

Chinese Bulletin of Botany ›› 2024, Vol. 59 ›› Issue (1): 122-133.DOI: 10.11983/CBB23063 cstr: 32102.14.CBB23063
• SPECIAL TOPICS • Previous Articles Next Articles
Yuejing Zhang1, Hetian Sang1, Hanqi Wang1, Zhenzhen Shi2, Li Li1, Xin Wang1, Kun Sun1, Ji Zhang1,3, Hanqing Feng1,3,*( )
)
Received:2023-05-15
Accepted:2023-09-19
Online:2024-01-10
Published:2024-01-10
Contact:
*E-mail: Yuejing Zhang, Hetian Sang, Hanqi Wang, Zhenzhen Shi, Li Li, Xin Wang, Kun Sun, Ji Zhang, Hanqing Feng. Research Progress of Plant Signaling in Systemic Responses to Abiotic Stresses[J]. Chinese Bulletin of Botany, 2024, 59(1): 122-133.
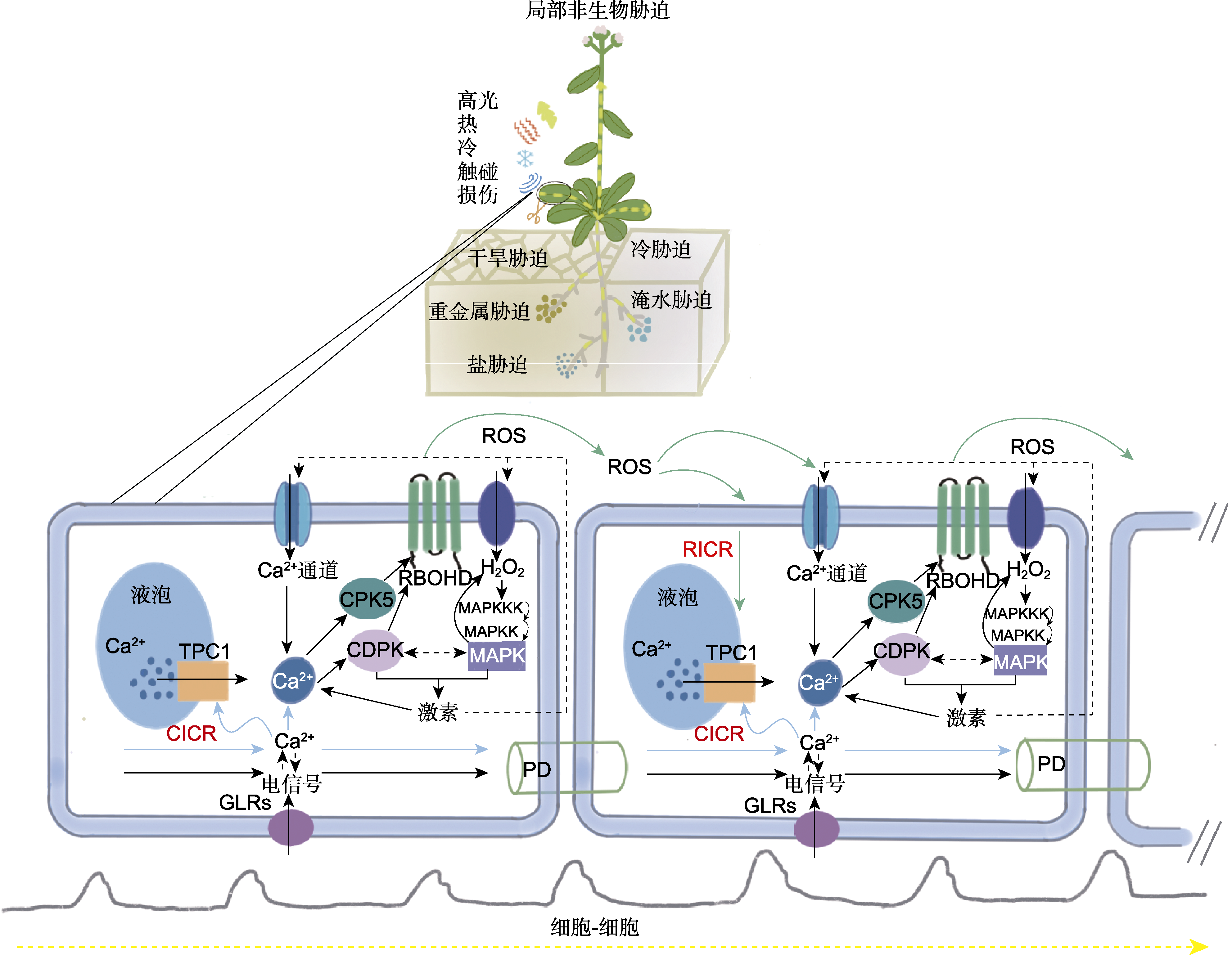
Figure 1 Conceptual models of systemic signal transduction (Ca2+ signal, ROS signal, electrical signals and plant hormones) in plants under different abiotic stresses When the local plant is stimulated by various abiotic stresses, the stimulated cells activate GLR, Ca2+ channels and TPC1, which leads to an increase in the concentration of Ca2+ in the cytoplasm, accompanied by changes in electrical signals. Cytoplasmic Ca2+ and CDPK, CPK5 and other proteins work together to activate RBOHD, which leads to an increase in extracellular ROS. Extracellular ROS can further increase cytoplasmic Ca2+ levels by activating Ca2+ channels and cause a phosphorylation cascade reaction of MAPK. CDPK and MAPK may work together to regulate the biosynthesis of plant hormones, which further increases the intensity of Ca2+ signals, ROS signals and electrical signals. At the same time, cells directly stimulated by stresses may induce similar changes in neighboring cells through PD and extracellular ROS-mediated intercellular interactions. This intercellular action extends further, thus the formation of systemic signals in the plant can travel long distances and regulate the systemic response of the plant to local stresses. In the systemic signaling pathway, the cells on the left are directly stressed cells, and the cells on the right are neighboring cells. The solid lines indicate the known action pathway, the dotted lines indicate the possible action pathway, the yellow dotted lines indicate long-distance transportation, and the gray curve indicates the electrical signal transmission pathway. ROS: Reactive oxygen species; PD: Plasmodesmata; RBOHD: Respiratory burst oxidase homolog D; GLR: Glutamate-like receptor; CPK5: Calmodulin domain protein kinase 5; TPC1: Two pore channel 1; CDPK: Ca2+-dependent protein kinase; MAPK: Mitogen-activated protein kinase; RICR: ROS-induced calcium release; CICR: Calcium-induced calcium release
| [1] |
代宇佳, 罗晓峰, 周文冠, 陈锋, 帅海威, 杨文钰, 舒凯 (2019). 生物和非生物逆境胁迫下的植物系统信号. 植物学报 54, 255-264.
DOI |
| [2] |
吴楠, 覃磊, 彭志红, 夏石头 (2022). 系统获得性抗性移动信号Pip/NHP研究进展. 植物学报 57, 412-421.
DOI |
| [3] | Baba AI, Rigó G, Andrási N, Tietz O, Palme K, Szabados L, Cséplő Á (2019). Striving towards abiotic stresses:role of the plant CDPK superfamily members. In: Palocz-AndresenM, SzalayD, GosztomA, SíposL, TaligásT,eds. International Climate Protection. Cham: Springer. pp. 99-105. |
| [4] |
Balla T (2006). Phosphoinositide-derived messengers in endocrine signaling. J Endocrinol 188, 135-153.
PMID |
| [5] | Barbaglia AM (2015). Long-Distance Signaling in Plants: Elucidating the Function of Lipid-Binding Proteins in the Phloem and Their Response to Abiotic Stress. Master’s thesis. Michigan: Michigan State University. pp. 1-30. |
| [6] | Barbaglia AM, Hoffmann-Benning S (2016). Long-distance lipid signaling and its role in plant development and stress response. In: NakamuraY, Li-BeissonY,eds. Lipids in Plant and Algae Development. Cham: Springer. pp. 339-361. |
| [7] |
Białasek M, Górecka M, Mittler R, Karpiński S (2017). Evidence for the involvement of electrical, calcium and ROS signaling in the systemic regulation of non-photochemical quenching and photosynthesis. Plant Cell Physiol 58, 207-215.
DOI PMID |
| [8] |
Campbell AK, Trewavas AJ, Knight MR (1996). Calcium imaging shows differential sensitivity to cooling and communication in luminous transgenic plants. Cell Calcium 19, 211-218.
DOI PMID |
| [9] |
Chauvin A, Caldelari D, Wolfender JL, Farmer EE (2013). Four 13-lipoxygenases contribute to rapid jasmonate synthesis in wounded Arabidopsis thaliana leaves: a role for lipoxygenase 6 in responses to long-distance wound signals. New Phytol 197, 566-575.
DOI URL |
| [10] |
Chen J, Wang LH, Yuan M (2021). Update on the roles of rice MAPK cascades. Int J Mol Sci 22, 1679.
DOI URL |
| [11] |
Chico JM, Raıces M, Téllez-Inón MT, Ulloa RM (2002). A calcium-dependent protein kinase is systemically induced upon wounding in tomato plants. Plant Physiol 128, 256-270.
DOI PMID |
| [12] |
Choi WG, Hilleary R, Swanson SJ, Kim SH, Gilroy S (2016). Rapid, long-distance electrical and calcium signaling in plants. Annu Rev Plant Biol 67, 287-307.
DOI URL |
| [13] |
Choi WG, Miller G, Wallace I, Harper J, Mittler R, Gilroy S (2017). Orchestrating rapid long-distance signaling in plants with Ca2+, ROS and electrical signals. Plant J 90, 698-707.
DOI URL |
| [14] |
Choi WG, Toyota M, Kim SH, Hilleary R, Gilroy S (2014). Salt stress-induced Ca2+ waves are associated with rapid, long-distance root-to-shoot signaling in plants. Proc Natl Acad Sci USA 111, 6497-6502.
DOI URL |
| [15] |
Dekomah SD, Bi ZZ, Dormatey R, Wang YH, Haider FU, Sun C, Yao PF, Bai JP (2022). The role of CDPKs in plant development, nutrient and stress signaling. Front Genet 13, 996203.
DOI URL |
| [16] |
Dempsey DA, Klessig DF (2012). SOS—too many signals for systemic acquired resistance? Trends Plant Sci 17, 538-545.
DOI URL |
| [17] |
Devireddy AR, Zandalinas SI, Fichman Y, Mittler R (2021). Integration of reactive oxygen species and hormone signaling during abiotic stress. Plant J 105, 459-476.
DOI URL |
| [18] | Dolfi M, Dini C, Morosi S, Comparini D, Masi E, Pandolfi C, Mancuso S (2021). Electrical signaling related to water stress acclimation. Sens Bio-Sens Res 32, 100420. |
| [19] |
Du HW, Chen JJ, Zhan HY, Li S, Wang YS, Wang W, Hu XL (2023). The roles of CDPKs as a convergence point of different signaling pathways in maize adaptation to abiotic stress. Int J Mol Sci 24, 2325.
DOI URL |
| [20] |
Durrant WE, Dong X (2004). Systemic acquired resistance. Annu Rev Phytopathol 42, 185-209.
PMID |
| [21] |
Fichman Y, Miller G, Mittler R (2019). Whole-plant live imaging of reactive oxygen species. Mol Plant 12, 1203-1210.
DOI PMID |
| [22] |
Fichman Y, Mittler R (2020). Noninvasive live ROS imaging of whole plants grown in soil. Trends Plant Sci 25, 1052-1053.
DOI PMID |
| [23] |
Fichman Y, Mittler R (2021). Integration of electric, calcium, reactive oxygen species and hydraulic signals during rapid systemic signaling in plants. Plant J 107, 7-20.
DOI URL |
| [24] |
Fichman Y, Myers RJ Jr, Grant DAG, Mittler R (2021). Plasmodesmata-localized proteins and ROS orchestrate light-induced rapid systemic signaling in Arabidopsis. Sci Signal 14, eabf0322.
DOI URL |
| [25] | Fraire-Velázquez S, Rodríguez-Guerra R, Sánchez-Calderón L (2011). Abiotic and biotic stress response crosstalk in plants. In: ShankerA, VenkateswarluB, eds. Abiotic Stress Response in Plants-Physiological, Biochemical and Genetic Perspectives. Croatia: InTech. pp. 3-26. |
| [26] |
Furch ACU, van Bel AJE, Fricker MD, Felle HH, Fuchs M, Hafke JB (2009). Sieve element Ca2+ channels as relay stations between remote stimuli and sieve tube occlusion in Vicia faba. Plant Cell 21, 2118-2132.
DOI URL |
| [27] |
Gasperini D, Chauvin A, Acosta IF, Kurenda A, Stolz S, Chételat A, Wolfender JL, Farmer EE (2015). Axial and radial oxylipin transport. Plant Physiol 169, 2244-2254.
DOI PMID |
| [28] |
Gaupels F, Durner J, Kogel KH (2017). Production, amplification and systemic propagation of redox messengers in plants? The phloem can do it all! New Phytol 214, 554-560.
DOI PMID |
| [29] |
Geilfus CM, Mithöfer A, Ludwig-Müller J, Zörb C, Muehling KH (2015). Chloride-inducible transient apoplastic alkalinizations induce stomata closure by controlling abscisic acid distribution between leaf apoplast and guard cells in salt-stressed Vicia faba. New Phytol 208, 803-816.
DOI URL |
| [30] |
Gilroy S, Białasek M, Suzuki N, Górecka M, Devireddy AR, Karpiński S, Mittler R (2016). ROS, calcium, and electric signals: key mediators of rapid systemic signaling in plants. Plant Physiol 171, 1606-1615.
DOI PMID |
| [31] |
Gilroy S, Suzuki N, Miller G, Choi WG, Toyota M, Devireddy AR, Mittler R (2014). A tidal wave of signals: calcium and ROS at the forefront of rapid systemic signaling. Trends Plant Sci 19, 623-630.
DOI PMID |
| [32] |
Glauser G, Dubugnon L, Mousavi SAR, Rudaz S, Wolfender JL, Farmer EE (2009). Velocity estimates for signal propagation leading to systemic jasmonic acid accumulation in wounded Arabidopsis. J Biol Chem 284, 34506-34513.
DOI PMID |
| [33] |
Gong ZZ (2021). Plant abiotic stress: new insights into the factors that activate and modulate plant responses. J Integr Plant Biol 63, 429-430.
DOI |
| [34] | Großkinsky DK, van der Graaff E, Roitsch T (2016). Regulation of abiotic and biotic stress responses by plant hormones. In: CollingeDB, ed. Plant Pathogen Resistance Biotechnology. Hoboken: John Wiley & Sons, Inc. pp. 131-154. |
| [35] |
He XW, Wang CZ, Wang HB, Li LG, Wang C (2020). The function of MAPK cascades in response to various stresses in horticultural plants. Front Plant Sci 11, 952.
DOI PMID |
| [36] |
Hettenhausen C, Schuman MC, Wu JQ (2015). MAPK signaling: a key element in plant defense response to insects. Insect Sci 22, 157-164.
DOI PMID |
| [37] |
Hou QC, Ufer G, Bartels D (2016). Lipid signaling in plant responses to abiotic stress. Plant Cell Environ 39, 1029-1048.
DOI URL |
| [38] |
Ikegami K, Okamoto M, Seo M, Koshiba T (2009). Activation of abscisic acid biosynthesis in the leaves of Arabidopsis thaliana in response to water deficit. J Plant Res 122, 235-243.
DOI URL |
| [39] |
Jia WS, Davies WJ (2007). Modification of leaf apoplastic pH in relation to stomatal sensitivity to root-sourced abscisic acid signals. Plant Physiol 143, 68-77.
DOI PMID |
| [40] |
Kiep V, Vadassery J, Lattke J, Maaß JP, Boland W, Peiter E, Mithöfer A (2015). Systemic cytosolic Ca2+ elevation is activated upon wounding and herbivory in Arabidopsis. New Phytol 207, 996-1004.
DOI URL |
| [41] |
Kimura S, Waszczak C, Hunter K, Wrzaczek M (2017). Bound by fate: the role of reactive oxygen species in receptor-like kinase signaling. Plant Cell 29, 638-654.
DOI URL |
| [42] |
Kollist H, Zandalinas SI, Sengupta S, Nuhkat M, Kangasjärvi J, Mittler R (2019). Rapid responses to abiotic stress: priming the landscape for the signal transduction network. Trends Plant Sci 24, 25-37.
DOI PMID |
| [43] |
Koo AJK, Gao XL, Jones AD, Howe GA (2009). A rapid wound signal activates the systemic synthesis of bioactive jasmonates in Arabidopsis. Plant J 59, 974-986.
DOI URL |
| [44] |
Kronzucker HJ, Britto DT (2011). Sodium transport in plants: a critical review. New Phytol 189, 54-81.
DOI PMID |
| [45] | Kumar K, Raina SK, Sultan SM (2020). Arabidopsis MAPK signaling pathways and their cross talks in abiotic stress response. J Plant Biochem Biot 29, 700-714. |
| [46] |
Lacombe B, Achard P (2016). Long-distance transport of phytohormones through the plant vascular system. Curr Opin Plant Biol 34, 1-8.
DOI PMID |
| [47] |
Lautner S, Grams TEE, Matyssek R, Fromm J (2005). Characteristics of electrical signals in poplar and responses in photosynthesis. Plant Physiol 138, 2200-2209.
DOI PMID |
| [48] |
Lee S, Suh S, Kim S, Crain RC, Kwak JM, Nam HG, Lee Y (1997). Systemic elevation of phosphatidic acid and lysophospholipid levels in wounded plants. Plant J 12, 547-556.
DOI URL |
| [49] |
Li HY, Liu Y, Li XY, Li XH, Ma HM (2021). Design, synthesis and application of a dual-functional fluorescent probe for reactive oxygen species and viscosity. Spectrochim Acta A 246, 119059.
DOI URL |
| [50] |
Li YQ, Liu YN, Jin LB, Peng RY (2022). Crosstalk between Ca2+ and other regulators assists plants in responding to abiotic stress. Plants 11, 1351.
DOI URL |
| [51] |
Liang XX, Zhang J (2022). Regulation of plant responses to biotic and abiotic stress by receptor-like cytoplasmic kinases. Stress Biol 2, 25.
DOI PMID |
| [52] |
Medina E, Kim SH, Yun M, Choi WG (2021). Recapitulation of the function and role of ROS generated in response to heat stress in plants. Plants 10, 371.
DOI URL |
| [53] | Miller G, Schlauch K, Tam R, Cortes D, Torres MA, Shulaev V, Dangl JL, Mittler R (2009). The plant NADPH oxidase RBOHD mediates rapid systemic signaling in response to diverse stimuli. Sci Signal 2, ra45. |
| [54] |
Mittler R, Blumwald E (2015). The roles of ROS and ABA in systemic acquired acclimation. Plant Cell 27, 64-70.
DOI URL |
| [55] |
Mittler R, Vanderauwera S, Suzuki N, Miller G, Tognetti VB, Vandepoele K, Gollery M, Shulaev V, Van Breusegem F (2011). ROS signaling: the new wave? Trends Plant Sci 16, 300-309.
DOI PMID |
| [56] |
Mousavi SAR, Chauvin A, Pascaud F, Kellenberger S, Farmer EE (2013). GLUTAMATE RECEPTOR-LIKE genes mediate leaf-to-leaf wound signaling. Nature 500, 422-426.
DOI |
| [57] |
Munnik T, Nielsen E (2011). Green light for polyphosphoinositide signals in plants. Curr Opin Plant Biol 14, 489-497.
DOI PMID |
| [58] |
Myers RJ, Fichman Y, Stacey G, Mittler R (2022). Extracellular ATP plays an important role in systemic wound response activation. Plant Physiol 189, 1314-1325.
DOI PMID |
| [59] |
Nguyen CT, Kurenda A, Stolz S, Chételat A, Farmer EE (2018). Identification of cell populations necessary for leaf-to-leaf electrical signaling in a wounded plant. Proc Natl Acad Sci USA 115, 10178-10183.
DOI PMID |
| [60] |
Peláez-Vico MÁ, Fichman Y, Zandalinas SI, Van Breusegem F, Karpiński SM, Mittler R (2022). ROS and redox regulation of cell-to-cell and systemic signaling in plants during stress. Free Radic Biol Med 193, 354-362.
DOI URL |
| [61] |
Ren HB, Wei KF, Jia WS, Davies WJ, Zhang JH (2007). Modulation of root signals in relation to stomatal sensitivity to root-sourced abscisic acid in drought-affected plants. J Integr Plant Biol 49, 1410-1420.
DOI |
| [62] |
Rivero RM, Mittler R, Blumwald E, Zandalinas SI (2022). Developing climate-resilient crops: improving plant tolerance to stress combination. Plant J 109, 373-389.
DOI URL |
| [63] | Sadak MS (2016). Physiological role of signal molecules in improving plant tolerance under abiotic stress. Int J Chem Tech Res 9, 46-60. |
| [64] |
Seo M, Koshiba T (2011). Transport of ABA from the site of biosynthesis to the site of action. J Plant Res 124, 501-507.
DOI PMID |
| [65] |
Shah J, Chaturvedi R, Chowdhury Z, Venables B, Petros RA (2014). Signaling by small metabolites in systemic acquired resistance. Plant J 79, 645-658.
DOI URL |
| [66] |
Shao QL, Gao QF, Lhamo D, Zhang HS, Luan S (2020). Two glutamate- and pH-regulated Ca2+ channels are required for systemic wound signaling in Arabidopsis. Sci Signal 13, eaba1453.
DOI URL |
| [67] | Strahl T, Thorner J (2007). Synthesis and function of membrane phosphoinositides in budding yeast, Saccharomyces cerevisiae. Biochim Biophys Acta-Mol Cell Biol Lipids 1771, 353-404. |
| [68] |
Sukhov V, Sherstneva O, Surova L, Katicheva L, Vodeneev V (2014). Proton cellular influx as a probable mechanism of variation potential influence on photosynthesis in pea. Plant Cell Environ 37, 2532-2541.
DOI URL |
| [69] |
Sukhov V, Sukhova E, Vodeneev V (2019). Long-distance electrical signals as a link between the local action of stressors and the systemic physiological responses in higher plants. Prog Biophys Mol Biol 146, 63-84.
DOI URL |
| [70] |
Sukhova E, Sukhov V (2021). Electrical signals, plant tolerance to actions of stressors, and programmed cell death: is interaction possible? Plants 10, 1704.
DOI URL |
| [71] |
Suzuki N, Bassil E, Hamilton JS, Inupakutika MA, Zandalinas SI, Tripathy D, Luo YT, Dion E, Fukui G, Kumazaki A, Nakano R, Rivero RM, Verbeck GF, Azad RK, Blumwald E, Mittler R (2016). ABA is required for plant acclimation to a combination of salt and heat stress. PLoS One 11, e0147625.
DOI URL |
| [72] |
Suzuki N, Miller G, Salazar C, Mondal HA, Shulaev E, Cortes DF, Shuman JL, Luo XZ, Shah J, Schlauch K, Shulaev V, Mittler R (2013). Temporal-spatial interaction between reactive oxygen species and abscisic acid regulates rapid systemic acclimation in plants. Plant Cell 25, 3553-3569.
DOI URL |
| [73] |
Suzuki N, Rivero RM, Shulaev V, Blumwald E, Mittler R (2014). Abiotic and biotic stress combinations. New Phytol 203, 32-43.
DOI PMID |
| [74] |
Szechyńska-Hebda M, Ghalami RZ, Kamran M, Van Breusegem F, Karpiński S (2022). To be or not to be? Are reactive oxygen species, antioxidants, and stress signaling universal determinants of life or death? Cells 11, 4105.
DOI URL |
| [75] |
Szechyńska-Hebda M, Kruk J, Górecka M, Karpińska B, Karpiński S (2010). Evidence for light wavelength-specific photoelectrophysiological signaling and memory of excess light episodes in Arabidopsis. Plant Cell 22, 2201-2218.
DOI URL |
| [76] |
Szechyńska-Hebda M, Lewandowska M, Karpiński S (2017). Electrical signaling, photosynthesis and systemic acquired acclimation. Front Physiol 8, 684.
DOI PMID |
| [77] |
Takahashi F, Kuromori T, Urano K, Yamaguchi-Shinozaki K, Shinozaki K (2020). Drought stress responses and resistance in plants: from cellular responses to long-distance intercellular communication. Front Plant Sci 11, 556972.
DOI URL |
| [78] | Todaka D, Takahashi F, Yamaguchi-Shinozaki K, Shinozaki K (2019). ABA-responsive gene expression in response to drought stress: cellular regulation and long- distance signaling. Adv Bot Res 92, 83-113. |
| [79] |
Toyota M, Spencer D, Sawai-Toyota S, Wang JQ, Zhang T, Koo AJ, Howe GA, Gilroy S (2018). Glutamate triggers long-distance, calcium-based plant defense signaling. Science 361, 1112-1115.
DOI PMID |
| [80] |
Tuteja N, Sopory SK (2008). Chemical signaling under abiotic stress environment in plants. Plant Signal Behav 3, 525-536.
DOI PMID |
| [81] |
Vincill ED, Bieck AM, Spalding EP (2012). Ca2+ conduction by an amino acid-gated ion channel related to glutamate receptors. Plant Physiol 159, 40-46.
DOI PMID |
| [82] |
Volkov AG (2019). Signaling in electrical networks of the Venus flytrap (Dionaea muscipula Ellis). Bioelectrochemistry 125, 25-32.
DOI URL |
| [83] |
Wilkinson S, Davies WJ (1997). Xylem sap pH increase: a drought signal received at the apoplastic face of the guard cell that involves the suppression of saturable abscisic acid uptake by the epidermal symplast. Plant Physiol 113, 559-573.
PMID |
| [84] |
Wilkinson S, Davies WJ (2002). ABA-based chemical signaling: the co-ordination of responses to stress in plants. Plant Cell Environ 25, 195-210.
DOI URL |
| [85] |
Xia XJ, Zhou YH, Shi K, Zhou J, Foyer CH, Yu JQ (2015). Interplay between reactive oxygen species and hormones in the control of plant development and stress tolerance. J Exp Bot 66, 2839-2856.
DOI URL |
| [86] |
Zandalinas SI, Mittler R (2018). ROS-induced ROS release in plant and animal cells. Free Radic Biol Med 122, 21-27.
DOI URL |
| [87] |
Zandalinas SI, Mittler R (2021). Vascular and nonvascular transmission of systemic reactive oxygen signals during wounding and heat stress. Plant Physiol 186, 1721-1733.
DOI PMID |
| [88] |
Zimmermann MR, Maischak H, Mithofer A, Boland W, Felle HH (2009). System potentials, a novel electrical long- distance apoplastic signal in plants, induced by wounding. Plant Physiol 149, 1593-1600.
DOI PMID |
| [89] |
Zinkevich NS, Gutterman DD (2011). ROS-induced ROS release in vascular biology: redox-redox signaling. Am J Physiol Heart Circ Physiol 301, H647-H653.
DOI URL |
| [1] | Xiong Lianglin, Liang Guolu, Guo Qigao, Jing Danlong. Advances in the Regulation of Alternative Splicing of Genes in Plants in Response to Abiotic Stress [J]. Chinese Bulletin of Botany, 2025, 60(3): 435-448. |
| [2] | Qingguo Du, Wenxue Li. Research Progress in the Regulation of Development and Stress Responses by Long Non-coding RNAs in Maize [J]. Chinese Bulletin of Botany, 2024, 59(6): 950-962. |
| [3] | Wenjie Zhou, Wenhan Zhang, Wei Jia, Zicheng Xu, Wuxing Huang. Advances in Plant miRNAs Responses to Abiotic Stresses [J]. Chinese Bulletin of Botany, 2024, 59(5): 810-833. |
| [4] | Zhaoxuan Zhong, Dongrui Zhang, Lu Li, Ying Su, Daining Wang, Zeran Wang, Yang Liu, Ying Chang. Bioinformatic and Expression Pattern Analysis of dfr-miR160a and Target Gene DfARF10 in Dryopteris fragrans [J]. Chinese Bulletin of Botany, 2024, 59(1): 22-33. |
| [5] | Yanan Xu, Jiarong Yan, Xin Sun, Xiaomei Wang, Yufeng Liu, Zhouping Sun, Mingfang Qi, Tianlai Li, Feng Wang. Red and Far-red Light Regulation of Plant Growth, Development, and Abiotic Stress Responses [J]. Chinese Bulletin of Botany, 2023, 58(4): 622-637. |
| [6] | Jia Zhang, Qidong Li, Cui Li, Qinghai Wang, Xincun Hou, Chunqiao Zhao, Shuhe Li, Qiang Guo. Research Progress on MATE Transporters in Plants [J]. Chinese Bulletin of Botany, 2023, 58(3): 461-474. |
| [7] | Xiaotong Ren, Ranran Zhang, Shaowei Wei, Xiaofeng Luo, Jiahui Xu, Kai Shu. Research Progress of Spermosphere Microorganisms [J]. Chinese Bulletin of Botany, 2023, 58(3): 499-509. |
| [8] | Dai Chen, Wang Jin, Lu Yaping. Determination of Acidic Plant Hormones by Derivative UPLC-MS [J]. Chinese Bulletin of Botany, 2022, 57(4): 500-507. |
| [9] | WU Lin-Sheng, ZHANG Yong-Guang, ZHANG Zhao-Ying, ZHANG Xiao-Kang, WU Yun-Fei. Remote sensing of solar-induced chlorophyll fluorescence and its applications in terrestrial ecosystem monitoring [J]. Chin J Plant Ecol, 2022, 46(10): 1167-1199. |
| [10] | Lingling Xie, Jinlong Wang, Guoqiang Wu. Regulatory Mechanisms of the Plant CBL-CIPK Signaling System in Response to Abiotic Stress [J]. Chinese Bulletin of Botany, 2021, 56(5): 614-626. |
| [11] | Fei Zhao, Liuyi Dang, Minhui Wei, Chunying Liu, Wei Leng, Chenjing Shang. Expression of Amaranthin-like Lectins Gene and Responses to Abiotic Stresses in Cucumber [J]. Chinese Bulletin of Botany, 2021, 56(2): 183-190. |
| [12] | Yingyan Xiao, Weina Yuan, Jing Liu, Jian Meng, Qiming Sheng, Yehuan Tan, Chunxiang Xu. Xyloglucan and the Advances in Its Roles in Plant Tolerance to Stresses [J]. Chinese Bulletin of Botany, 2020, 55(6): 777-787. |
| [13] | Lin Hong,Lei Yang,Haijian Yang,Wu Wang. Research Advances in AP2/ERF Transcription Factors in Regulating Plant Responses to Abiotic Stress [J]. Chinese Bulletin of Botany, 2020, 55(4): 481-496. |
| [14] | Menglong Wang,Xiaoqun Peng,Zhufeng Chen,Xiaoyan Tang. Research Advances on Lectin Receptor-like Kinases in Plants [J]. Chinese Bulletin of Botany, 2020, 55(1): 96-105. |
| [15] | Xun Zhang,Juanjuan Yu,Sizhu Wang,Ying Li,Shaojun Dai. Research Advances in DREPP Gene Family in Plants [J]. Chinese Bulletin of Botany, 2019, 54(5): 582-595. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||