

植物学报 ›› 2020, Vol. 55 ›› Issue (4): 407-420.DOI: 10.11983/CBB20009 cstr: 32102.14.CBB20009
收稿日期:2020-01-15
接受日期:2020-05-15
出版日期:2020-07-01
发布日期:2020-05-21
通讯作者:
韩榕
基金资助:
Fangfang He,Huize Chen,Jinlin Feng,Lin Gao,Jiao Niu,Rong Han( )
)
Received:2020-01-15
Accepted:2020-05-15
Online:2020-07-01
Published:2020-05-21
Contact:
Rong Han
摘要: UV-B辐射对植物的影响体现在多个水平, 其会引起植物DNA损伤, 造成有丝分裂异常, 最终影响植物的生长发育及生理生化过程。RAD21.3是黏连蛋白复合物的一个亚基, 参与有丝分裂中染色体的分离。该研究以哥伦比亚生态型拟南芥(Arabidopsis thaliana)和atrad21.3突变体为材料, 设置对照(CK)及UV-B处理组, 对野生型(WT)、atrad21.3及过表达株系的根长、株高、抽薹时间和生理生化指标进行统计分析。利用碱性品红染色观察拟南芥根尖的有丝分裂现象, 并统计畸变率。SPSS分析结果表明, UV-B处理后, WT UV-B和atrad21.3 CK的抽薹时间、株高及各项生理生化指标与WT CK相比无显著差异, 但atrad21.3 UV-B与之相比差异显著。通过烟草(Nicotiana benthamiana)的瞬时表达和亚细胞定位观察, 发现RAD21.3集中在细胞核; 进一步观察分裂期细胞发现落后染色体、染色体桥和游离染色体等异常现象。统计结果表明, 与WT CK相比, WT UV-B和atrad21.3 CK的畸变率较高, 但atrad21.3 UV-B的畸变率更高, 表明RAD21.3可能响应UV-B辐射诱导的异常有丝分裂。
贺芳芳,陈慧泽,冯金林,高琳,牛娇,韩榕. 拟南芥黏连蛋白RAD21对增强UV-B辐射后细胞分裂的响应. 植物学报, 2020, 55(4): 407-420.
Fangfang He,Huize Chen,Jinlin Feng,Lin Gao,Jiao Niu,Rong Han. Response of Arabidopsis Cohesin RAD21 to Cell Division after Enhanced UV-B Radiation. Chinese Bulletin of Botany, 2020, 55(4): 407-420.
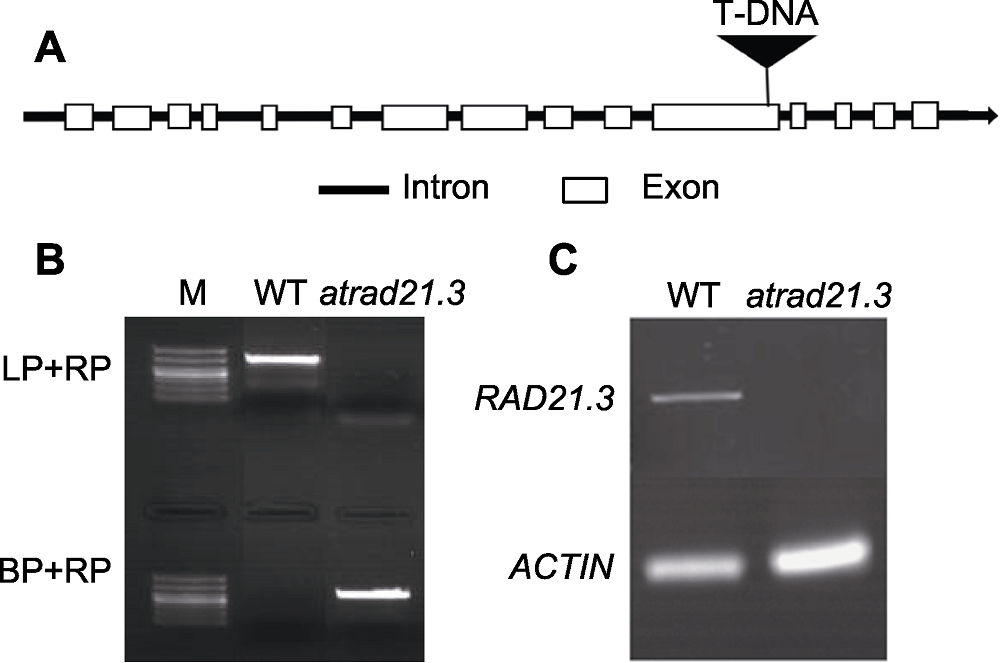
图1 atrad21.3的T-DNA插入示意图及鉴定 (A) atrad21.3基因结构以及T-DNA插入位点示意图; (B) atrad21.3 DNA水平鉴定(M: 分子量标记); (C) atrad21.3中RAD 21.3基因的转录本水平。WT: 野生型
Figure 1 T-DNA insertions and identification of atrad21.3 (A) Gene structure and T-DNA insertions of atrad21.3; (B) Identification of DNA level in atrad21.3(M: Marker); (C) Transcriptional level of RAD21.3 gene in atrad21.3. WT: Wild type

图2 野生型(WT)和atrad21.3的表型分析 (A) WT种子; (B) 第12天的WT莲座叶; (C) WT抽薹; (D) atrad21.3种子; (E) 第12天的atrad21.3莲座叶; (F) atrad21.3未抽薹; (G) WT和atrad21.3的株高. (A), (D) Bars=10 μm; (B), (C), (E)-(G) Bars=1.0 cm
Figure 2 Phenotypic analysis of wild type (WT) and atrad21.3 (A) Seed of WT; (B) Rosette leaf (day 12) of WT; (C) Bolting of WT; (D) Seed of atrad21.3; (E) Rosette leaf (day 12) of atrad21.3; (F) Bolting of atrad21.3; (G) Plant height of WT and atrad21.3. (A), (D) Bars=10 μm; (B), (C), (E)-(G) Bars=1.0 cm
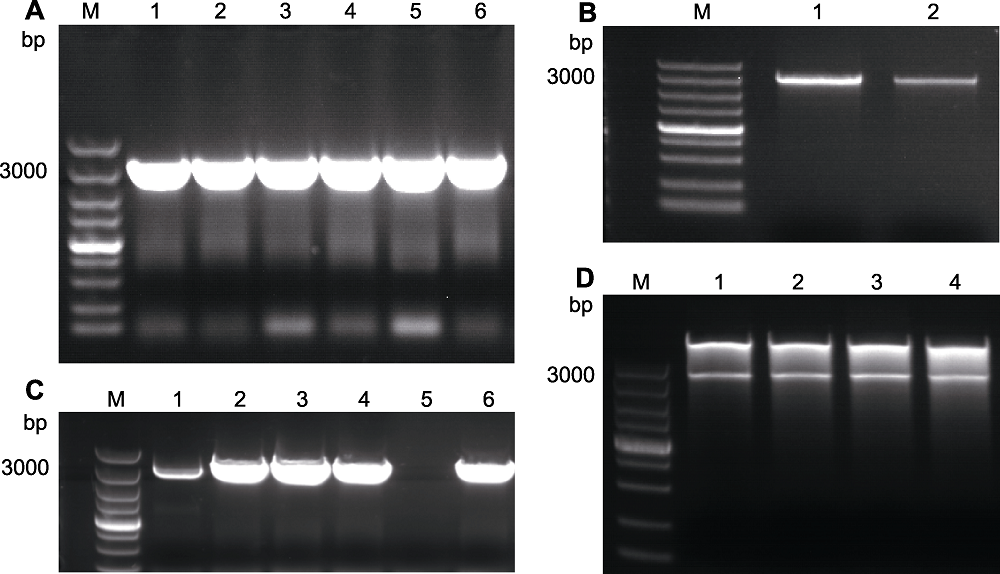
图3 AtRAD21.3的克隆 (A) AtRAD21.3的扩增产物(1-6: 产物); (B) AtRAD21.3切胶回收电泳结果(1, 2: 切胶回收产物); (C) 菌液PCR结果(1-4, 6: 阳性单菌落; 5: 阴性对照); (D) 质粒双酶切验证(1-4: 双酶切结果)。M: 分子量标记
Figure 3 Cloning of AtRAD21.3 (A) PCR products of AtRAD21.3 (1-6: Products); (B) Gel cutting recovery result of AtRAD21.3 (1, 2: Gel cutting recovery products); (C) Bacterial PCR result (1-4, 6: Positive single colony; 5: Negative control products); (D) Dual-restriction digestion of plasmid (1-4: Dual-restriction result). M: DNA marker
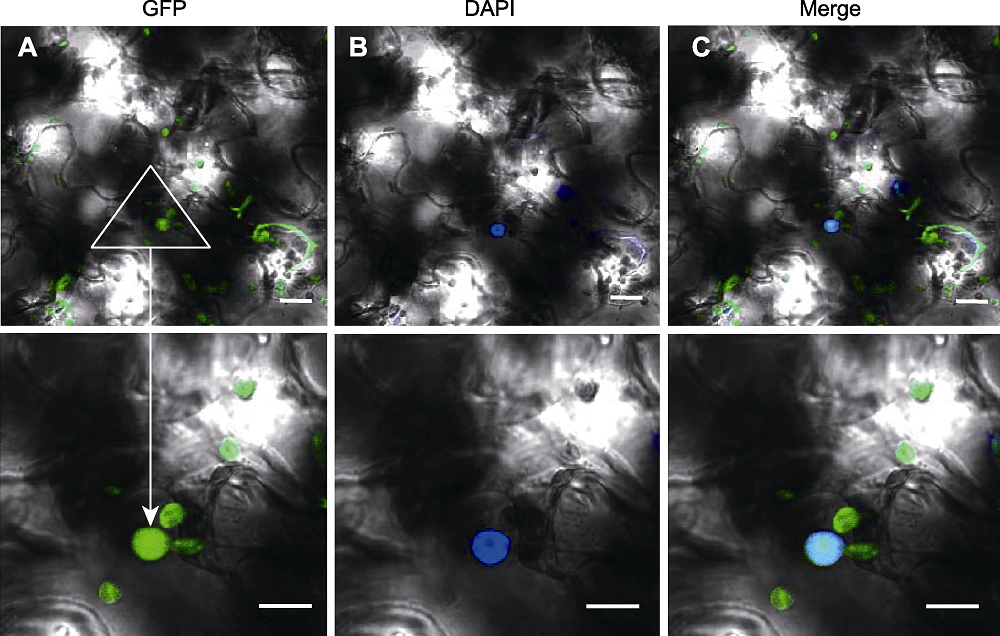
图4 pSuper1300-RAD21.3-GFP在烟草叶片的瞬时表达 (A) pSuper1300-RAD21.3-GFP的GFP荧光信号图像; (B) pSuper1300-RAD21.3-GFP在烟草叶片的DAPI染色图像; (C) A和B叠加的图像。Bars=10 μm
Figure 4 Transient expression of pSuper1300-RAD21.3-GFP in leaves of Nicotiana benthamiana (A) GFP signal of pSuper1300-RAD21.3-GFP; (B) DAPI staining of pSuper1300-RAD21.3-GFP in leaves of Nicotiana benthamiana; (C) Merged image of A and B. Bars=10 μm
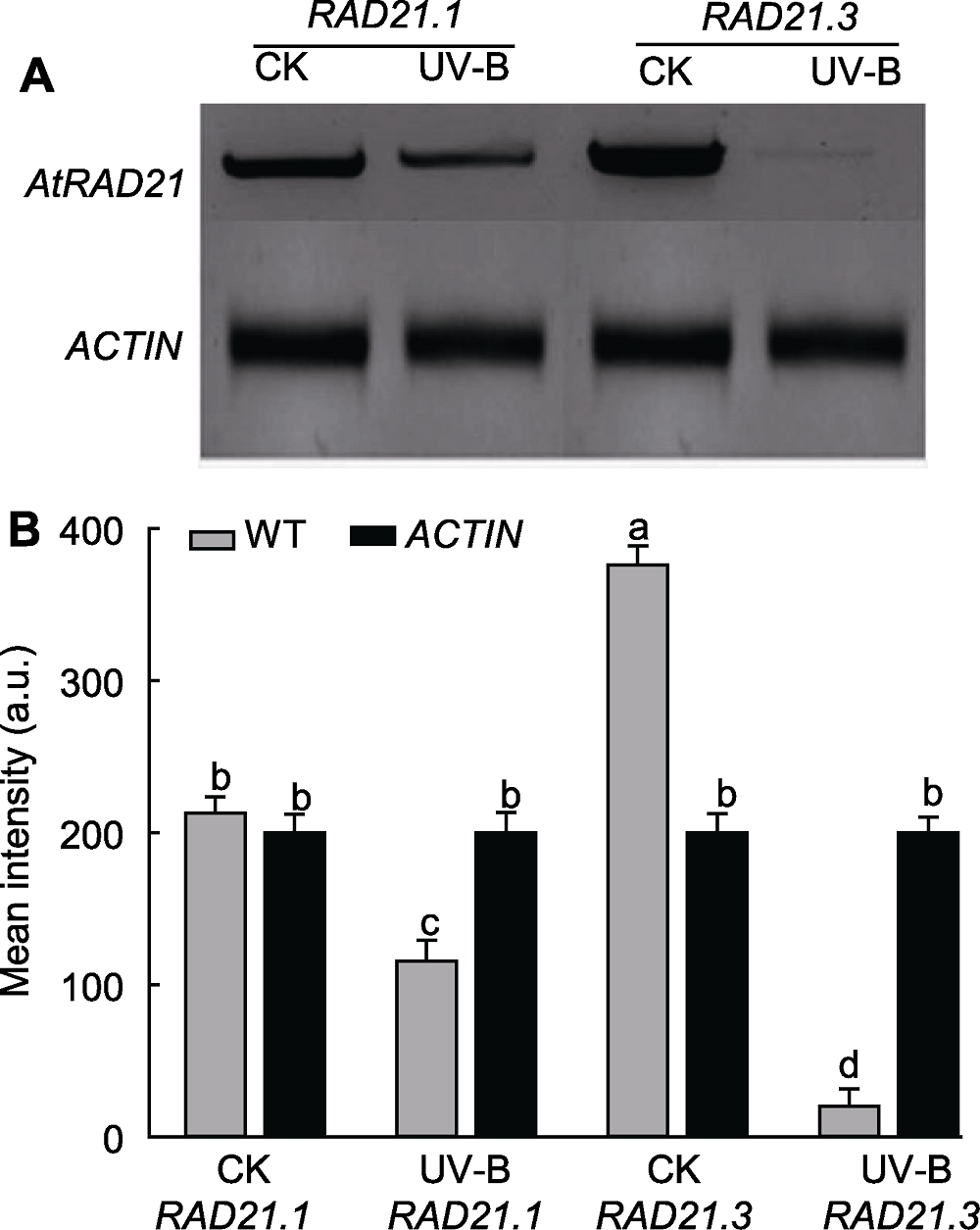
图6 UV-B辐射后AtRAD21基因表达量检测 (A) 半定量RT-PCR电泳图; (B) 灰度值分析。WT: 野生型。不同小写字母表示差异显著(Duncan法, P<0.05)。
Figure 6 Detection of UV-B radiation on AtRAD21 expres-sion quantity (A) Result of RT-PCR; (B) Gray value analysis. WT: Wild type. Different lowercase letters show significant differences (Duncan method, P<0.05).
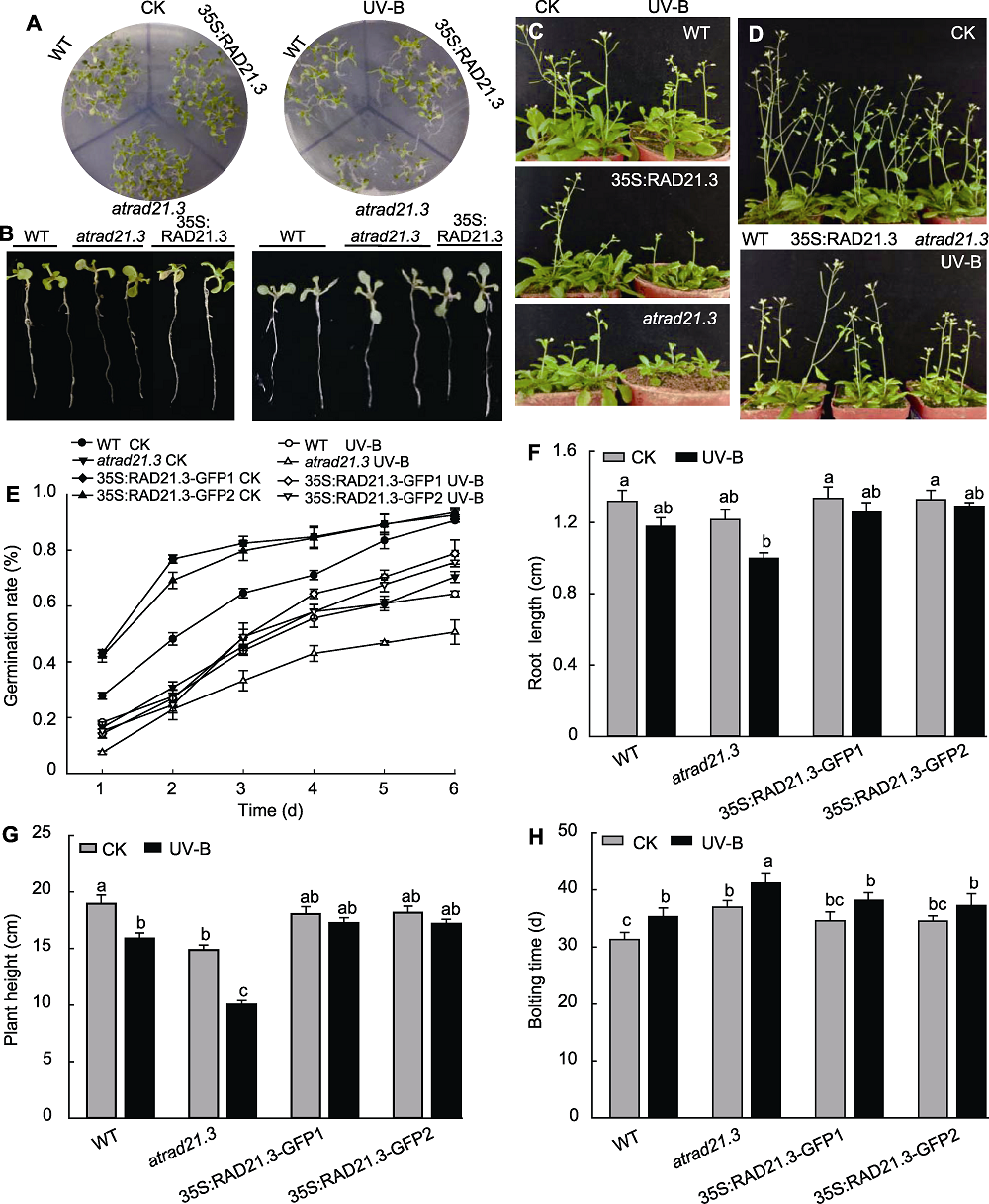
图7 UV-B处理对拟南芥种子萌发率及幼苗根长、株高和抽薹时间的影响 (A), (E) UV-B处理对拟南芥种子萌发率的影响; (B), (F) UV-B处理对拟南芥幼苗根长的影响; (C), (H) UV-B处理对拟南芥抽薹时间的影响; (D), (G) UV-B处理对拟南芥株高的影响。WT: 野生型。不同小写字母表示差异显著(Duncan法, P<0.05)。
Figure 7 The seed germination rate, root length, plant height and bolting time of Arabidopsis thaliana with UV-B treatment (A), (E) The seed germination rate of Arabidopsis thaliana with UV-B treatment; (B), (F) The root length of Arabidopsis thaliana seedling with UV-B treatment; (C), (H) The bolting time of Arabidopsis thaliana with UV-B treatment; (D), (G) The plant height of Arabidopsis thaliana with UV-B treatment. WT: Wild type. Different lowercase letters show significant differences (Duncan method, P<0.05).
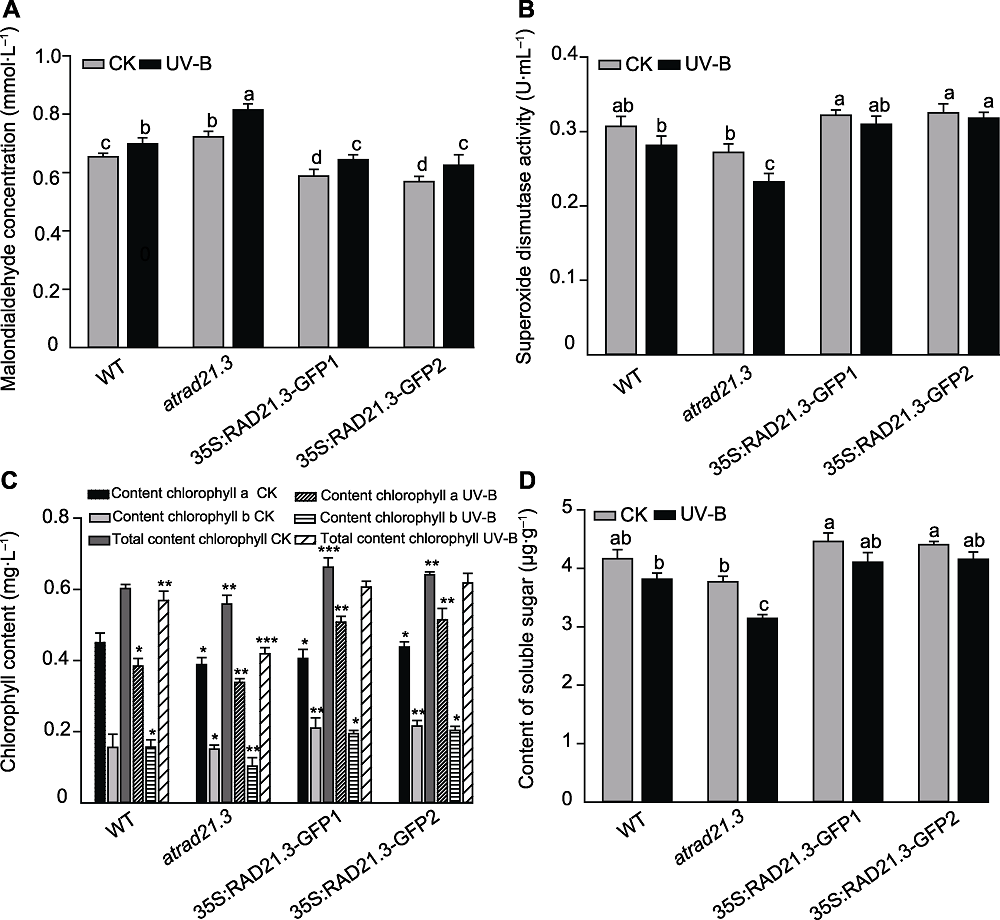
图8 UV-B辐射对拟南芥植株生理生化指标的影响 (A) UV-B辐射对拟南芥丙二醛(MDA)浓度的影响; (B) UV-B辐射对拟南芥超氧化物歧化酶(SOD)活性的影响; (C) UV-B辐射对拟南芥叶绿素含量的影响; (D) UV-B辐射对拟南芥叶片可溶性糖含量的影响。WT: 野生型。不同小写字母(或*)表示差异显著(Duncan法, P<0.05)。
Figure 8 The influence of physiological and biochemical in Arabidopsis thaliana with UV-B treatment (A) Effect of UV-B radiation on Arabidopsis thaliana malondialdehyde (MDA) concentration; (B) Effect of UV-B radiation on Arabidopsis thaliana superoxide dismutase (SOD) activity; (C) Effect of UV-B radiation on chlorophyll content in Arabidopsis thaliana; (D) Effect of UV-B radiation on content of soluble sugar of Arabidopsis thaliana. WT: Wild type. Different lowercase letters (or * ) show significant differences (Duncan method, P<0.05).
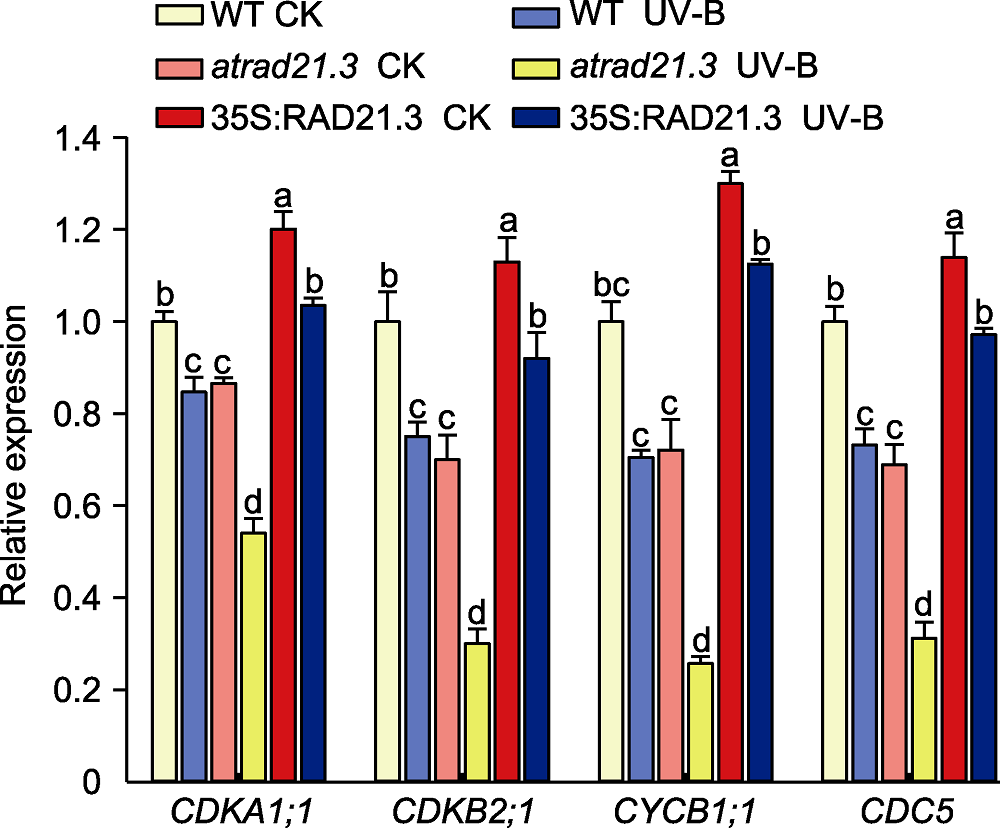
图9 拟南芥细胞周期相关基因的相对表达量 WT: 野生型。不同小写字母表示差异显著(Duncan法, P<0.05)。
Figure 9 Relative expression of cell cycle related genes in Arabidopsis thaliana WT: Wild type. Different lowercase letters show significant differences (Duncan method, P<0.05).
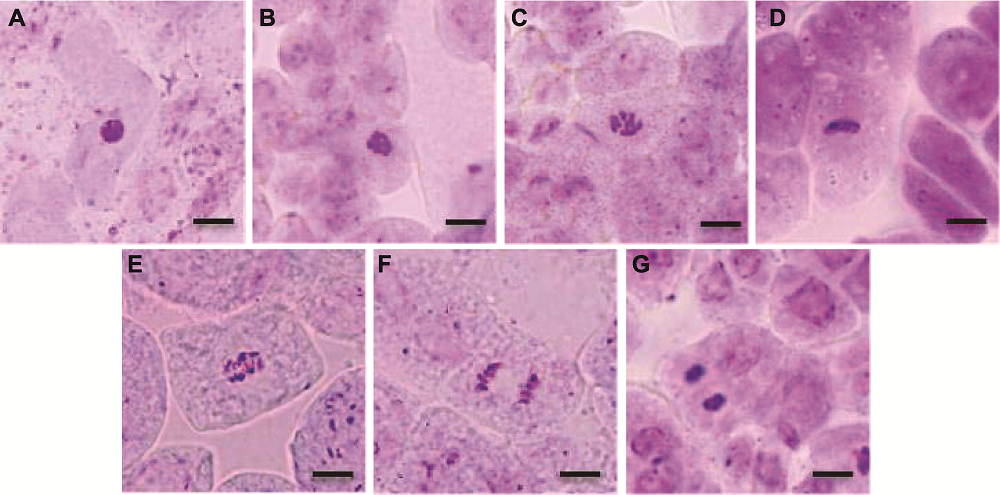
图10 拟南芥根尖正常有丝分裂各时期图 (A) 间期; (B), (C) 前期; (D) 中期; (E) 中后期; (F) 后期; (G) 末期。Bars=20 μm
Figure 10 Normal phase types of mitosis in root tip of Arabidopsis thaliana (A) Interphase; (B), (C) Prophase; (D) Metaphase; (E) Meta-anaphase; (F) Anaphase; (G) Telephase. Bars=20 μm
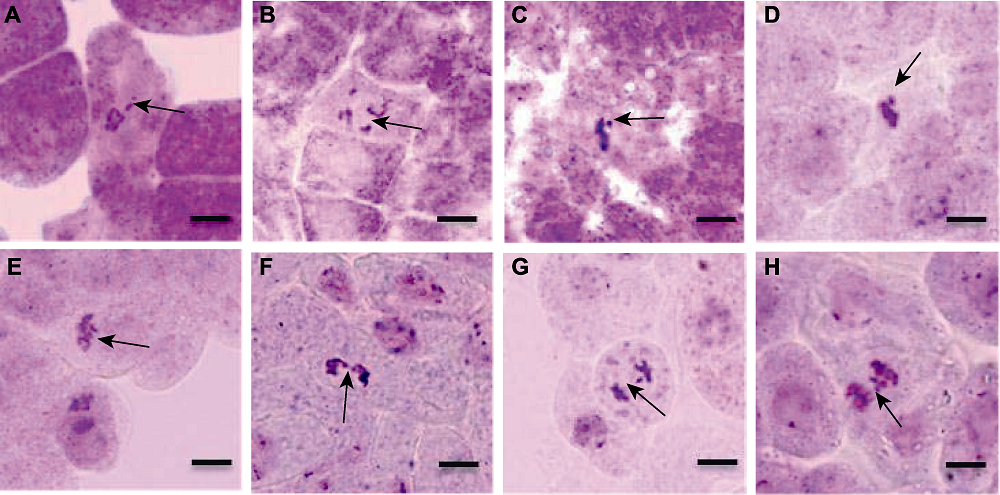
图11 拟南芥根尖染色体畸变的不同类型 (A) 前期不定向(箭头); (B) 前期散乱(箭头); (C) 游离染色体(箭头); (D) 游离染色体(箭头); (E) 后期不对称(箭头); (F) 染色体桥(箭头); (G) 落后染色体(箭头); (H) 落后染色体(箭头)。Bars=20 μm
Figure 11 Different types of chromosome aberration in root tip of Arabidopsis thaliana (A) Unorientation at prophase (arrow); (B) Cofusion at prophase (arrow); (C) Fragments chromosomes (arrow); (D) Fragments chromosomes (arrow); (E) Asymmetric at anaphase (arrow); (F) Chromosome bridge (arrow); (G) Lagging chromosome (arrow); (H) Lagging chromosome (arrow). Bars=20 μm
| Treatment | Total of observing cells | Total of dividing cells | Total of aberration cells | Percentage of dividing cells (%) | Percentage of chromosomal aberration (%) |
|---|---|---|---|---|---|
| WT CK | 5390 | 195 | 8 | 3.6±0.202 a | 0.148±0.102 c |
| WT UV-B | 5376 | 166 | 25 | 3.08±0.258 c | 0.465±0.056 b |
| atrad21.3 CK | 6998 | 215 | 32 | 3.07±0.195 c | 0.457±0.085 b |
| atrad21.3 UV-B | 7044 | 194 | 45 | 2.75±0.182 d | 0.638±0.105 a |
| 35S:RAD21.3 CK | 5603 | 186 | 7 | 3.3±0.132 ab | 0.125±0.136 c |
| 35S:RAD21.3 UV-B | 5536 | 193 | 21 | 3.48±0.129 ab | 0.379±0.125 bc |
表1 UV-B辐射对拟南芥有丝分裂的影响
Table1 Effects of the UV-B radiation on mitosis of Arabidopsis thaliana
| Treatment | Total of observing cells | Total of dividing cells | Total of aberration cells | Percentage of dividing cells (%) | Percentage of chromosomal aberration (%) |
|---|---|---|---|---|---|
| WT CK | 5390 | 195 | 8 | 3.6±0.202 a | 0.148±0.102 c |
| WT UV-B | 5376 | 166 | 25 | 3.08±0.258 c | 0.465±0.056 b |
| atrad21.3 CK | 6998 | 215 | 32 | 3.07±0.195 c | 0.457±0.085 b |
| atrad21.3 UV-B | 7044 | 194 | 45 | 2.75±0.182 d | 0.638±0.105 a |
| 35S:RAD21.3 CK | 5603 | 186 | 7 | 3.3±0.132 ab | 0.125±0.136 c |
| 35S:RAD21.3 UV-B | 5536 | 193 | 21 | 3.48±0.129 ab | 0.379±0.125 bc |
| [1] | 陈慧泽, 韩榕 (2015). 植物响应UV-B辐射的研究进展. 植物学报 50, 790-801. |
| [2] |
陈建权, 程晨, 张梦恬, 张向前, 张尧, 王爱英, 祝建波 (2018). 天山雪莲SiSAD基因与拟南芥AtFAB2基因转化烟草的抗寒性分析. 植物学报 53, 603-611.
DOI URL |
| [3] | 方荧, 刘风珍, 张昆, 张秀荣, 朱素青, 赵炎, 万勇善 (2018). UV-B辐射增强影响作物生长发育的研究进展. 山东农业科学 50, 183-188. |
| [4] | 韩榕 (2002). He-Ne激光对小麦增强UV-B辐射损伤的修复效应及机理. 博士论文. 西安: 西北大学. pp. 52-55. |
| [5] |
李晓阳, 陈慧泽, 韩榕 (2013). UV-B辐射对拟南芥种子萌发和幼苗生长的影响. 植物学报 48, 52-58.
DOI URL |
| [6] | 马兰, 黄华孙, 程汉 (2018). 拟南芥突变体L1.3的表型分析及遗传定位. 分子植物育种 12, 4023-4028. |
| [7] |
王静, 蒋磊, 王艳, 李韶山 (2009). UV-B辐射对拟南芥细胞周期G1/S期转变的影响. 植物学报 44, 426-433.
DOI URL |
| [8] | 徐金龙, 梁爽, 郁飞 (2019). 拟南芥细胞周期基因AtCDC5的功能研究及抗体制备. 江苏农业学报 35, 26-32. |
| [9] | 张亮然 (2006). 水稻RAD21/REC8家族基因的分离与功能分析. 博士论文. 北京: 中国科学院研究生院(植物研究所). pp. 1-3. |
| [10] | 张志良, 瞿伟菁, 李小方 (2009). 植物生理学实验指导(第4版). 北京: 高等教育出版社. pp. 54-229. |
| [11] |
Björn LO, Callaghan TV, Johnsen I, Lee JA, Manetas Y, Paul ND, Sonesson M, Wellburn AR, Coop D, Heide-Jørgensen HS, Gehrke C, Gwynn-Jones D, Johanson U, Kyparissis A, Levizou E, Nikolopoulos D, Petropoulou Y, Stephanou M (1997). The effects of UV-B radiation on European heathland species. Plant Ecol 128, 253-264.
DOI URL |
| [12] |
Björn OL (1996). Effects of ozone depletion and increased UV-B on terrestrial ecosystems. Int J Environ Stud 51, 217-243.
DOI URL |
| [13] | Caldwell MM, Teramura AH, Tevini M, Bornman JF, Björn LO, Kulandaivelu G (1995). Effects of increased solar ultraviolet radiation on terrestrial plants. Ambio 24, 166-173. |
| [14] |
Casadevall R, Rodriguez RE, Debernardi JM, Palatnik JF, Casati P (2013). Repression of growth regulating factors by the microRNA396 inhibits cell proliferation by UV-B radiation in Arabidopsis leaves. Plant Cell 25, 3570-3583.
DOI URL |
| [15] |
Čejka C, Ardan T, Širc J, Michálek J, Beneš J, Brůnová B, Rosina J (2011). Hydration and transparency of the rabbit cornea irradiated with UVB-doses of 0.25 J/cm2 and 0.5 J/cm2 compared with equivalent UVB radiation exposure reaching the human cornea from sunlight. Curr Eye Res 36, 607-613.
DOI URL |
| [16] |
Chen F, Kamradt M, Mulcahy M, Byun Y, Xu HL, McKay MJ, Cryns VL (2002). Caspase proteolysis of the cohesin component RAD21 promotes apoptosis. J Biol Chem 277, 16775-16781.
DOI URL PMID |
| [17] |
Da Costa-Nunes JA, Bhatt AM, O'Shea S, West CE, Bray CM, Grossniklaus U, Dickinson HG (2006). Characterization of the three Arabidopsis thaliana RAD21 cohesins reveals differential responses to ionizing radiation. J Exp Bot 57, 971-983.
URL PMID |
| [18] |
Da Costa-Nunes JA, Capitão C, Kozak J, Costa-Nunes P, Ducasa GM, Pontes O, Angelis KJ (2014). The At- RAD21.1 and AtRAD21.3 Arabidopsis cohesins play a synergistic role in somatic DNA double strand break damage repair. BMC Plant Biol 14, 353.
DOI URL PMID |
| [19] |
Frohnmeyer H, Staiger D (2003). Ultraviolet-B radiation- mediated responses in plants. Balancing damage and protection. Plant Physiol 133, 1420-1428.
DOI URL PMID |
| [20] |
Hauf S, Waizenegger C, Peters JM (2001). Cohesin cleavage by separase required for anaphase and cytokinesis in human cells. Science 293, 1320-1323.
DOI URL PMID |
| [21] |
Hectors K, Jacques E, Prinsen E, Guisez Y, Verbelen JP, Jansen MK, Vissenberg K (2010). UV radiation reduces epidermal cell expansion in leaves of Arabidopsis thaliana. J Exp Bot 61, 4339-4349.
DOI URL PMID |
| [22] |
Hirano T (2000). Chromosome cohesion, condensation, and separation. Annu Rev Biochem 69, 115-144.
DOI URL PMID |
| [23] |
Hoque MT, Ishikawa F (2002). Cohesin defects lead to premature sister chromatid separation, kinetochore dysfunction, and spindle-assembly checkpoint activation. J Biol Chem 277, 42306-42314.
DOI URL PMID |
| [24] |
Jallepalli PV, Waizenegger IC, Bunz F, Langer S, Speicher MR, Peters JM, Kinzler KW, Vogelstein B, Lengauer C (2001). Securin is required for chromosomal stability in human cells. Cell 105, 445-457.
URL PMID |
| [25] |
Jiang L, Wang Y, Björn LO, Li SS (2011). UV-B-induced DNA damage mediates expression changes of cell cycle regulatory genes in Arabidopsis root tips. Planta 233, 831-841.
DOI URL |
| [26] |
Jiang L, Xia M, Strittmatter LI, Makaroff CA (2007). The Arabidopsis cohesin protein SYN3 localizes to the nucleolus and is essential for gametogenesis. Plant J 50, 1020-1034.
DOI URL PMID |
| [27] | Liu X, Yue M, Ji QR, He JF (2013). Effects of ultraviolet-B radiation on primary photophysical process in photosystem II: a fluorescence spectrum analysis. In: Kuang TY, Lu CM, Zhang LX, eds. Photosynthesis Research for Food, Fuel and the Future. Berlin, Heidelberg: Springer. pp. 642-649. |
| [28] |
Losada A (2007). Cohesin regulation: fashionable ways to wear a ring. Chromosoma 116, 321-329.
DOI URL |
| [29] |
Nasmyth K, Peters JM, Uhlmann F (2000). Splitting the chromosome: cutting the ties that bind sister chromatids. Science 288, 1379-1384.
DOI URL PMID |
| [30] |
Nogués S, Allen DJ, Morison JL, Baker NR (1998). Ultraviolet-B radiation effects on water relations, leaf development, and photosynthesis in droughted pea plants. Plant Physiol 117, 173-181.
URL PMID |
| [31] |
Rao H, Uhlmann F, Nasmyth K, Varshavsky A (2001). Degradation of a cohesin subunit by the N-end rule pathway is essential for chromosome stability. Nature 410, 955-959.
DOI URL PMID |
| [32] |
Robson TM, Klem K, Urban O, Jansen MAK (2015). Re-interpreting plant morphological responses to UV-B radiation. Plant Cell Environ 38, 856-866.
DOI URL PMID |
| [33] |
Sadano H, Sugimoto H, Sakai F, Nomura N, Osumi T (2000). NXP-1, a human protein related to Rad21/Scc1/ Mcd1, is a component of the nuclear matrix. Biochem Biophys Res Commun 267, 418-422.
URL PMID |
| [34] |
Searles PS, Flint SD, Caldwell MM (2001). A meta-analysis of plant field studies simulating stratospheric ozone depletion. Oecologia 127, 1-10.
DOI URL PMID |
| [35] |
Skibbens RV (2009). Establishment of sister chromatid cohesion. Curr Biol 19, R1126-R1132.
DOI URL PMID |
| [36] |
Sugimoto-Shirasu K, Roberts K (2003). ‘Big it up’: endoreduplication and cell-size control in plants. Curr Opin Plant Biol 6, 544-553.
DOI URL PMID |
| [37] |
Suzuki G, Nishiuchi C, Tsuru A, Kako E, Li J, Yamamoto M, Mukai Y (2013). Cellular localization of mitotic RAD21 with repetitive amino acid motifs in Allium cepa. Gene 514, 75-81.
DOI URL |
| [38] |
Uhlmann F, Lottspeich F, Nasmyth K (1999). Sister- chromatid separation at anaphase onset is promoted by cleavage of the cohesin subunit Scc1. Nature 400, 37-42.
DOI URL PMID |
| [39] |
Uhlmann F, Wernic D, Poupart MA, Koonin EV, Nasmyth K (2000). Cleavage of cohesin by the CD clan protease separin triggers anaphase in yeast. Cell 103, 375-386.
DOI URL PMID |
| [40] |
Vandenbussche F, Yu N, Li WD, Vanhaelewyn L, Hamshou M, Van Der Straeten D, Smagghe G (2018). An ultraviolet B condition that affects growth and defense in Arabidopsis. Plant Sci 268, 54-63.
DOI URL PMID |
| [41] |
Waizenegger IC, Hauf S, Meinke A, Peters JM (2000). Two distinct pathways remove mammalian cohesin from chromosome arms in prophase and from centromeres in ana- phase. Cell 103, 399-410.
DOI URL PMID |
| [42] |
Warren WD, Steffensen S, Lin E, Coelho P, Loupart ML, Cobbe N, Lee JY, McKay M, Orr-Weaver TL, Heck MMS, Sunkel CE (2000). The Drosophila RAD21 cohesin persists at the centromere region in mitosis. Curr Biol 10, 1463-1466.
URL PMID |
| [43] |
Xu HL, Beasley M, Verschoor S, Inselman A, Handel MA, McKay MJ (2004). A new role for the mitotic RAD21/ SCC1 cohesin in meiotic chromosome cohesion and segregation in the mouse. EMBO Rep 5, 378-384.
DOI URL PMID |
| [44] |
Xu HL, Yan YQ, Deb S, Rangasamy D, Germann M, Malaterre J, Eder NC, Ward RL, Hawkins NJ, Tothill RW, Chen L, Mortensen NJ, Fox SB, McKay MJ, Ramsay RG (2014). Cohesin rad21 mediates loss of heterozygosity and is upregulated via Wnt promoting transcriptional dysregulation in gastrointestinal tumors. Cell Rep 9, 1781-1797.
URL PMID |
| [1] | 刘雨函, 曹启江, 张诗晗, 李益慧, 王菁, 谭晓萌, 刘筱儒, 王显玲. 拟南芥AtFTCD-L参与根系响应土壤紧实度的机制研究[J]. 植物学报, 2025, 60(4): 1-0. |
| [2] | 陈鹏翔, 王波, 王子俊, 韩榕. 转录因子在植物响应UV-B辐射中的调控作用[J]. 植物学报, 2025, 60(3): 449-459. |
| [3] | 景艳军, 林荣呈. 蓝光受体CRY2化身“暗黑舞者”[J]. 植物学报, 2024, 59(6): 878-882. |
| [4] | 罗燕, 刘奇源, 吕元兵, 吴越, 田耀宇, 安田, 李振华. 拟南芥光敏色素突变体种子萌发的光温敏感性[J]. 植物学报, 2024, 59(5): 752-762. |
| [5] | 陈艳晓, 李亚萍, 周晋军, 解丽霞, 彭永彬, 孙伟, 和亚男, 蒋聪慧, 王增兰, 郑崇珂, 谢先芝. 拟南芥光敏色素B氨基酸位点突变对其结构与功能的影响[J]. 植物学报, 2024, 59(3): 481-494. |
| [6] | 杨继轩, 王雪霏, 顾红雅. 西藏野生拟南芥开花时间变异的遗传基础[J]. 植物学报, 2024, 59(3): 373-382. |
| [7] | 罗兰莎, 宋雯佩, 化青珠, 李大卫, 梁红, 张宪智. 植物性别决定基因及其表观遗传调控研究进展[J]. 植物学报, 2024, 59(2): 278-290. |
| [8] | 孙尚, 胡颖颖, 韩阳朔, 薛超, 龚志云. 水稻染色体双链寡核苷酸荧光原位杂交技术[J]. 植物学报, 2023, 58(3): 433-439. |
| [9] | 王钢, 王二涛. “卫青不败由天幸”——WeiTsing的广谱抗根肿病机理被揭示[J]. 植物学报, 2023, 58(3): 356-358. |
| [10] | 张凡凡, 邢新滢, 石文清, 沈懿, 程祝宽. 植物寡核苷酸荧光原位杂交技术方法[J]. 植物学报, 2023, 58(2): 274-284. |
| [11] | 杨永青, 郭岩. 植物细胞质外体pH感受机制的解析[J]. 植物学报, 2022, 57(4): 409-411. |
| [12] | 彭丹, 武志强. 植物雌雄异株性别决定研究进展[J]. 生物多样性, 2022, 30(3): 21416-. |
| [13] | 支添添, 周舟, 韩成云, 任春梅. PAD4突变加速拟南芥酪氨酸降解缺陷突变体sscd1的程序性细胞死亡[J]. 植物学报, 2022, 57(3): 288-298. |
| [14] | 李艳艳, 齐艳华. 植物Aux/IAA基因家族生物学功能研究进展[J]. 植物学报, 2022, 57(1): 30-41. |
| [15] | 李秋信, 迟伟, 季代丽. CURT1调控类囊体膜弯曲的研究进展[J]. 植物学报, 2021, 56(4): 462-469. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||