

植物学报 ›› 2020, Vol. 55 ›› Issue (4): 397-402.DOI: 10.11983/CBB20099 cstr: 32102.14.CBB20099
• 热点评述 • 下一篇
收稿日期:2020-05-28
接受日期:2020-06-02
出版日期:2020-07-01
发布日期:2020-06-11
通讯作者:
姚瑞枫,谢道昕
基金资助:
Ruifeng Yao1,*( ),Daoxin Xie2,*(
),Daoxin Xie2,*( )
)
Received:2020-05-28
Accepted:2020-06-02
Online:2020-07-01
Published:2020-06-11
Contact:
Ruifeng Yao,Daoxin Xie
摘要: 植物激素信号传导途径中的抑制子(repressor) DELLA、AUX/IAA、JAZ和D53/SMXL均结合下游转录因子并抑制其转录活性, 从而阻遏激素响应基因的表达; 激素分子则激活信号传导链降解抑制子、释放转录因子, 从而诱导响应基因表达并介导相应的生物学功能。中国科学院遗传与发育生物学研究所李家洋研究团队最新的研究发现, 独脚金内酯(SL)信号途径中的SMXL6、SMXL7和SMXL8是具有抑制子和转录因子双重功能的新型抑制子, 他们还通过研究SL转录调控网络发现了大量新的SL响应基因, 揭示了SL调控植物分枝、叶片伸长和花色素苷积累的分子机制。这些重要发现为探索植物激素作用机理提供了新思路, 具有重要科学意义和应用前景。
姚瑞枫,谢道昕. 独脚金内酯信号途径的新发现——抑制子也是转录因子. 植物学报, 2020, 55(4): 397-402.
Ruifeng Yao,Daoxin Xie. New Insight into Strigolactone Signaling. Chinese Bulletin of Botany, 2020, 55(4): 397-402.
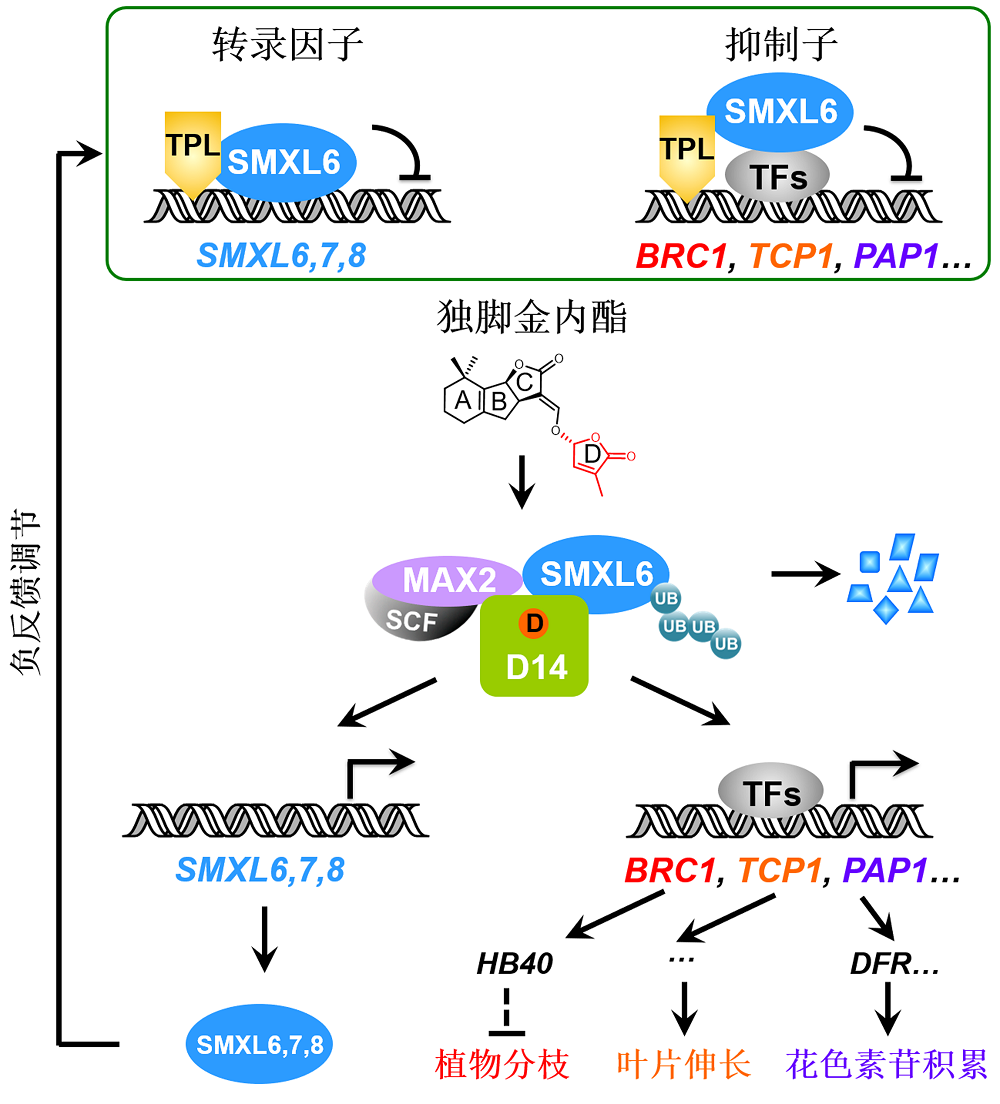
图1 独脚金内酯信号通路中抑制子SMXL6,7,8的双重功能工作模型 独脚金内酯信号通路中的SMXL6,7,8是具有双重功能的新型抑制子: SMXL6,7,8作为抑制子招募TPL共抑制子并直接结合下游转录因子抑制其转录活性, 从而阻遏独脚金内酯(SL)响应基因的表达; 同时SMXL6,7,8又作为转录因子直接结合并抑制SMXL6,7,8基因的启动子。SL被D14感知, 诱导SMXL6,7, 8-D14-MAX2复合体形成, 导致SMXL6,7,8通过泛素化-蛋白酶体途径降解, 从而解除SMXL6,7,8对下游转录因子以及自身基因启动子的抑制, 一方面激活BRC1、TCP1和PAP1等响应基因的转录, 最终调控植物分枝、叶片伸长和花色素苷积累等生物学过程; 另一方面解除对SMXL6,7,8启动子的抑制, 激活SMXL6,7,8自身基因的表达, 形成维持SL通路稳态的负反馈调控体系。SCF: Skp1-Cullin-F-box; UB: 泛素
Figure 1 Working model for the dual-function repressors SMXL6,7,8 in strigolactone signaling SMXL6,7,8 in the strigolactone signaling pathway act as novel repressors with dual functions: SMXL6,7,8 act as repressors that recruit TPL co-repressor proteins and bind transcription factors to inhibit their transcriptional activity, thereby suppressing expression of strigolactone (SL)-responsive genes; meanwhile, SMXL6,7,8 also serve as transcription factors that directly bind and inhibit the promoters of SMXL6,7,8 genes. SL is perceived by D14 to trigger formation of SMXL6,7,8-D14-MAX2 complex and further induce SMXL6,7,8 degradation via the ubiquitination-proteasome pathway. The SL-induced SMXL6,7,8 degradation releases transcription factors to activate expression of the SL-responsive genes such as BRC1, TCP1 and PAP1 essential for plant branching, leaf elongation, and anthocyanin biosynthesis, respectively. Such SMXL6,7,8 degradation also de- represses the SMXL6,7,8 suppression on the SMXL6,7,8 promoters to activate the expression of SMXL6,7,8 genes, which forms a negative feedback regulation loop that maintains the homeostasis of SL pathway. SCF: Skp1-Cullin-F- box; UB: Ubiquitin
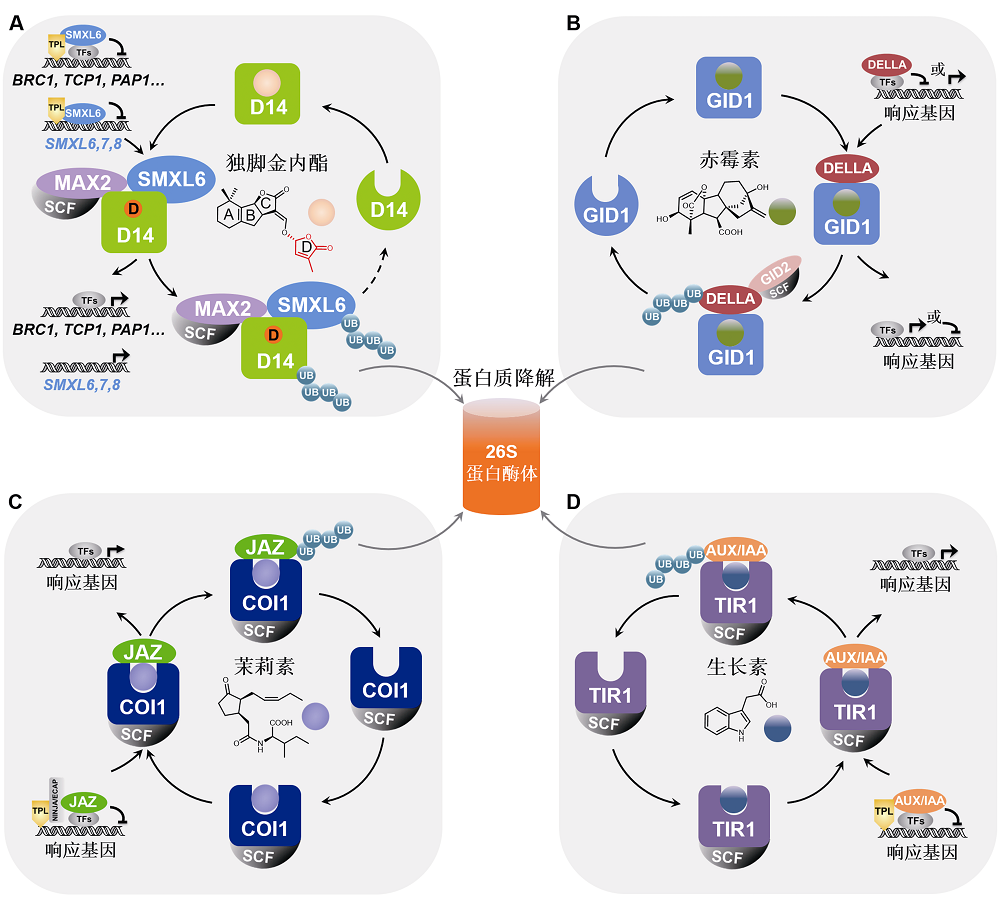
图2 独脚金内酯、赤霉素、茉莉素及生长素信号途径中抑制子的功能比较 植物激素独脚金内酯(A)、赤霉素(B)、茉莉素(C)及生长素(D)信号传导途径中的抑制子DELLA、AUX/IAA、JAZ和D53/SMXL均通过结合下游信号蛋白(转录因子)调控其转录活性, 从而阻遏激素响应基因的表达。激素分子被相应的受体识别后激活其信号传导链,诱导抑制子通过泛素化-蛋白酶体途径降解, 促进响应基因表达并介导相应的生物学功能。独脚金内酯(SL)信号传导途径中的抑制子SMXL6,7,8同时还作为转录因子直接结合并抑制SMXL6,7,8基因的启动子; SL诱导SMXL6,7,8降解, 从而解除SMXL6,7,8对自身基因启动子的抑制, 激活SMXL6,7,8自身基因的表达, 形成维持SL通路稳态的负反馈调控体系(A)。
Figure 2 Comparison of the repressor proteins in strigolactone, gibberellin, jasmonate and auxin signaling pathways The repressor proteins D53/SMXL, DELLA, JAZ, and AUX/IAA in the signaling pathways of strigolactone (A), gibberellin (B), jasmonate (C) and auxin (D) bind and inhibit downstream transcription factors, thereby suppressing the expression of hormone-responsive genes. Hormone molecule is recognized by corresponding receptor protein and activates the signal transduction chain to induce the degradation of the repressor protein via ubiquitination-proteasome pathway, then triggering response gene expression and related biological processes. Moreover, the repressor proteins SMXL6,7,8 in strigolactone (SL) signaling pathway can also directly bind and inhibit the promoter of SMXL6,7,8 gene as transcription factors. SL induces the degradation of SMXL6,7,8 to release its repression on the SMXL6,7,8 promoters to activate the expression of SMXL6,7,8 genes, forming a negative feedback regulation loop (A) essential for the homeostasis of SL pathway.
| [1] | 黎家, 李传友 (2019). 新中国成立70年来植物激素研究进展. 中国科学: 生命科学 49, 1227-1281. |
| [2] |
Bürger M, Chory J (2020). The many models of strigolactone signaling. Trends Plant Sci 25, 395-405.
DOI URL PMID |
| [3] |
de Saint Germain A, Clavé G, Badet-Denisot MA, Pillot JP, Cornu D, Le Caer JP, Burger M, Pelissier F, Retailleau P, Turnbull C, Bonhomme S, Chory J, Rameau C, Boyer FD (2016). A histidine covalent receptor and butenolide complex mediates strigolactone perception. Nat Chem Biol 12, 787-794.
DOI URL PMID |
| [4] |
Duan J, Yu H, Yuan K, Liao Z, Meng X, Jing Y, Liu G, Chu J, Li J (2019). Strigolactone promotes cytokinin degradation through transcriptional activation of CYTOKININ OXIDASE/DEHYDROGENASE 9 in rice. Proc Natl Acad Sci USA 116, 14319-14324.
DOI URL PMID |
| [5] |
Fang Z, Ji Y, Hu J, Guo R, Sun S, Wang X (2020). Strigolactones and brassinosteroids antagonistically regulate the stability of the D53-OsBZR1 complex to determine FC1 expression in rice tillering. Mol Plant 13, 586-597.
URL PMID |
| [6] |
Gomez-Roldan V, Fermas S, Brewer PB, Puech-Pages V, Dun EA, Pillot JP, Letisse F, Matusova R, Danoun S, Portais JC, Bouwmeester H, Bécard G, Beveridge CA, Rameau C, Rochange SF (2008). Strigolactone inhibition of shoot branching. Nature 455, 189-194.
DOI URL PMID |
| [7] |
Hamiaux C, Drummond RSM, Janssen BJ, Ledger SE, Cooney JM, Newcomb RD, Snowden KC (2012). DAD2 is an α/β hydrolase likely to be involved in the perception of the plant branching hormone, strigolactone. Curr Biol 22, 2032-2036.
DOI URL PMID |
| [8] |
Hu J, Ji Y, Hu X, Sun S, Wang X (2020). BES1 functions as the co-regulator of D53-like SMXLs to inhibit BRC1 expression in strigolactone-regulated shoot branching in Arabidopsis. Plant Commun 1, 100014.
DOI URL |
| [9] |
Jiang L, Liu X, Xiong G, Liu H, Chen F, Wang L, Meng X, Liu G, Yu H, Yuan Y, Yi W, Zhao L, Ma H, He Y, Wu Z, Melcher K, Qian Q, Xu H, Wang Y, Li J (2013). DWARF 53 acts as a repressor of strigolactone signaling in rice. Nature 504, 401-405.
DOI URL |
| [10] |
Ma H, Duan J, Ke J, He Y, Gu X, Xu TH, Yu H, Wang Y, Brunzelle JS, Jiang Y, Rothbart SB, Xu H, Li J, Melcher K (2017). A D53 repression motif induces oligomerization of TOPLESS corepressors and promotes assembly of a corepressor-nucleosome complex. Sci Adv 3, e1601217.
DOI URL PMID |
| [11] |
Seto Y, Yasui R, Kameoka H, Tamiru M, Cao MM, Terauchi R, Sakurada A, Hirano R, Kisugi T, Hanada A, Umehara M, Seo E, Akiyama K, Burke J, Takeda-Kamiya N, Li WQ, Hirano Y, Hakoshima T, Mashiguchi K, Noel JP, Kyozuka J, Yamaguchi S (2019). Strigolactone perception and deactivation by a hydrolase receptor DWARF14. Nat Commun 10, 191.
DOI URL PMID |
| [12] |
Shabek N, Ticchiarelli F, Mao HB, Hinds TR, Leyser O, Zheng N (2018). Structural plasticity of D3-D14 ubiquitin ligase in strigolactone signaling. Nature 563, 652-656.
DOI URL PMID |
| [13] |
Song X, Lu Z, Yu H, Shao G, Xiong J, Meng X, Jing Y, Liu G, Xiong G, Duan J, Yao X, Liu C, Li H, Wang Y, Li J (2017). IPA1 functions as a downstream transcription factor repressed by D53 in strigolactone signaling in rice. Cell Res 27, 1128-1141.
URL PMID |
| [14] |
Stanga JP, Smith SM, Briggs WR, Nelson DC (2013). SUPPRESSOR OF MORE AXILLARY GROWTH2 1 controls seed germination and seedling development in Arabidopsis. Plant Physiol 163, 318-330.
DOI URL |
| [15] |
Umehara M, Hanada A, Yoshida S, Akiyama K, Arite T, Takeda-Kamiya N, Magome H, Kamiya Y, Shirasu K, Yoneyama K, Kyozuka J, Yamaguchi S (2008). Inhibition of shoot branching by new terpenoid plant hormones. Nature 455, 195-200.
URL PMID |
| [16] |
Uraguchi D, Kuwata K, Hijikata Y, Yamaguchi R, Imaizumi H, Am S, Rakers C, Mori N, Akiyama K, Irle S, McCourt P, Kinoshita T, Ooi T, Tsuchiya Y (2018). A femtomolar-range suicide germination stimulant for the parasitic plant Striga hermonthica. Science 362, 1301-1305.
URL PMID |
| [17] | Wang B, Wang Y, Li J (2017). Strigolactones. In: Li JY, Li CY, Smith SM, eds. Hormone Metabolism and Signaling in Plants. London: Academic Press. pp. 327-359. |
| [18] |
Wang L, Wang B, Jiang L, Liu X, Li X, Lu Z, Meng X, Wang Y, Smith SM, Li J (2015). Strigolactone signaling in Arabidopsis regulates shoot development by targeting D53-like SMXL repressor proteins for ubiquitination and degradation. Plant Cell 27, 3128-3142.
DOI URL PMID |
| [19] |
Wang L, Wang B, Yu H, Guo H, Lin T, Kou L, Wang A, Shao N, Ma H, Xiong G, Li X, Yang J, Chu J, Li J (2020a). Transcriptional regulation of strigolactone signaling in Arabidopsis. Nature 583, 277-281.
DOI URL PMID |
| [20] |
Wang L, Xu Q, Yu H, Ma H, Li X, Yang J, Chu J, Xie Q, Wang Y, Smith SM, Li J, Xiong G, Wang B (2020b). Strigolactone and karrikin signaling pathways elicit ubiquitination and proteolysis of SMXL2 to regulate hypocotyl elongation in Arabidopsis thaliana. Plant Cell 32, 2251-2270.
DOI URL PMID |
| [21] |
Waters MT, Gutjahr C, Bennett T, Nelson DC (2017). Strigolactone signaling and evolution. Annu Rev Plant Biol 68, 291-322.
DOI URL PMID |
| [22] |
Xie Y, Liu Y, Ma M, Zhou Q, Zhao Y, Zhao B, Wang B, Wei H, Wang H (2020). Arabidopsis FHY3 and FAR1 integrate light and strigolactone signaling to regulate branching. Nat Commun 11, 1955.
URL PMID |
| [23] | Yao R, Ming Z, Yan L, Li S, Wang F, Ma S, Yu C, Yang M, Chen L, Chen L, Li Y, Yan C, Miao D, Sun Z, Yan J, Sun Y, Wang L, Chu J, Fan S, He W, Deng H, Nan F, Li J, Rao Z, Lou Z, Xie D (2016). DWARF14 is a non-canonical hormone receptor for strigolactone. Nature 536, 469-473. |
| [24] |
Yao R, Wang F, Ming Z, Du X, Chen L, Wang Y, Zhang W, Deng H, Xie D (2017). ShHTL7 is a non-canonical receptor for strigolactones in root parasitic weeds. Cell Res 27, 838-841.
DOI URL PMID |
| [25] |
Yao R, Wang L, Li Y, Chen L, Li S, Du X, Wang B, Yan J, Li J, Xie D (2018). Rice DWARF14 acts as an unconventional hormone receptor for strigolactone. J Exp Bot 69, 2355-2365.
DOI URL PMID |
| [26] |
Zhao LH, Zhou XE, Wu ZS, Yi W, Xu Y, Li SL, Xu TH, Liu Y, Chen RZ, Kovach A, Kang YY, Hou L, He YZ, Xie C, Song WL, Zhong DF, Xu YC, Wang YH, Li JY, Zhang CH, Melcher K, Xu HE (2013). Crystal structures of two phytohormone signal-transducing α/β hydrolases: karrikin-signaling KAI2 and strigolactone-signaling DWARF14. Cell Res 23, 436-439.
DOI URL PMID |
| [27] |
Zhou F, Lin Q, Zhu L, Ren Y, Zhou K, Shabek N, Wu F, Mao H, Dong W, Gan L, Ma W, Gao H, Chen J, Yang C, Wang D, Tan J, Zhang X, Guo X, Wang J, Jiang L, Liu X, Chen W, Chu J, Yan C, Ueno K, Ito S, Asami T, Cheng Z, Wang J, Lei C, Zhai H, Wu C, Wang H, Zheng N, Wan J (2013). D14-SCFD3-dependent degradation of D53 regulates strigolactone signaling. Nature 504, 406-410.
DOI URL |
| [28] |
Zwanenburg B, Blanco-Ania D (2018). Strigolactones: new plant hormones in the spotlight. J Exp Bot 69, 2205-2218.
DOI URL PMID |
| [1] | 陈鹏翔, 王波, 王子俊, 韩榕. 转录因子在植物响应UV-B辐射中的调控作用[J]. 植物学报, 2025, 60(3): 449-459. |
| [2] | 刘旭鹏, 王敏, 韩守安, 朱学慧, 王艳蒙, 潘明启, 张雯. 植物器官脱落调控因素及分子机理研究进展[J]. 植物学报, 2025, 60(3): 472-482. |
| [3] | 姚瑞枫, 谢道昕. 水稻独脚金内酯信号感知的激活和终止[J]. 植物学报, 2024, 59(6): 873-877. |
| [4] | 陈婷欣, 符敏, 李娜, 杨蕾蕾, 李凌飞, 钟春梅. 铁甲秋海棠DNA甲基转移酶全基因组鉴定及表达分析(长英文摘要)[J]. 植物学报, 2024, 59(5): 726-737. |
| [5] | 陈雯, 周颖盈, 罗平, 崔永一. 被子植物花朵重瓣化分子调控机制[J]. 植物学报, 2024, 59(2): 257-277. |
| [6] | 张悦婧, 桑鹤天, 王涵琦, 石珍珍, 李丽, 王馨, 孙坤, 张继, 冯汉青. 植物对非生物胁迫系统性反应中信号传递的研究进展[J]. 植物学报, 2024, 59(1): 122-133. |
| [7] | 曾鑫海, 陈锐, 师宇, 盖超越, 范凯, 李兆伟. 植物SPL转录因子的生物功能研究进展[J]. 植物学报, 2023, 58(6): 982-997. |
| [8] | 李季蔓, 靳楠, 胥毛刚, 霍举颂, 陈小云, 胡锋, 刘满强. 不同干旱水平下蚯蚓对番茄抗旱能力的影响[J]. 生物多样性, 2022, 30(7): 21488-. |
| [9] | 于淼, 阮成江, 丁健, 李景滨, 卢顺光, 温秀凤. 沙棘hrh-miRn458靶向转录因子WRI1调控油脂合成[J]. 植物学报, 2022, 57(5): 635-648. |
| [10] | 戴琛, 汪瑾, 卢亚萍. 衍生化UPLC-MS法测定酸性植物激素[J]. 植物学报, 2022, 57(4): 500-507. |
| [11] | 李月, 胡德升, 谭金芳, 梅浩, 王祎, 李慧, 李芳, 韩燕来. 单列毛壳菌通过促进秸秆降解并调控激素响应基因表达促进玉米生长[J]. 植物学报, 2022, 57(4): 422-433. |
| [12] | 孟彦彦, 张楠, 熊延. 植物TOR激酶响应上游信号的研究进展[J]. 植物学报, 2022, 57(1): 1-11. |
| [13] | 王静文, 王兴军, 马长乐, 李膨呈. 植物核糖体应激响应机制研究进展[J]. 植物学报, 2022, 57(1): 80-89. |
| [14] | 王田幸子, 朱峥, 陈悦, 刘玉晴, 燕高伟, 徐珊, 张彤, 马金姣, 窦世娟, 李莉云, 刘国振. 水稻OsWRKY42是Xa21介导的抗白叶枯病途径新元件[J]. 植物学报, 2021, 56(6): 687-698. |
| [15] | 刘栋. 既主内政, 又辖外交——以PHR为中心的基因网络调控植物-菌根真菌的共生[J]. 植物学报, 2021, 56(6): 647-650. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||