

Chinese Bulletin of Botany ›› 2020, Vol. 55 ›› Issue (2): 192-198.DOI: 10.11983/CBB19223 cstr: 32102.14.CBB19223
• TECHNIQUES AND METHODS • Previous Articles Next Articles
Yan Xiao1,Zhenxing Wang1,Dongming Li2,Yanhua Qi2, Enhebayaer1( )
)
Received:2019-11-17
Accepted:2020-02-26
Online:2020-03-01
Published:2020-02-26
Contact:
Enhebayaer
Yan Xiao,Zhenxing Wang,Dongming Li,Yanhua Qi, Enhebayaer. Optimization of Tissue Culture and Plant Regeneration System of Mature Embryo of Leymus chinensis[J]. Chinese Bulletin of Botany, 2020, 55(2): 192-198.
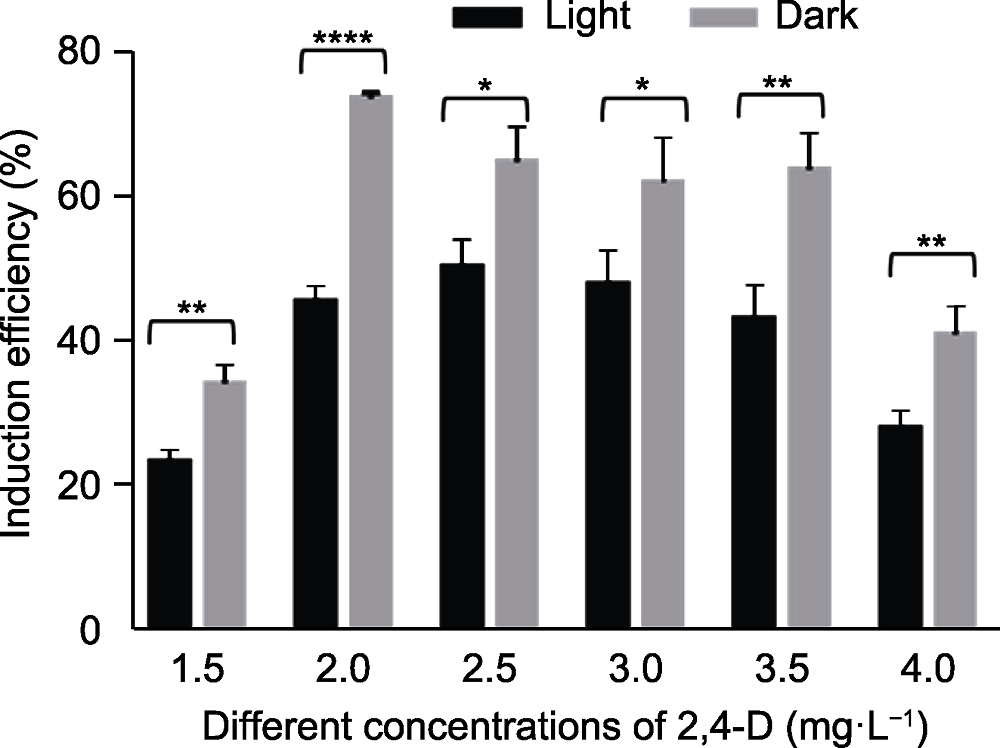
Figure 1 Induction efficiency of different 2,4-D concentra- tions in mature embryos of Leymus chinensis under light/dark conditions for 20 days * P<0.05; ** P<0.01; ****P<0.0001
| 6-BA/NAA (mg·L-1) | Callus number | The number of adventitious buds in proliferation | The number of roots in reproduction | Differentiation efficiency (%) |
|---|---|---|---|---|
| 0.5/0.25 | 21 | 0 | 0 | 0 |
| 1.0/0.25 | 21 | 1A | 4+ | 4.8±4.8 ab |
| 2.0/0.25 | 21 | 0 | 0 | 0 |
| 3.0/0.25 | 21 | 3AB | 0 | 14.3±8.3 abcd |
| 4.0/0.25 | 21 | 8AB | 0 | 38.1±4.8 efgh |
| 5.0/0.25 | 21 | 4A | 0 | 19.1±4.8 abcde |
| 0.5/0.5 | 21 | 0 | 0 | 0 |
| 1.0/0.5 | 21 | 7BC | 6+ | 33.3±9.5 defg |
| 2.0/0.5 | 21 | 10BC | 5+ | 47.6±9.5 gh |
| 3.0/0.5 | 21 | 7AB | 4 | 33.3±4.8 defg |
| 4.0/0.5 | 21 | 10AB | 0 | 47.6±9.5 gh |
| 5.0/0.5 | 21 | 3BC | 0 | 14.3±0 abcd |
| 0.5/1.0 | 21 | 2A | 8+ | 9.5±4.8 abc |
| 1.0/1.0 | 21 | 12AB | 4 | 57.1±4.8 h |
| 2.0/1.0 | 21 | 9A | 0 | 42.9±8.3 fgh |
| 3.0/1.0 | 21 | 5AC | 3 | 23.8±8.3 bcdef |
| 4.0/1.0 | 21 | 4A | 1+ | 19.1±4.8 abcde |
| 5.0/1.0 | 21 | 5A | 0 | 23.8±12.6 bcdef |
| 0.5/2.0 | 21 | 5ABC | 9+ | 23.8±4.8 bcdef |
| 1.0/2.0 | 21 | 1A | 5 | 4.8±4.8 ab |
| 2.0/2.0 | 21 | 0 | 0 | 0 |
| 3.0/2.0 | 21 | 2A | 1+ | 9.5±4.8 abc |
| 4.0/2.0 | 21 | 2AB | 4 | 9.5±4.8 abc |
| 5.0/2.0 | 21 | 0 | 0 | 0 |
| 0.5/3.0 | 21 | 1A | 3 | 4.8±4.8 ab |
| 1.0/3.0 | 21 | 4A | 3 | 19.1±4.8 abcde |
| 2.0/3.0 | 21 | 7AB | 3 | 33.3±4.8 defg |
| 3.0/3.0 | 21 | 10ABC | 1 | 47.6±9.5 gh |
| 4.0/3.0 | 21 | 0 | 0 | 0 |
| 5.0/3.0 | 21 | 0 | 0 | 0 |
| 0.5/4.0 | 21 | 6A | 3 | 28.6±8.2 cdefg |
| 1.0/4.0 | 21 | 0 | 0 | 0 |
| 2.0/4.0 | 21 | 8AB | 2 | 38.1±4.8 efgh |
| 3.0/4.0 | 21 | 0 | 0 | 0 |
| 4.0/4.0 | 21 | 3AB | 5 | 14.3±8.2 abcd |
| 5.0/4.0 | 21 | 6AB | 0 | 28.6±8.2 cdefg |
Table 1 Differentiation efficiency of Leymus chinensis callus under different concentrations of 6-BA and NAA (means±SE)
| 6-BA/NAA (mg·L-1) | Callus number | The number of adventitious buds in proliferation | The number of roots in reproduction | Differentiation efficiency (%) |
|---|---|---|---|---|
| 0.5/0.25 | 21 | 0 | 0 | 0 |
| 1.0/0.25 | 21 | 1A | 4+ | 4.8±4.8 ab |
| 2.0/0.25 | 21 | 0 | 0 | 0 |
| 3.0/0.25 | 21 | 3AB | 0 | 14.3±8.3 abcd |
| 4.0/0.25 | 21 | 8AB | 0 | 38.1±4.8 efgh |
| 5.0/0.25 | 21 | 4A | 0 | 19.1±4.8 abcde |
| 0.5/0.5 | 21 | 0 | 0 | 0 |
| 1.0/0.5 | 21 | 7BC | 6+ | 33.3±9.5 defg |
| 2.0/0.5 | 21 | 10BC | 5+ | 47.6±9.5 gh |
| 3.0/0.5 | 21 | 7AB | 4 | 33.3±4.8 defg |
| 4.0/0.5 | 21 | 10AB | 0 | 47.6±9.5 gh |
| 5.0/0.5 | 21 | 3BC | 0 | 14.3±0 abcd |
| 0.5/1.0 | 21 | 2A | 8+ | 9.5±4.8 abc |
| 1.0/1.0 | 21 | 12AB | 4 | 57.1±4.8 h |
| 2.0/1.0 | 21 | 9A | 0 | 42.9±8.3 fgh |
| 3.0/1.0 | 21 | 5AC | 3 | 23.8±8.3 bcdef |
| 4.0/1.0 | 21 | 4A | 1+ | 19.1±4.8 abcde |
| 5.0/1.0 | 21 | 5A | 0 | 23.8±12.6 bcdef |
| 0.5/2.0 | 21 | 5ABC | 9+ | 23.8±4.8 bcdef |
| 1.0/2.0 | 21 | 1A | 5 | 4.8±4.8 ab |
| 2.0/2.0 | 21 | 0 | 0 | 0 |
| 3.0/2.0 | 21 | 2A | 1+ | 9.5±4.8 abc |
| 4.0/2.0 | 21 | 2AB | 4 | 9.5±4.8 abc |
| 5.0/2.0 | 21 | 0 | 0 | 0 |
| 0.5/3.0 | 21 | 1A | 3 | 4.8±4.8 ab |
| 1.0/3.0 | 21 | 4A | 3 | 19.1±4.8 abcde |
| 2.0/3.0 | 21 | 7AB | 3 | 33.3±4.8 defg |
| 3.0/3.0 | 21 | 10ABC | 1 | 47.6±9.5 gh |
| 4.0/3.0 | 21 | 0 | 0 | 0 |
| 5.0/3.0 | 21 | 0 | 0 | 0 |
| 0.5/4.0 | 21 | 6A | 3 | 28.6±8.2 cdefg |
| 1.0/4.0 | 21 | 0 | 0 | 0 |
| 2.0/4.0 | 21 | 8AB | 2 | 38.1±4.8 efgh |
| 3.0/4.0 | 21 | 0 | 0 | 0 |
| 4.0/4.0 | 21 | 3AB | 5 | 14.3±8.2 abcd |
| 5.0/4.0 | 21 | 6AB | 0 | 28.6±8.2 cdefg |
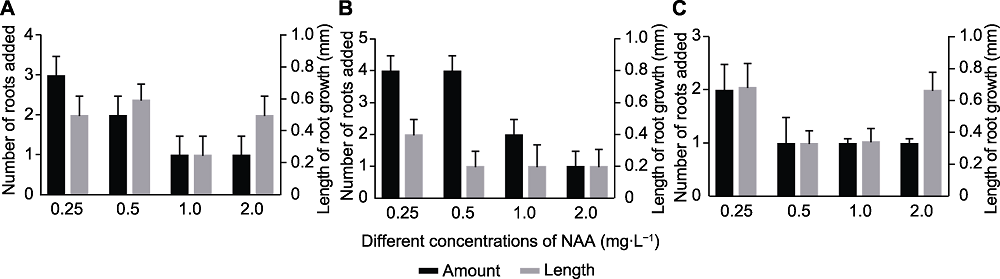
Figure 2 Effect of different NAA concentrations on rooting of tissue culture seedlings in Leymus chinensis (A) Rooting of 2.0-3.0 cm of seedlings; (B) Rooting of 3.1-5.0 cm of seedlings; (C) Rooting of 5.1-7.0 cm of seedlings. All seedlings with (0.5±0.1) cm of primary root and 3-5 of adventitious roots were used to the related experiments.
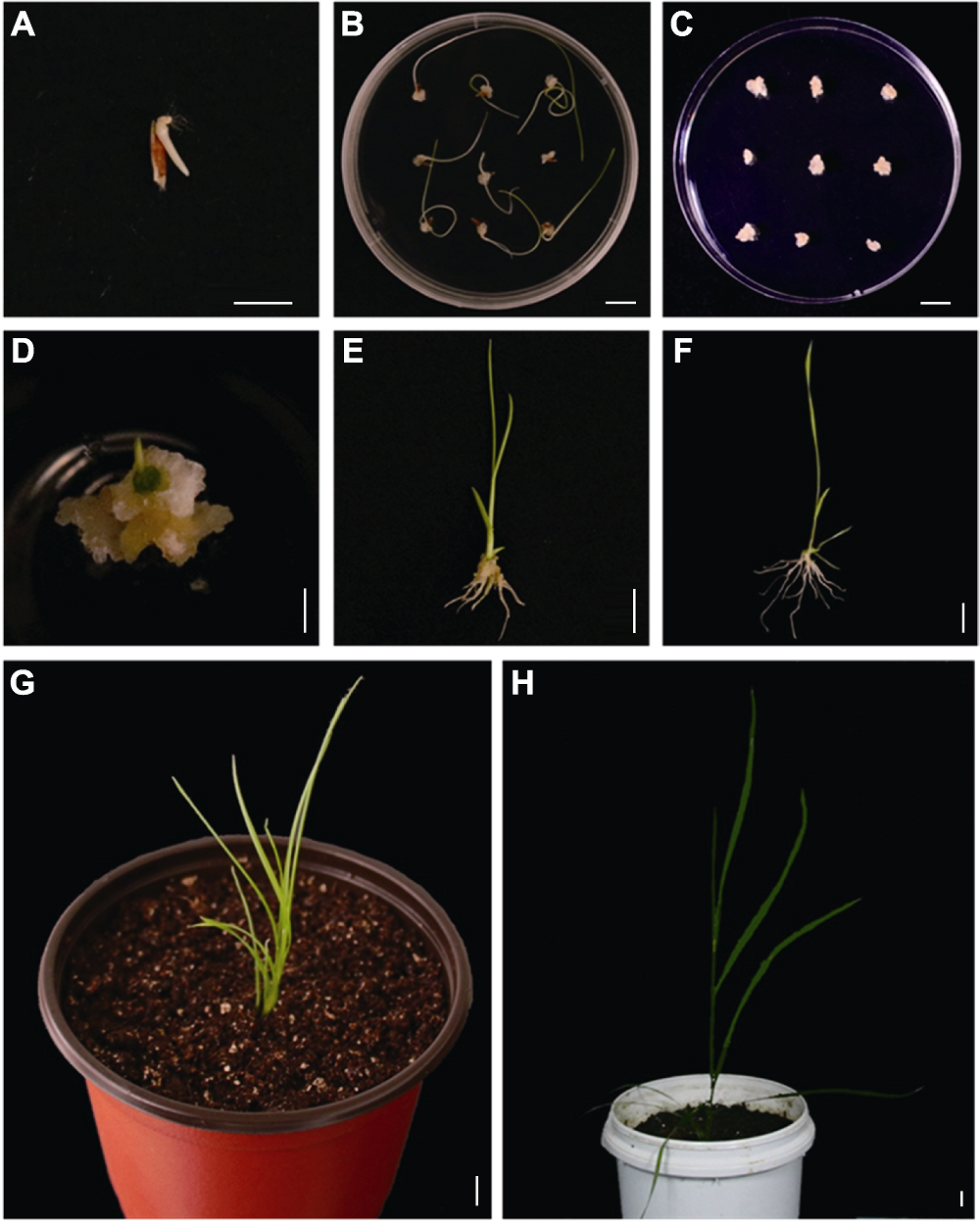
Figure 3 Induction, subculture, differentiation and transplanting of mature embryos of Leymus chinensis (A) Mature embryo induction for 5 days; (B) Mature embryo induction for 20 days; (C) Subculture of callus; (D) Callus differentiation; (E) The differentiated 40-day seedlings; (F) Rooting culture for 14 days; (G) Plants transplanted for 14 days; (H) Plants transplanted for 2 months. Bars=1 cm
| [8] | 马红媛, 梁正伟 ( 2007). 不同pH值土壤及其浸提液对羊草种子萌发和幼苗生长的影响. 植物学通报 24, 181-188. |
| [9] | 孟宪宝 ( 2010). 优质饲草羊草栽培技术. 黑龙江畜牧兽医 13, 97-98. |
| [10] | 曲同宝 ( 2004). 羊草遗传转化受体系统的建立及转BADH基因的研究. 硕士论文. 吉林: 吉林农业大学. pp.12-25. |
| [11] | 曲同宝, 孟繁勇, 张友民, 王丕武 ( 2010). 影响羊草愈伤组织分化因素的研究. 安徽农业科学 38, 6125-6127, 6130. |
| [12] | 曲同宝, 王丕武, 关淑艳, 刘玲芝 ( 2004). 羊草组织培养及再生系统的建立. 草业学报 13(5), 91-94. |
| [13] | 汪恩华 ( 2002). 羊草繁殖生物学特性的研究.硕士论文. 北京: 中国科学院植物研究所. pp.39-45. |
| [14] | 魏琪, 胡国富, 李凤兰, 胡宝忠 ( 2005). 羊草种子愈伤组织的诱导及植株再生. 东北农业大学学报 36, 41-44. |
| [15] | 张莹, 李晓峰, 刘公社, 陈耀锋 ( 2007). 羊草愈伤组织状态的调控. 西北农林科技大学学报(自然科学版) 35(5), 111-114. |
| [16] | 张玉芬, 周道玮 ( 2002). 羊草分化及育种研究进展. 中国草地 24(2), 54-58, 74. |
| [17] | 周道玮, 李强, 宋彦涛, 王学志 ( 2011). 松嫩平原羊草草地盐碱化过程. 应用生态学报 22, 1423-1430. |
| [18] | 邹吉祥 ( 2012). 羊草高频再生体系建立及转CodA基因的初步探索. 硕士论文. 吉林: 延边大学. pp.3-4. |
| [19] | Hiei Y, Ohta S, Komari T, Kumashiro T ( 1994). Efficient transformation of rice ( Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J 6, 271-282. |
| [20] | Liu BS, Kang CL, Wang X, Bao GZ ( 2015). Tolerance mechanisms of Leymus chinensis to salt-alkaline stress. Acta Agric Scand Sect B-Soil Plant Sci 65, 723-734. |
| [21] | Mathur S, Tomar RS, Jajoo A ( 2019). Arbuscularmycorrhizal fungi (AMF) protects photosynthetic apparatus of wheat under drought stress. Photosyn Res 139, 227-238. |
| [22] | Olson RE, Rudney H ( 1983). Biosynthesis of ubiquinone. Vitam Horm 40, 1-43. |
| [23] | Zhu TC, Li JD, Yang DC ( 1981). A study of the ecology of Yang-cao (Leymus chinensis) grassland in Northern China. In: Proceedings of the 14th International Grassland Congress. Lexington: Westview. pp. 429-431. |
| [1] | 崔秋华, 张玉珍, 朴铁夫, 顾德峰, 张为群, 许耀奎, 孙振雷, 刘海学 ( 1990). 羊草胚性愈伤组织的形成及植株再生. 吉林农业大学学报 12(3), 1-5. |
| [2] | 韩德复 ( 1996). 羊草组织培养的研究. 吉林农业大学学报 18(S1), 140-141. |
| [3] | 孔祥军, 梁正伟 ( 2007). 羊草分子生物学研究进展. 生命科学研究 11, 289-294. |
| [4] | 孔祥军, 梁正伟, 马红媛, 刘淼 ( 2008). 变温培养对羊草胚性愈伤组织诱导率的影响. 生物技术 10(5), 60-62. |
| [5] | 李艳波, 李凤芹 ( 1998). 松嫩盐碱草地羊草群落的产量动态. 黑龙江大学自然科学学报 15(2), 103-106. |
| [6] | 刘滨硕, 康春莉, 王鑫, 包国章 ( 2014). 羊草对盐碱胁迫的生理生化响应特征. 农业工程学报 30(23), 166-173. |
| [7] | 刘公社, 汪恩华, 刘杰, 齐冬梅, 李芳芳 ( 2002). 羊草幼穗离体培养诱导植株再生的研究. 草地学报 10, 198-202. |
| [1] | Zheng Guo, Xiangjun Shao, Haiwen Lu, Dan Hou, Simeng Kong, Xiangyu Li, Huaqian Liu, Xinchun Lin. Efficient Induction and Identification of Polyploids in Dendrocalamus asper [J]. Chinese Bulletin of Botany, 2025, 60(2): 246-255. |
| [2] | XU Hong, SU Hua, LI Yong-Geng, SU Ben-Ying, YANG Jing-Cheng, LI Yu-Qiang, WANG Zheng-Wen. Exploration of livestock-poultry-grassland systems: the influence of different land use types on the grassland dominated by Leymus chinensis in northern China [J]. Chin J Plant Ecol, 2025, 49(1): 211-220. |
| [3] | BAI Hao-Ran, HOU Meng, LIU Yan-Jie. Mechanisms of the invasion of Cenchrus spinifex and drought effects on productivity of Leymus chinensis community [J]. Chin J Plant Ecol, 2024, 48(5): 577-589. |
| [4] | Yuchen Li, Haixia Zhao, Xiping Jiang, Xintian Huang, Yaling Liu, Zhenying Wu, Yan Zhao, Chunxiang Fu. Establishment of Agrobacterium-mediated Transformation System for Agropyron mongolicum [J]. Chinese Bulletin of Botany, 2024, 59(4): 600-612. |
| [5] | Xuping Tian, Kangjie Yue, Jiali Wang, Huixin Liu, Ziyin Shi, Hongwei Kang. Callus Induction and Plant Regeneration of Dracocephalum rupestre [J]. Chinese Bulletin of Botany, 2024, 59(4): 613-625. |
| [6] | Hao Zeng, Peifang Li, Zhihui Guo, Chunlin Liu, Ying Ruan. Establishment of a Regeneration System for Lunaria annua [J]. Chinese Bulletin of Botany, 2024, 59(3): 433-440. |
| [7] | YAO Zhen-Yu, XIN Yue, MU Wen-Kui, ZHANG Quan-Min, YANG Liu, ZHAO Li-Qing. Community characteristics of Leymus chinensis steppe in Nei Mongol, China [J]. Chin J Plant Ecol, 2024, 48(10): 1385-1392. |
| [8] | Yuejing Zhang, Hetian Sang, Hanqi Wang, Zhenzhen Shi, Li Li, Xin Wang, Kun Sun, Ji Zhang, Hanqing Feng. Research Progress of Plant Signaling in Systemic Responses to Abiotic Stresses [J]. Chinese Bulletin of Botany, 2024, 59(1): 122-133. |
| [9] | Shangwen Zhang, Shiyu Huang, Tianwei Yang, Ting Li, Xiangjun Zhang, Manrong Gao. Establishment of a Tissue Culture and Rapid Propagation System for Erythropalum scandens Based on Orthogonal Test [J]. Chinese Bulletin of Botany, 2024, 59(1): 99-109. |
| [10] | Liu Xiaofei, Sun Yingbo, Huang Lili, Yang Yuchai, Zhu Genfa, Yu Bo. Efficient Plant Regeneration via Somatic Embryogenesis in Alocasia reginula cv. ‘Black Velvet’ [J]. Chinese Bulletin of Botany, 2023, 58(5): 750-759. |
| [11] | Jiming Cheng, Huimin He, Hongyu Niu, Hongmao Zhang. Research progress on the effect of intraspecific personality differences on seed dispersal in rodents [J]. Biodiv Sci, 2023, 31(4): 22446-. |
| [12] | Yefei Liu, Haixia Zhao, Xiping Jiang, Rui Qiu, Xinyue Zhou, Yan Zhao, Chunxiang Fu. Establishment of Highly Efficient Tissue Culture and Agrobacterium-mediated Callus Infection Systems for Hordeum brevisubulatum [J]. Chinese Bulletin of Botany, 2023, 58(3): 440-448. |
| [13] | Jiman Li, Nan Jin, Maogang Xu, Jusong Huo, Xiaoyun Chen, Feng Hu, Manqiang Liu. Effects of earthworm on tomato resistance under different drought levels [J]. Biodiv Sci, 2022, 30(7): 21488-. |
| [14] | Dai Chen, Wang Jin, Lu Yaping. Determination of Acidic Plant Hormones by Derivative UPLC-MS [J]. Chinese Bulletin of Botany, 2022, 57(4): 500-507. |
| [15] | Jinchun Lu, Lina Cao, Guanjie Tong, Xinying Wang, Liying Zhang, Xin Yu, Huifang Li, Yanhui Li. Establishment of Callus Induction and Regeneration System of Anemone silvestris [J]. Chinese Bulletin of Botany, 2022, 57(2): 217-226. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||