

Chinese Bulletin of Botany ›› 2019, Vol. 54 ›› Issue (6): 711-722.DOI: 10.11983/CBB19042 cstr: 32102.14.CBB19042
• EXPERIMENTAL COMMUNICATIONS • Previous Articles Next Articles
Tong Zhang1,Yalu Guo1,2,Yue Chen1,Jinjiao Ma1,Jinping Lan1,3,Gaowei Yan1,Yuqing Liu1,Shan Xu1,Liyun Li1,Guozhen Liu1,*( ),Shijuan Dou1,*(
),Shijuan Dou1,*( )
)
Received:2019-02-27
Accepted:2019-05-06
Online:2019-11-01
Published:2020-07-09
Contact:
Guozhen Liu,Shijuan Dou
Tong Zhang,Yalu Guo,Yue Chen,Jinjiao Ma,Jinping Lan,Gaowei Yan,Yuqing Liu,Shan Xu,Liyun Li,Guozhen Liu,Shijuan Dou. Expression Characterization of Rice OsPR10A and Its Function in Response to Drought Stress[J]. Chinese Bulletin of Botany, 2019, 54(6): 711-722.
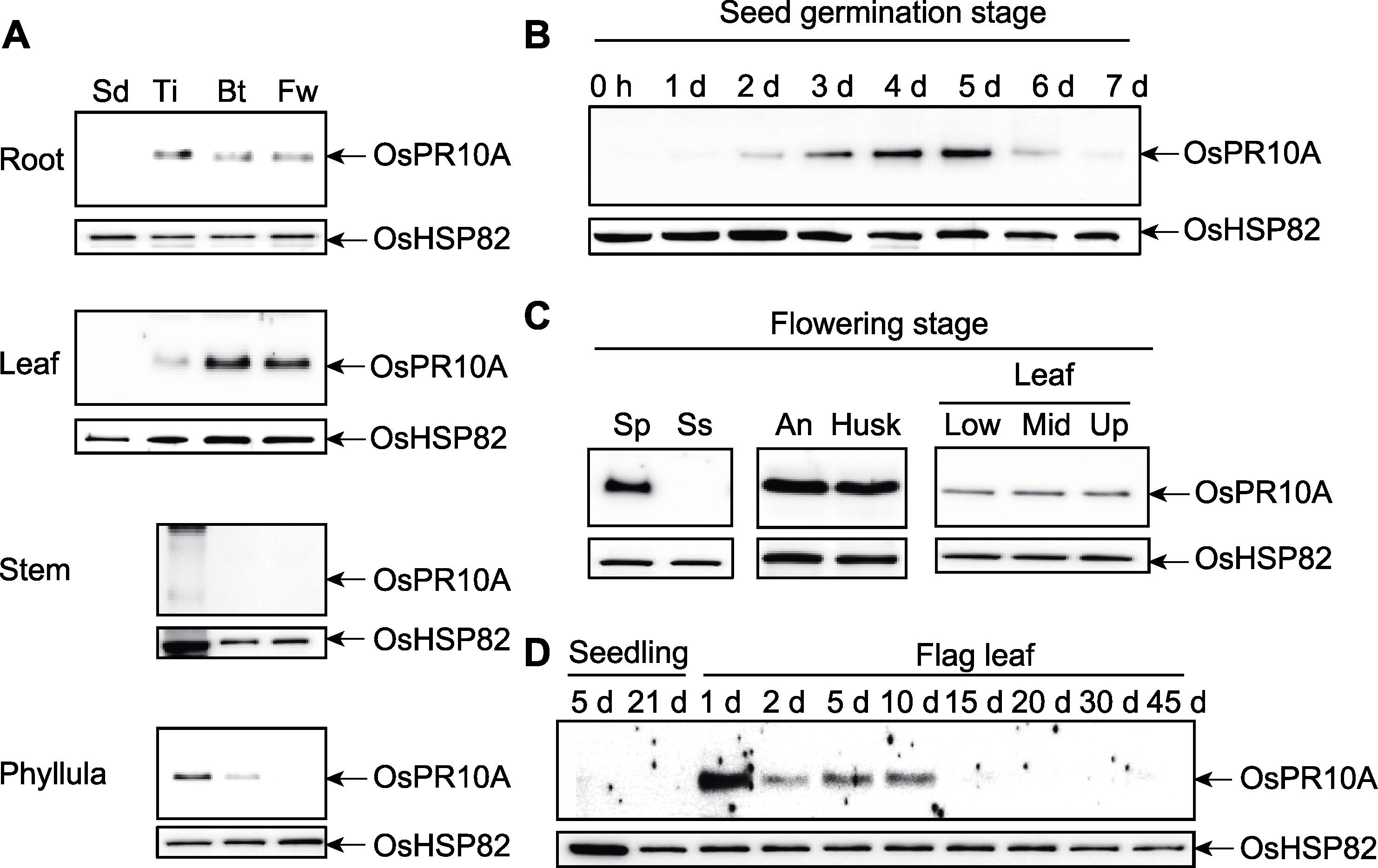
Figure 1 Expression of OsPR10A during rice growth and development (A) Expression of OsPR10A in different growth stages and tissue parts of rice (Sd: Seedling stage; Ti: Tillering stage; Bt: Booting stage; Fw: Flowering stage); (B) Expression of OsPR10A in seeds during germination; (C) Expression of OsPR10A in tissues during flowering stage (Sp: Spike; Ss: Spike-stalk; An: Anther; Husk: Husk; Low: Lower part of leaf; Mid: Middle part of leaf; Up: Upper part of leaf); (D) Expression of OsPR10A in seedling and flag leaf
| Library description | FPKM |
|---|---|
| Shoots | 50.1661 |
| Leaves-20 day | 144.7290 |
| Pre-emergence inflorescence | 4.1989 |
| Post-emergence inflorescence | 88.9886 |
| Anther | 0.5100 |
| Pistil | 2.3483 |
| Seed-5 DAP | 77.4876 |
| Seed-10 DAP | 0.0000 |
| Embryo-25 DAP | 1.5052 |
| Endosperm-25 DAP | 2.3371 |
Table 1 Transcriptome analysis of the OsPR10A gene
| Library description | FPKM |
|---|---|
| Shoots | 50.1661 |
| Leaves-20 day | 144.7290 |
| Pre-emergence inflorescence | 4.1989 |
| Post-emergence inflorescence | 88.9886 |
| Anther | 0.5100 |
| Pistil | 2.3483 |
| Seed-5 DAP | 77.4876 |
| Seed-10 DAP | 0.0000 |
| Embryo-25 DAP | 1.5052 |
| Endosperm-25 DAP | 2.3371 |
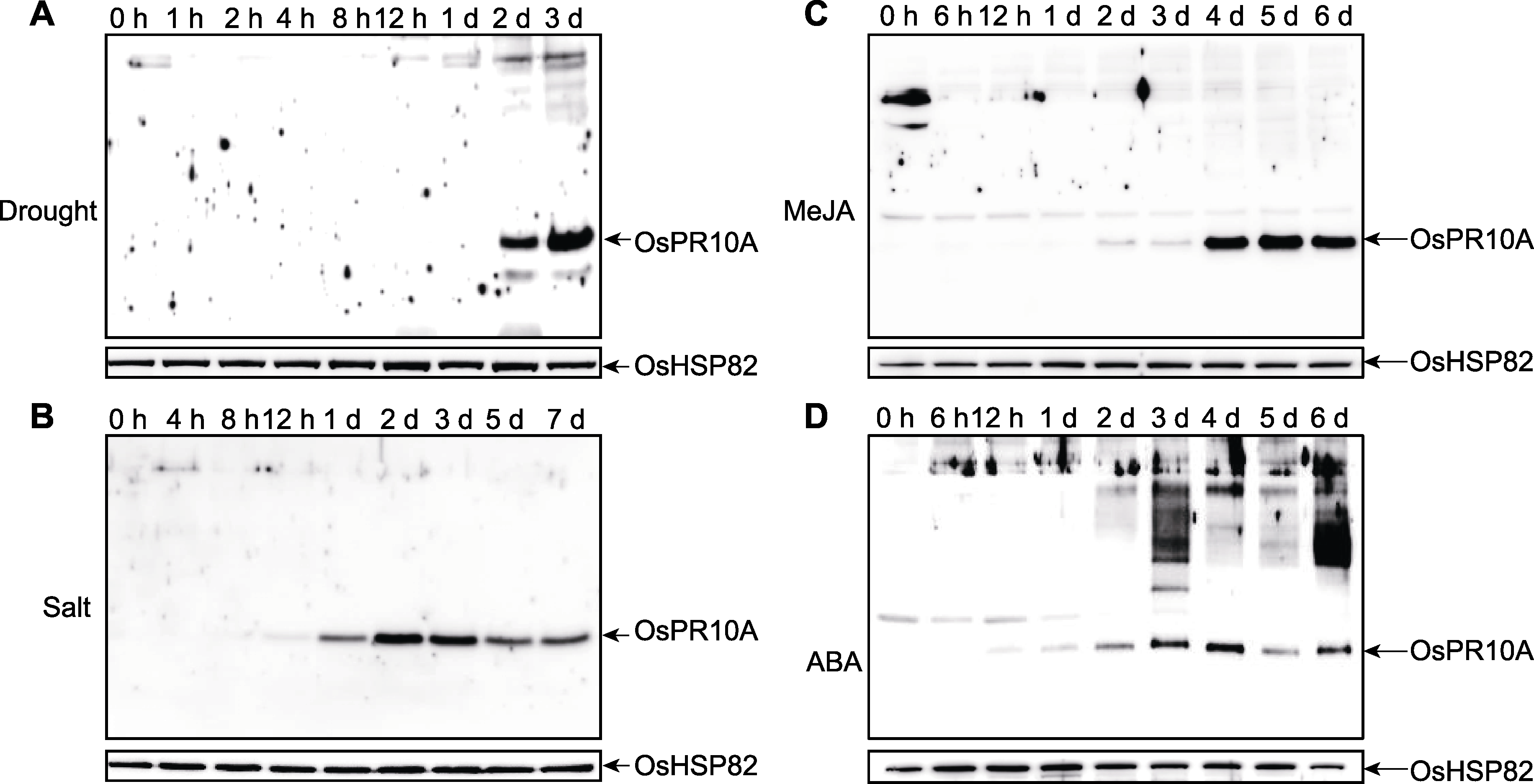
Figure 2 Dynamic expression of rice OsPR10A under abiotic stress (A) Expression of OsPR10A under drought stress; (B) Expression of OsPR10A under salt stress; (C) Expression of OsPR10A modulated by exogenous MeJA; (D) Expression of OsPR10A modulated by exogenous ABA. MeJA: Methyl jasmonate; ABA: Abscisic acid
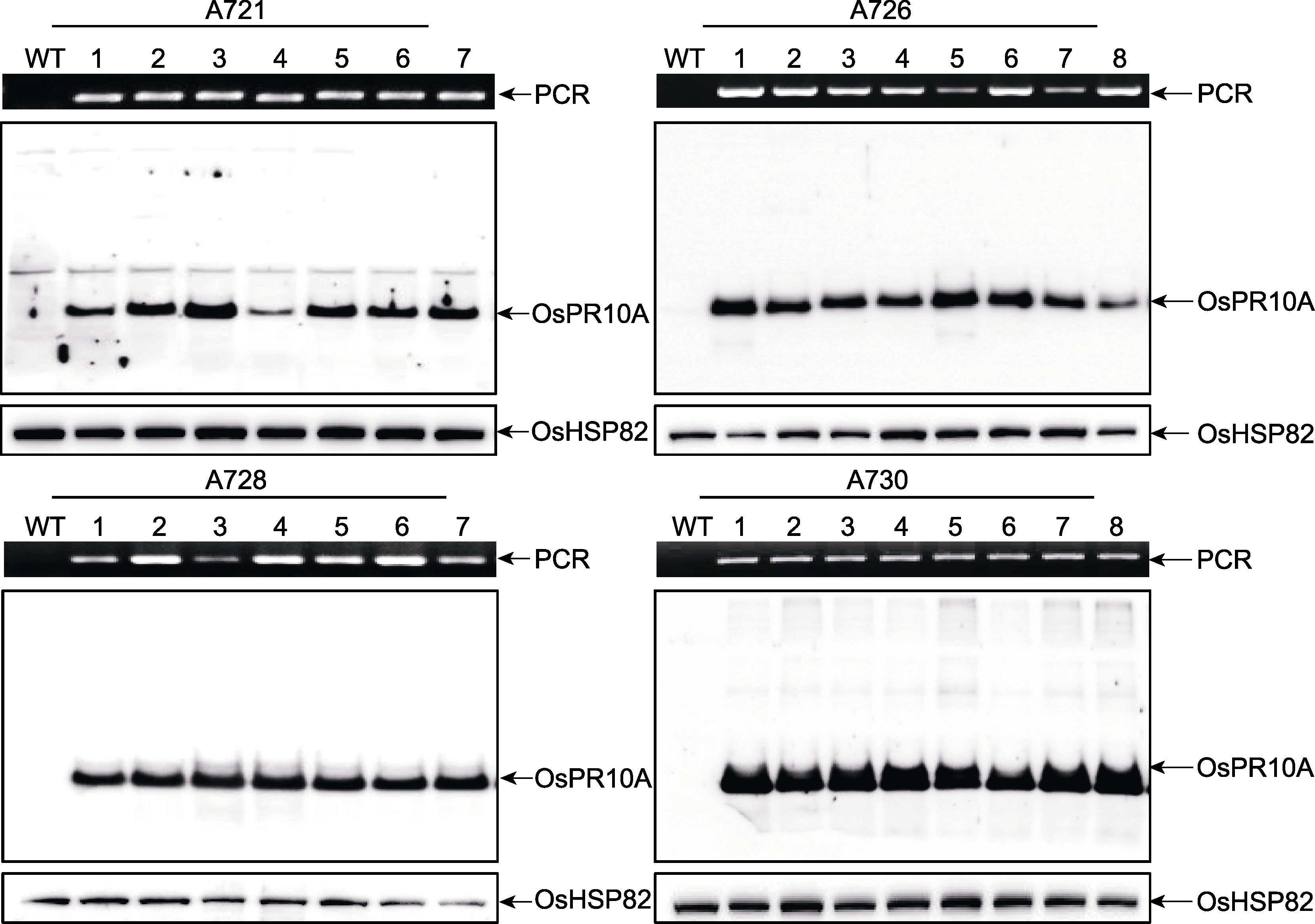
Figure 3 Identification of OsPR10A overexpression transgenic rice A721, A726, A728 and A730 represent different transgenic lines, respectively; Lane 1-7 or 1-8 represent different plants of the same transgenic line, respectively; WT: Wildtype
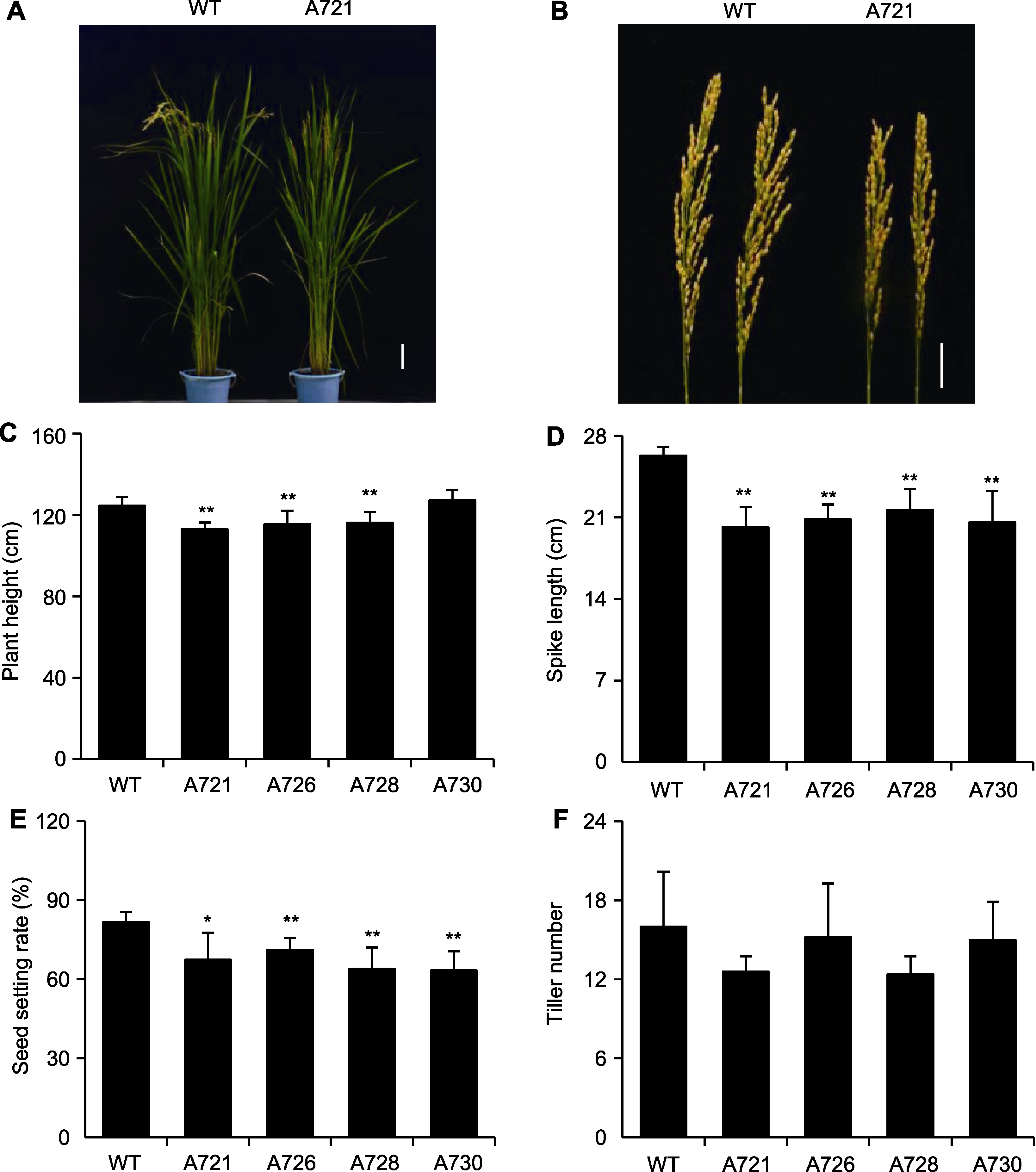
Figure 4 Phenotype of OsPR10A overexpression transgenic rice (A) Mature plant (Bar=10 cm); (B) Spike (Bar=4 cm); (C)-(F) Represent plant height, spike length, seed setting rate and tiller number, respectively. WT: Wildtype. * P<0.05; ** P<0.01
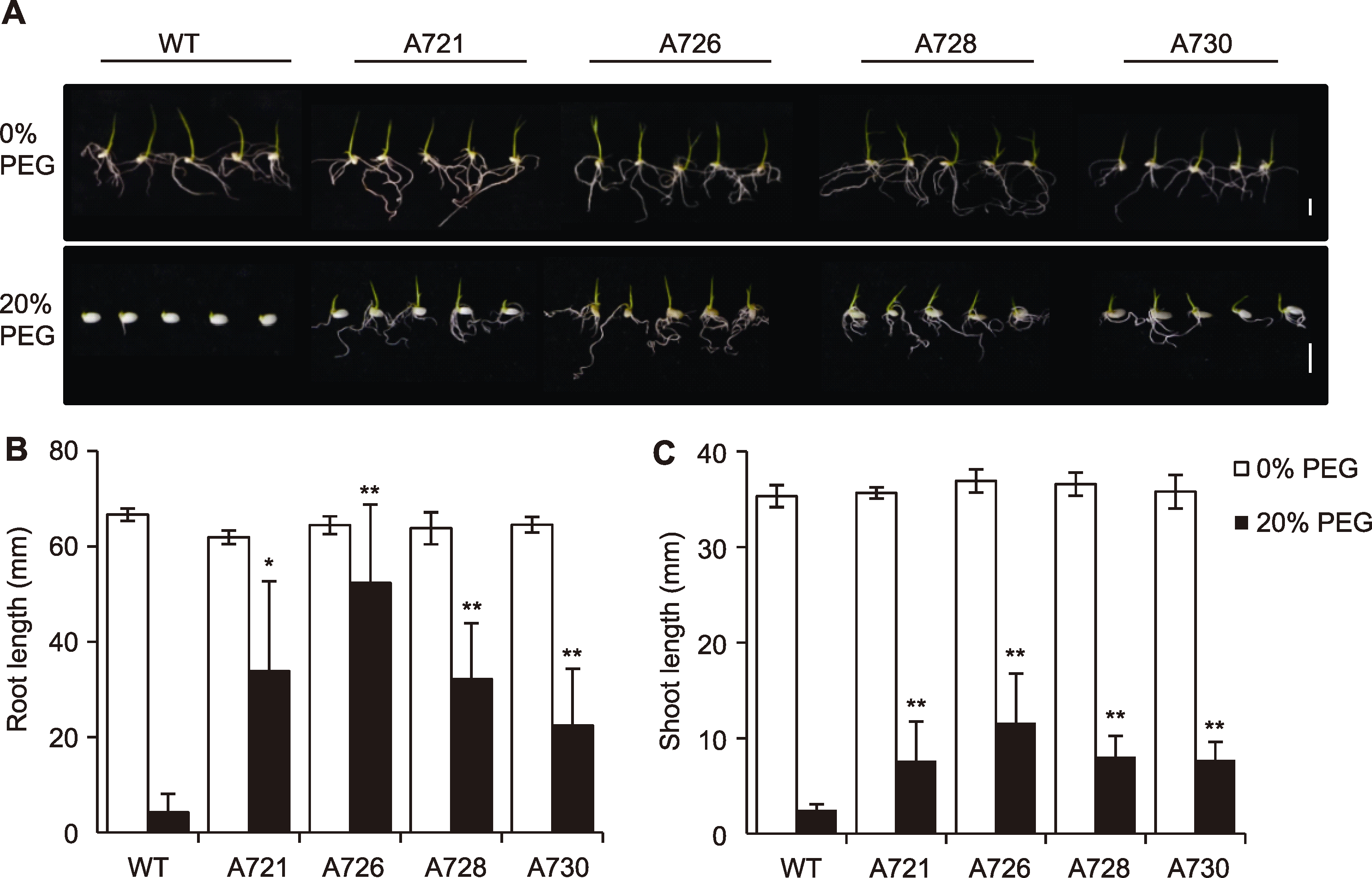
Figure 5 Overexpression of OsPR10A enhanced the drought tolerance at rice seed germination stage (A) The germinated rice seeds at 7 days treated with 20% PEG6000 (Bar=1 cm); (B) Root length; (C) Shoot length. WT: Wildtype. * P<0.05; ** P<0.01
| [1] | 白辉, 王宪云, 曹英豪, 李晓明, 李莉云, 陈浩, 刘丽娟, 朱健辉, 刘国振 ( 2010). 水稻叶绿体蛋白质在生长发育过程中的表达研究. 生物化学与生物物理进展 37, 988-995. |
| [2] | 窦世娟, 关明俐, 李莉云, 刘国振 ( 2014). 水稻的病程相关基因. 科学通报 59, 245-258. |
| [3] | 高庆华, 曾祥然, 贾霖, 牛东东, 李雪姣, 关明俐, 贾盟, 兰金苹, 窦世娟, 李莉云, 刘丽娟, 刘国振 ( 2013). 水稻病程相关蛋白质在逆境胁迫下的表达研究. 生物化学与生物物理进展 40, 1140-1147. |
| [4] | 李雪姣, 范伟, 牛东东, 关明俐, 缪刘杨, 史佳楠, 窦世娟, 魏健, 刘丽娟, 李莉云, 刘国振 ( 2014). 水稻病程相关PR1家族蛋白质在叶片生长及与白叶枯病菌互作反应中的表达. 植物学报 49, 127-138. |
| [5] | 刘国振, 刘斯奇, 吴琳, 徐宁志 ( 2011). 基于抗体的水稻蛋白质组学——开端与展望. 中国科学: 生命科学 41, 173-177. |
| [6] | 刘巧泉, 张景六, 王宗阳, 洪孟民, 顾铭洪 ( 1998). 根癌农杆菌介导的水稻高效转化系统的建立. 植物生理学报 24, 259-271. |
| [7] | 魏健, 李莉云, 曹英豪, 刘雨萌, 巩校东, 刘丽娟, 张园园, 刘国振 ( 2011). 水稻类Tubby蛋白质在叶片生长和白叶枯病抗性反应中的表达. 植物学报 46, 525-533. |
| [8] | 谢纯政, 刘海燕, 李玲, 梁炫强 ( 2008). 植物病程相关蛋白PR10研究进展. 分子植物育种 6, 949-953. |
| [9] | 张剑硕, 马金姣, 张彤, 陈悦, 魏健, 张柳, 史佳楠, 徐珊, 燕高伟, 杜铁民, 窦世娟, 李莉云, 刘丽娟, 刘国振 ( 2018). 水稻蛋白质样品资源库RiceS-A300的建立与应用. 中国农业科学 51, 3625-3638. |
| [10] | Agrawal GK, Jwa NS, Rakwal R ( 2000a). A novel rice (Oryza sativa L.) acidic PR1 gene highly responsive to cut, phytohormones, and protein phosphatase inhibitors. Biochem Biophys Res Commun 274, 157-165. |
| [11] | Agrawal GK, Rakwal R, Jwa NS ( 2000b). Rice (Oryza sativa L.) OsPR1b gene is phytohormonally regulated in close interaction with light signals. Biochem Biophys Res Commun 278, 290-298. |
| [12] | Ausubel FM ( 2005). Are innate immune signaling pathways in plants and animals conserved? Nat Immunol 6, 973-979. |
| [13] | Banerjee J, Das N, Dey P, Maiti MK ( 2010). Transgenically expressed rice germin-like protein 1 in tobacco causes hyper-accumulation of H2O2 and reinforcement of the cell wall components. Biochem Biophys Res Commun 402, 637-643. |
| [14] | Cai SL, Jiang GB, Ye NH, Chu ZZ, Xu XZ, Zhang JH, Zhu GH ( 2015). A key ABA catabolic gene, OsABA8ox3 , is involved in drought stress resistance in rice. PLoS One 10, e0116646. |
| [15] | Choi C, Hwang SH, Fang IR, Kwon SI, Park SR, Ahn I, Kim JB, Hwang DJ ( 2015). Molecular characterization of Oryza sativa WRKY6, which binds to W-box-like element 1 of the Oryza sativa pathogenesis-related (PR) 10a promoter and confers reduced susceptibility to pathogens. New Phytol 208, 846-859. |
| [16] | Dansana PK, Kothari KS, Vij S, Tyagi AK ( 2014). OsiSAP1 overexpression improves water-deficit stress tolerance in transgenic rice by affecting expression of endogenous stress-related genes. Plant Cell Rep 33, 1425-1440. |
| [17] | Datta K, Tu JM, Oliva N, Ona I, Velazhahan R, Mew TW, Muthukrishnan S, Datta SK ( 2001). Enhanced resistance to sheath blight by constitutive expression of infection-related rice chitinase in transgenic elite indica rice cultivars. Plant Sci 160, 405-414. |
| [18] | Duan YB, Zhai CG, Li H, Li J, Mei WQ, Gui HP, Ni DH, Song FS, Li L, Zhang WG, Yang JB ( 2012). An efficient and high-throughput protocol for Agrobacterium-mediated transformation based on phosphomannose isomerase positive selection in Japonica rice(Oryza sativa L.). Plant Cell Rep 31, 1611-1624. |
| [19] | Fu J, Wu H, Ma SQ, Xiang DH, Liu RY, Xiong LZ ( 2017). OsJAZ1 attenuates drought resistance by regulating JA and ABA signaling in rice. Front Plant Sci 8, 2108. |
| [20] | Hamamouch N, Li CY, Seo PJ, Park CM, Davis EL ( 2011). Expression of Arabidopsis pathogenesis-related genes during nematode infection. Mol Plant Pathol 12, 355-364. |
| [21] | Hashimoto M, Kisseleva L, Sawa S, Furukawa T, Komatsu S, Koshiba T ( 2004). A novel rice PR10 protein, RSOsPR10, specifically induced in roots by biotic and abiotic stresses, possibly via the jasmonic acid signaling pathway. Plant Cell Physiol 45, 550-559. |
| [22] | Huang LF, Lin KH, He SL, Chen JL, Jiang JZ, Chen BH, Hou YS, Chen RS, Hong CY, Ho SL ( 2016). Multiple patterns of regulation and overexpression of a ribonuclease-like pathogenesis-related protein gene,OsPR10a, conferring disease resistance in rice and Arabidopsis. PLoS One 11, e0156414. |
| [23] | Hwang SH, Lee IA, Yie SW, Hwang DJ ( 2008). Identification of an OsPR10a promoter region responsive to salicylic acid. Planta 227, 1141-1150. |
| [24] | Kim SG, Kim ST, Wang YM, Yu S, Choi IS, Kim YC, Kim WT, Agrawal GK, Rakwal R, Kang KY ( 2011). The RNase activity of rice probenazole-induced protein1 (PBZ1) plays a key role in cell death in plants. Mol Cells 31, 25-31. |
| [25] | Kim ST, Yu S, Kang YH, Kim SG, Kim JY, Kim SH, Kang KY ( 2008). The rice pathogen-related protein 10 (JIOsPR10) is induced by abiotic and biotic stresses and exhibits ribonuclease activity. Plant Cell Rep 27, 593-603. |
| [26] | Li GW, Xie XS ( 2011). Central dogma at the single-molecule level in living cells. Nature 475, 308-315. |
| [27] | Li JJ, Li Y, Yin ZG, Jiang JH, Zhang MH, Guo X, Ye ZJ, Zhao Y, Xiong HY, Zhang ZY, Shao YJ, Jiang CH, Zhang HL, An G, Paek NC, Ali J, Li ZC ( 2017). OsASR5 enhances drought tolerance through a stomatal closure pathway associated with ABA and H2O2 signaling in rice. Plant Biotechnol J 15, 183-196. |
| [28] | Li XM, Bai H, Wang XY, Li LY, Cao YH, Wei J, Liu YM, Liu LJ, Gong XD, Wu L, Liu SQ, Liu GZ ( 2011). Identification and validation of rice reference proteins for western blotting. J Exp Bot 62, 4763-4772. |
| [29] | Liu GZ, Pi LY, Walker JC, Ronald PC, Song WY ( 2002). Biochemical characterization of the kinase domain of the rice disease resistance receptor-like kinase XA21. J Biol Chem 277, 20264-20269. |
| [30] | Liu Q, Li X, Yan SJ, Yu T, Yang JY, Dong JF, Zhang SH, Zhao JL, Yang TF, Mao XX, Zhu XY, Liu B ( 2018). OsWRKY67 positively regulates blast and bacteria blight resistance by direct activation of PR genes in rice. BMC Plant Biol 18, 257. |
| [31] | Luan ZH, Zhou DW ( 2015). Screening of rice ( Oryza sativa L.) OsPR1b-interacting factors and their roles in resisting bacterial blight. Genet Mol Res 14, 1868-1874. |
| [32] | McGee JD, Hamer JE, Hodges TK ( 2001). Characterization of a PR-10 pathogenesis-related gene family induced in rice during infection with Magnaporthe grisea. Mol Plant Microbe Interact 14, 877-886. |
| [33] | Nishizawa Y, Saruta M, Nakazono K, Nishio Z, Soma M, Yoshida T, Nakajima E, Hibi T ( 2003). Characterization of transgenic rice plants over-expressing the stress-inducible β-glucanase gene Gns1. Plant Mol Biol 51, 143-152. |
| [34] | Nürnberger T, Brunner F, Kemmerling B, Piater L ( 2004). Innate immunity in plants and animals: striking similarities and obvious differences. Immunol Rev 198, 249-266. |
| [35] | Pandey A, Mann M ( 2000). Proteomics to study genes and genomes. Nature 405, 837-846. |
| [36] | Ponciano G, Yoshikawa M, Lee JL, Ronald PC, Whalen MC ( 2006). Pathogenesis-related gene expression in rice is correlated with developmentally controlled Xa21-mediated resistance against Xanthomonas oryzae pv. Oryzae. Physiol Mol Plant Pathol 69, 131-139. |
| [37] | Seo PJ, Lee AK, Xiang FN, Park CM ( 2008). Molecular and functional profiling of Arabidopsis Pathogenesis-related genes: insights into their roles in salt response of seed germination. Plant Cell Physiol 49, 334-344. |
| [38] | Takeuchi K, Gyohda A, Tominaga M, Kawakatsu M, Hatakeyama A, Ishii N, Shimaya K, Nishimura T, Riemann M, Nick P, Hashimoto M, Komano T, Endo A, Okamoto T, Jikumaru Y, Kamiya Y, Terakawa T, Koshiba T ( 2011). RSOsPR10 expression in response to environmental stresses is regulated antagonistically by jasmonate/ethylene and salicylic acid signaling pathways in rice roots. Plant Cell Physiol 52, 1686-1696. |
| [39] | Takeuchi K, Hasegawa H, Gyohda A, Komatsu S, Okamoto T, Okada K, Terakawa T, Koshiba T ( 2016). Overexpression of RSOsPR10, a root-specific rice PR10 gene, confers tolerance against drought stress in rice and drought and salt stresses in bentgrass. Plant Cell Tissue Organ Cult 127, 35-46. |
| [40] | Thakur P, Kumar S, Malik JA, Berger JD, Nayyar H ( 2010). Cold stress effects on reproductive development in grain crops: an overview. Environ Exp Bot 67, 429-443. |
| [41] | Uhlén M, Björling E, Agaton C, Szigyarto CAK, Amini B, Andersen E, Andersson AC, Angelidou P, Asplund A, Asplund C, Berglund L, Bergström K, Brumer H, Cerjan D, Ekström M, Elobeid A, Eriksson C, Fagerberg L, Falk R, Fall J, Forsberg M, Björklund MG, Gumbel K, Halimi A, Hallin I, Hamsten C, Hansson M, Hedhammar M, Hercules G, Kampf C, Larsson K, Lindskog M, Lodewyckx W, Lund J, Lundeberg J, Magnusson K, Malm E, Nilsson P, Ödling J, Oksvold P, Olsson I, Öster E, Ottosson J, Paavilainen L, Persson A, Rimini R, Rockberg J, Runeson M, Sivertsson Å, Sköllermo A, Steen J, Stenvall M, Sterky F, Strömberg S, Sundberg M, Tegel H, Tourle S, Wahlund E, Waldén A, Wan JH, Wernérus H, Westberg J, Wester K, Wrethagen U, Xu LL, Hober S, Pontén F ( 2005). A human protein atlas for normal and cancer tissues based on antibody proteomics. Mol Cell Proteomics 4, 1920-1932. |
| [42] | Wang NL, Xiao BZ, Xiong LZ ( 2011). Identification of a cluster of PR4-like genes involved in stress responses in rice. J Plant Physiol 168, 2212-2224. |
| [43] | Wu JN, Kim SG, Kang KY, Kim JG, Park SR, Gupta R, Kim YH, Wang YM, Kim ST ( 2016). Overexpression of a pathogenesis-related protein 10 enhances biotic and abiotic stress tolerance in rice. Plant Pathol J 32, 552-562. |
| [44] | Wu Q, Hou MM, Li LY, Liu LJ, Hou YX, Liu GZ ( 2011). Induction of pathogenesis-related proteins in rice bacterial blight resistant gene XA21-mediated interactions with Xanthomonas oryzae pv. oryzae. J Plant Pathol 93, 455-459. |
| [45] | Zong W, Tang N, Yang J, Peng L, Ma SQ, Xu Y, Li GL, Xiong LZ ( 2016). Feedback regulation of ABA signaling and biosynthesis by a bZIP transcription factor targets drought- resistance-related genes. Plant Physiol 171, 2810-2825. |
| [1] |
Juan Cui, Xiaoyu Yu, Yuejiao Yu, Chengwei Liang, Jian Sun, Wenfu Chen.
Analysis of Texture Factors and Genetic Basis Influencing the Differences in Eating Quality between Northeast China and Japanese Japonica Rice [J]. Chinese Bulletin of Botany, 2025, 60(4): 1-0. |
| [2] | Zhao Ling, Guan Ju, Liang Wenhua, Zhang Yong, Lu Kai, Zhao Chunfang, Li Yusheng, Zhang Yadong. Mapping of QTLs for Heat Tolerance at the Seedling Stage in Rice Based on a High-density Bin Map [J]. Chinese Bulletin of Botany, 2025, 60(3): 342-353. |
| [3] | Xinyu Li, Yue Gu, Feifei Xu, Jinsong Bao. Research Progress on Post-translational Modifications of Starch Biosynthesis-related Proteins in Rice Endosperm [J]. Chinese Bulletin of Botany, 2025, 60(2): 256-270. |
| [4] | Bei Fan, Min Ren, Yanfeng Wang, Fengfeng Dang, Guoliang Chen, Guoting Cheng, Jinyu Yang, Huiru Sun. Functions of SlWRKY45 in Response to Low-temperature and Drought Stress in Tomato [J]. Chinese Bulletin of Botany, 2025, 60(2): 186-203. |
| [5] | Jianguo Li, Yi Zhang, Wenjun Zhang. Iron Plaque Formation and Its Effects on Phosphorus Absorption in Rice Roots [J]. Chinese Bulletin of Botany, 2025, 60(1): 132-143. |
| [6] | LONG Ji-Lan, JIANG Zheng, LIU Ding-Qin, MIAO Yu-Xuan, ZHOU Ling-Yan, FENG Ying, PEI Jia-Ning, LIU Rui-Qiang, ZHOU Xu-Hui, FU Yu-Ling. Effects of drought on plant root exudates and associated rhizosphere priming effect: review and prospect [J]. Chin J Plant Ecol, 2024, 48(7): 817-827. |
| [7] | Ruifeng Yao, Daoxin Xie. Activation and Termination of Strigolactone Signal Perception in Rice [J]. Chinese Bulletin of Botany, 2024, 59(6): 873-877. |
| [8] | Jinjin Lian, Luyao Tang, Yinuo Zhang, Jiaxing Zheng, Chaoyu Zhu, Yuhan Ye, Yuexing Wang, Wennan Shang, Zhenghao Fu, Xinxuan Xu, Richeng Wu, Mei Lu, Changchun Wang, Yuchun Rao. Genetic Locus Mining and Candidate Gene Analysis of Antioxidant Traits in Rice [J]. Chinese Bulletin of Botany, 2024, 59(5): 738-751. |
| [9] | Laipeng Zhao, Baike Wang, Tao Yang, Ning Li, Haitao Yang, Juan Wang, Huizhuan Yan. Investigation of the Regulation of Drought Tolerance by the SlHVA22l Gene in Tomato [J]. Chinese Bulletin of Botany, 2024, 59(4): 558-573. |
| [10] | Jiahui Huang, Huimin Yang, Xinyu Chen, Chaoyu Zhu, Yanan Jiang, Chengxiang Hu, Jinjin Lian, Tao Lu, Mei Lu, Weilin Zhang, Yuchun Rao. Response Mechanism of Rice Mutant pe-1 to Low Light Stress [J]. Chinese Bulletin of Botany, 2024, 59(4): 574-584. |
| [11] | Jianmin Zhou. A Combat Vehicle with a Smart Brake [J]. Chinese Bulletin of Botany, 2024, 59(3): 343-346. |
| [12] | Chaoyu Zhu, Chengxiang Hu, Zhenan Zhu, Zhining Zhang, Lihai Wang, Jun Chen, Sanfeng Li, Jinjin Lian, Luyao Tang, Qianqian Zhong, Wenjing Yin, Yuexing Wang, Yuchun Rao. Mapping of QTLs Associated with Rice Panicle Traits and Candidate Gene Analysis [J]. Chinese Bulletin of Botany, 2024, 59(2): 217-230. |
| [13] | Yanli Fang, Chuanyu Tian, Ruyi Su, Yapei Liu, Chunlian Wang, Xifeng Chen, Wei Guo, Zhiyuan Ji. Mining and Preliminary Mapping of Rice Resistance Genes Against Bacterial Leaf Streak [J]. Chinese Bulletin of Botany, 2024, 59(1): 1-9. |
| [14] | Bao Zhu, Jiangzhe Zhao, Kewei Zhang, Peng Huang. OsCKX9 is Involved in Regulating the Rice Lamina Joint Development and Leaf Angle [J]. Chinese Bulletin of Botany, 2024, 59(1): 10-21. |
| [15] | Zhang Yingchuan, Wu Xiaomingyu, Tao Baolong, Chen Li, Lu Haiqin, Zhao Lun, Wen Jing, Yi Bin, Tu Jinxing, Fu Tingdong, Shen Jinxiong. Bna-miR43 Mediates the Response of Drought Tolerance in Brassica napus [J]. Chinese Bulletin of Botany, 2023, 58(5): 701-711. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||