidopsis. Proc Natl Acad Sci USA 115, 13123-13128.
DOI URL |
| 38 |
Zheng XY, Spivey NW, Zeng WQ, Liu PP, Fu ZQ, Klessig DF, He SY, Dong XN (2012). Coronatine promotes Pseu- domonas syringae virulence in plants by activating a signaling cascade that inhibits salicylic acid accumulation. Cell Host Microbe 11, 587-596.
DOI URL |
| [1] | 宋威, 程才, 王嘉伟, 吴纪华. 土壤微生物对植物多样性–生态系统功能关系的调控作用[J]. 生物多样性, 2025, 33(4): 24579-. |
| [2] | 马亮, 杨永青, 郭岩. “后绿色革命”基因——助力培育“气候智能”作物新品种[J]. 植物学报, 2025, 60(4): 489-498. |
| [3] | 何璐梅, 马伯军, 陈析丰. 植物执行者抗病基因研究进展[J]. 植物学报, 2024, 59(4): 671-680. |
| [4] | 周玉滢, 陈辉, 刘斯穆. 植物非典型Aux/IAA蛋白应答生长素研究进展[J]. 植物学报, 2024, 59(4): 651-658. |
| [5] | 王琪, 吴允哲, 刘学英, 孙丽莉, 廖红, 傅向东. 类受体激酶调控水稻生长发育和环境适应研究进展[J]. 植物学报, 2023, 58(2): 199-213. |
| [6] | 蔡畅, 张雪, 朱晨, 赵郁豪, 乔格侠, 丁平. 千岛湖片段化生境中蚜虫群落嵌套格局的形成: 岛屿面积和寄主植物多样性的作用[J]. 生物多样性, 2023, 31(12): 23183-. |
| [7] | 李聪, 齐立娟, 谷晓峰, 李继刚. 植物光信号途径重要新调控因子TZP的研究进展[J]. 植物学报, 2022, 57(5): 579-587. |
| [8] | 贾利霞, 齐艳华. 生长素代谢、运输及信号转导调控水稻粒型研究进展[J]. 植物学报, 2022, 57(3): 263-275. |
| [9] | 支添添, 周舟, 韩成云, 任春梅. PAD4突变加速拟南芥酪氨酸降解缺陷突变体sscd1的程序性细胞死亡[J]. 植物学报, 2022, 57(3): 288-298. |
| [10] | 宋松泉, 刘军, 杨华, 张文虎, 张琪, 高家东. 细胞分裂素调控种子发育、休眠与萌发的研究进展[J]. 植物学报, 2021, 56(2): 218-231. |
| [11] | 王伟, 唐定中. 两类免疫受体强强联手筑牢植物免疫防线[J]. 植物学报, 2021, 56(2): 142-146. |
| [12] | 李慢如, 张玲. 桑寄生植物繁殖物候研究概述[J]. 生物多样性, 2020, 28(7): 833-841. |
| [13] | 杨程惠子,唐先宇,李威,夏石头. NLR及其在植物抗病中的调控作用[J]. 植物学报, 2020, 55(4): 497-504. |
| [14] | 张娜,刘秀霞,陈学森,吴树敬. 基于转录组分析鉴定苹果茉莉素响应基因[J]. 植物学报, 2019, 54(6): 733-743. |
| [15] | 胡孔琴, 丁兆军. 非TIR1受体依赖型激活生长素信号的新机制[J]. 植物学报, 2019, 54(3): 293-295. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||