

Chinese Bulletin of Botany ›› 2018, Vol. 53 ›› Issue (6): 764-772.DOI: 10.11983/CBB17198 cstr: 32102.14.CBB17198
• EXPERIMENTAL COMMUNICATIONS • Previous Articles Next Articles
Liu Ming1, Liu Xia1, Sun Ran1,2, Li Yuling1, Du Kejiu1,2,*( )
)
Received:2017-10-27
Online:2018-11-01
Published:2018-12-05
Contact:
Du Kejiu
Liu Ming, Liu Xia, Sun Ran, Li Yuling, Du Kejiu. Polychlorinated Biphenyls Promotes Differentiation on Adventitious Roots of Populous tomentosa[J]. Chinese Bulletin of Botany, 2018, 53(6): 764-772.
| Treatments | The time of an initial root (d) | Period reaching 100% of adventitious roots differentiation rate (d) |
|---|---|---|
| CK- | 7 | 13 |
| CK+ | 5 | 9 |
| Aroclor1254 | 5 | 10 |
Table 1 Effect of different treatments on the differentiation of adventitious roots of Populous tomentosa seedlings
| Treatments | The time of an initial root (d) | Period reaching 100% of adventitious roots differentiation rate (d) |
|---|---|---|
| CK- | 7 | 13 |
| CK+ | 5 | 9 |
| Aroclor1254 | 5 | 10 |
| Treatments | IAA (ng·g-1 FW) | ZR (ng·g-1 FW) | dhZR (ng·g-1 FW) | IAA/(ZR+dhZR) |
|---|---|---|---|---|
| 0 d | 62.078±2.105a | 6.657±0.099a | 2.997±0.214a | 6.431±0.218a |
| 4 d CK- | 88.393±0.601b | 12.248±0.444b | 4.067±0.135b | 5.418±0.037c |
| 4 d CK+ | 102.893±2.517d | 12.283±0.479b | 5.040±0.280c | 5.940±0.145b |
| 4 d Aroclor1254 | 93.052±2.617c | 11.916±0.443b | 3.736±0.119b | 5.945±0.167b |
Table 2 Changes of phytohormonal contents in sections of stems of Populous tomentosa seedlings under different treatments
| Treatments | IAA (ng·g-1 FW) | ZR (ng·g-1 FW) | dhZR (ng·g-1 FW) | IAA/(ZR+dhZR) |
|---|---|---|---|---|
| 0 d | 62.078±2.105a | 6.657±0.099a | 2.997±0.214a | 6.431±0.218a |
| 4 d CK- | 88.393±0.601b | 12.248±0.444b | 4.067±0.135b | 5.418±0.037c |
| 4 d CK+ | 102.893±2.517d | 12.283±0.479b | 5.040±0.280c | 5.940±0.145b |
| 4 d Aroclor1254 | 93.052±2.617c | 11.916±0.443b | 3.736±0.119b | 5.945±0.167b |
| Treatments | P005g2489 | P005g2376 | P002g222700 | P006g142600 | P009g125900 |
|---|---|---|---|---|---|
| 0 d | 1.000±0.000a | 1.000±0.000a | 1.000±0.000a | 1.000±0.000a | 1.000±0.000a |
| CK- | 3.012±0.520b | 3.778±0.133c | 9.153±0.381c | 2.958±0.257c | 1.985±0.131b |
| CK+ | 1.299±0.201a | 1.010±0.045a | 3.444±0.517b | 2.240±0.496b | 4.298±0.091c |
| Aroclor1254 | 1.451±0.298a | 2.039±0.188b | 2.804±0.518b | 2.022±0.463b | 20.165±0.614d |
Table 3 Changes of the expression of genes related to auxin and cytokinin in sections of stems of Populous tomentosa seedlings under different treatments
| Treatments | P005g2489 | P005g2376 | P002g222700 | P006g142600 | P009g125900 |
|---|---|---|---|---|---|
| 0 d | 1.000±0.000a | 1.000±0.000a | 1.000±0.000a | 1.000±0.000a | 1.000±0.000a |
| CK- | 3.012±0.520b | 3.778±0.133c | 9.153±0.381c | 2.958±0.257c | 1.985±0.131b |
| CK+ | 1.299±0.201a | 1.010±0.045a | 3.444±0.517b | 2.240±0.496b | 4.298±0.091c |
| Aroclor1254 | 1.451±0.298a | 2.039±0.188b | 2.804±0.518b | 2.022±0.463b | 20.165±0.614d |
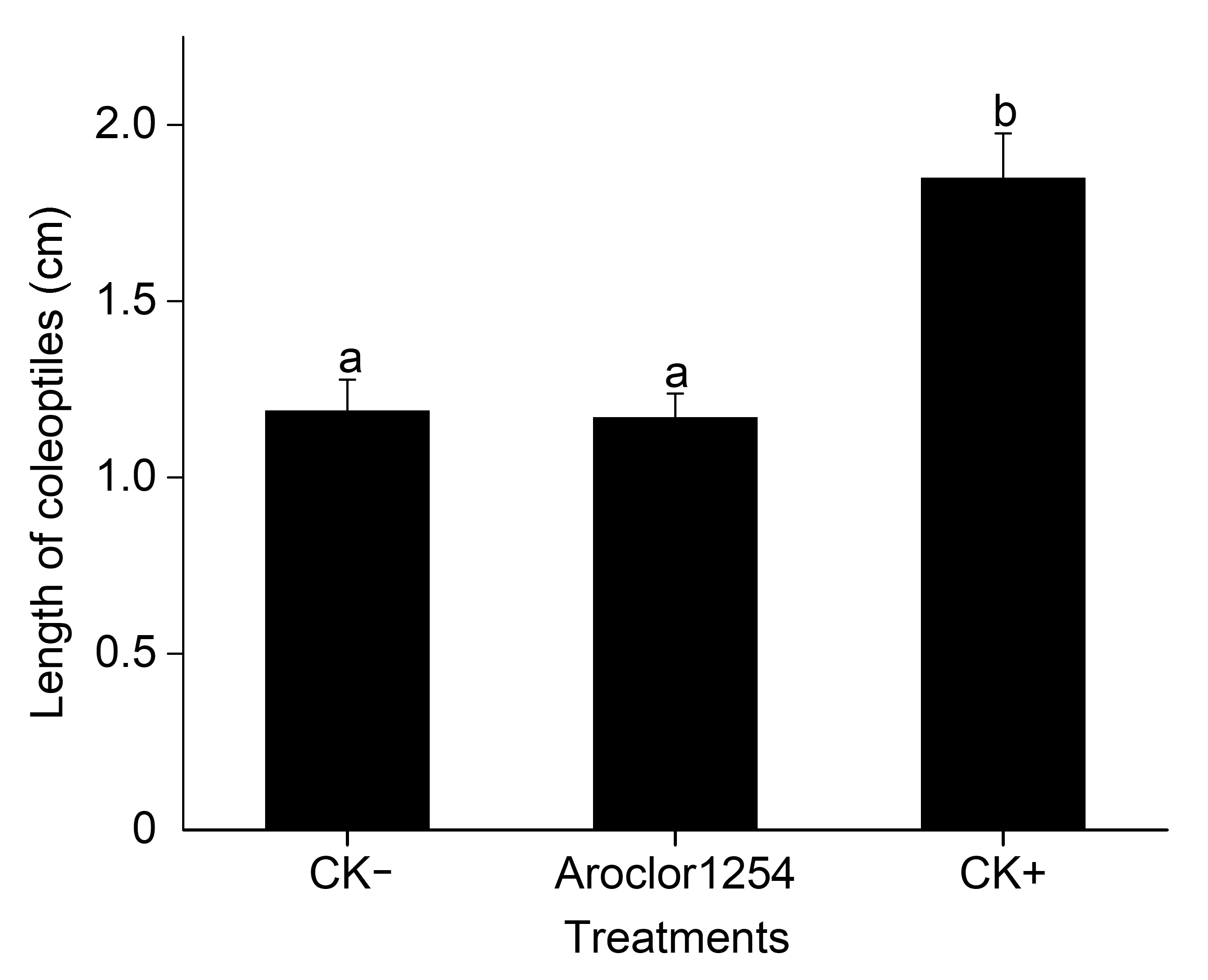
Figure 2 Effect of different treatments on the growth of coleoptiles in Zea mays Different lowercase letters indicate significant differences among different treatments (P<0.05).
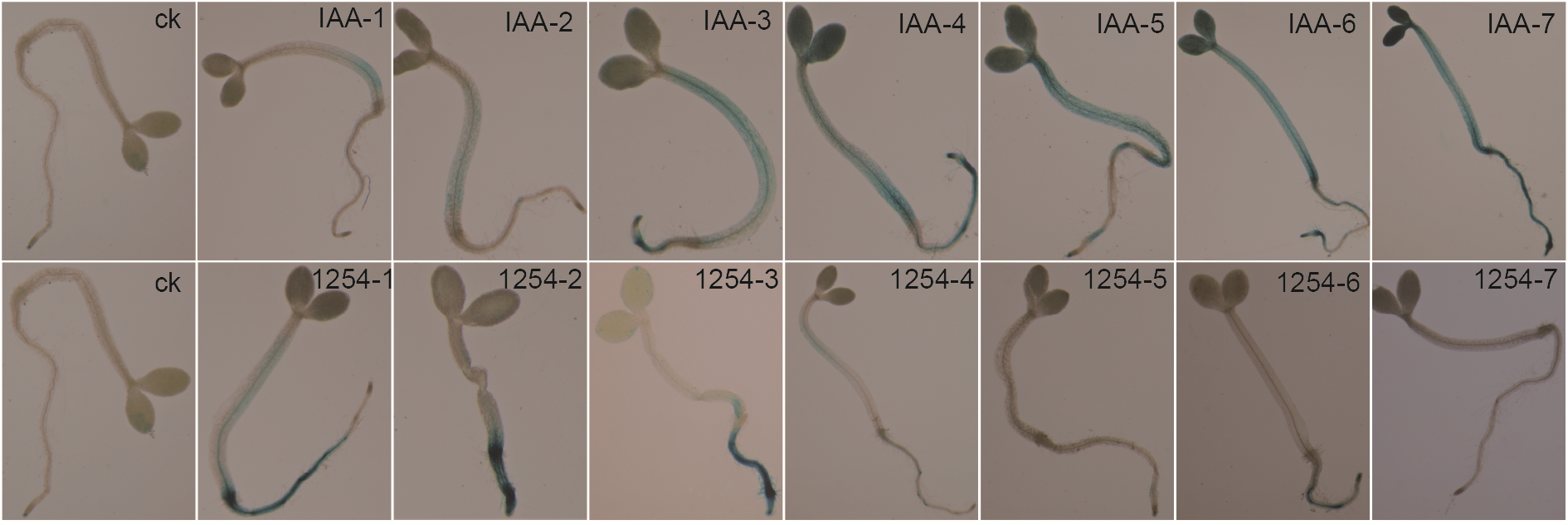
Figure 3 Effect of different concentration of Aroclor1254 on DR5::GUS genes response of Arabidopsis seedlings ck: Negative control; 1-7 indicate the working concentration of 10, 20, 40, 60, 80, 100 and 2 000 μg·L-1, respectively.
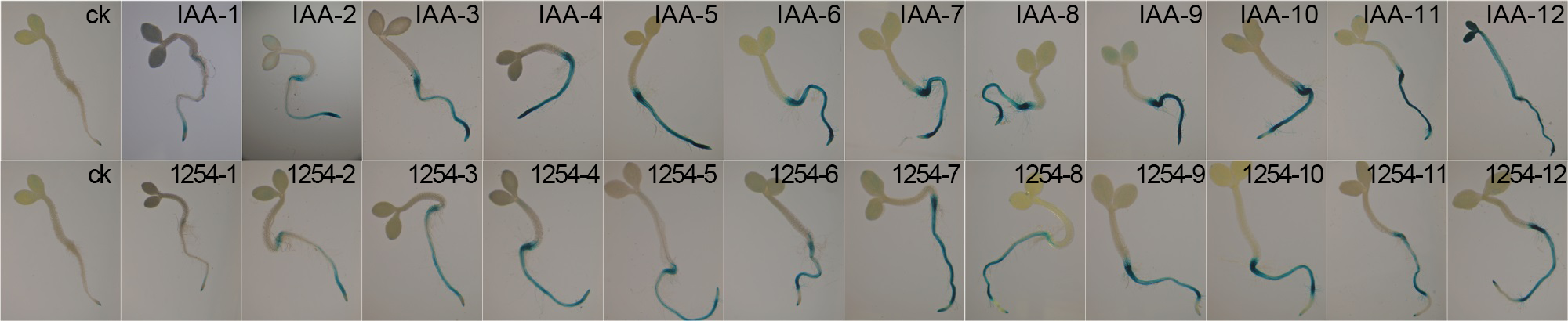
Figure 4 Effect of different induction time on DR5::GUS gene response of Arabidopsis seedlings ck: Negative control; 1-12 indicate the induction time of 2, 4, 6, 8, 10, 12, 14, 16, 18, 20, 22 and 24 h, respectively.
| [1] | 蔡卓平 (2009). 有机磷农药对海洋微藻的毒物兴奋效应及其机理研究. 博士论文. 广州: 暨南大学. pp. 59-98. |
| [2] | 丁娜 (2012). 多氯联苯在毫米级根际微域中的消减行为及生物响应机制研究. 博士论文. 杭州: 浙江大学. pp. 64-85. |
| [3] | 何佳 (2007). 多氯联苯(PCBs)对模式植物拟南芥的毒效应机制研究. 硕士论文. 杭州: 浙江大学. pp. 14-19. |
| [4] | 李雪梅, 刘熔山 (1994). 小麦幼穗胚性愈伤组织诱导及分化过程中内源激素的作用. 植物生理学报 30, 255-260. |
| [5] | 李叶, 张爽, 李玉灵, 杜克久 (2016). 多氯联苯暴露对绦柳初生根及显微结构的影响. 北方园艺 (24), 55-60. |
| [6] | 梁继仁 (2012). SD大鼠新生期联合暴露苯并芘和3, 3', 4, 4', 5, 5'-六氯联苯对睾丸抗氧化酶和精子形成的影响. 硕士论文. 上海: 复旦大学. pp. 7-44. |
| [7] | 刘亚云, 孙红斌, 陈桂珠 (2007a). 多氯联苯对桐花树幼苗生长及膜保护酶系统的影响. 应用生态学报 18, 123-128. |
| [8] |
刘亚云, 孙红斌, 陈桂珠, 赵波, 李伟煜 (2007b). 秋茄(Kan- delia candel)幼苗对多氯联苯污染的生理生态响应. 生态学报 27, 746-754.
DOI URL |
| [9] | 史树德, 孙亚卿, 魏磊 (2011). 植物生理学实验指导. 北京: 中国林业出版社. pp. 102-107. |
| [10] |
孙然, 池翠兰, 李燕玲, 杜克久 (2015). 苯并[a]芘暴露对绦柳生长发育的影响. 河北林果研究 30, 126-128.
DOI URL |
| [11] |
王传飞, 龚平, 王小萍, 姚檀栋 (2016). 西藏农田土和农作物中多氯联苯的分布、环境行为和健康风险评估. 生态毒理学报 11, 339-346.
DOI URL |
| [12] | 王亚红, 赵燕, 彭彦, 刘晓柱, 张学文 (2012). 5′UTR序列对DR5::GUS基因瞬时表达的影响. 湖南农业大学学报(自然科学版) 38, 146-149. |
| [13] | 王忠 (2009). 植物生理学(第2版). 北京: 中国农业出版社. pp. 315-329. |
| [14] | 王子岚 (2016). 多氯联苯的类植物生长素生物学效应研究. 硕士论文. 保定: 河北农业大学. pp. 36. |
| [15] |
曾凡锁, 钱晶晶, 康君, 王红艳, 王亦洲, 詹亚光 (2009). 转基因白桦中GUS基因表达的定量分析. 植物学报 44, 484-490.
DOI URL |
| [16] |
张晓丹, 才满, 张爽, 杜克久 (2017). 4-BDE胁迫对毛白杨组培苗不定根发生的影响. 环境化学 36, 514-520.
DOI URL |
| [17] |
周佳佳 (2013). 多氯联苯与邻苯二甲酸酯污染对油菜生长的影响及累积效应研究. 硕士论文. 泰安: 山东农业大学. pp. 15-25.
DOI URL |
| [18] | 周琼芝 (2016). 低浓度QACs对小球藻生长及氮磷去除的毒物兴奋效应. 硕士论文. 湘潭: 湘潭大学. pp. 30-45. |
| [19] | 朱鸿雁 (2012). 新生SD大鼠联合暴露苯并芘和多氯联苯对血清睾酮水平的影响及表观遗传机制对睾酮合成酶的调控作用. 硕士论文. 上海: 复旦大学. pp. 10-17. |
| [20] |
AKen BV, Correa PA, Schnoor JL (2010). Phytoremediation of polychlorinated biphenyls: new trends and promises.Environ Sci Technol 44, 2767-2776.
DOI URL |
| [21] |
Borja J, Taleon DM, Auresenia J, Gallardo S (2005). Polychlorinated biphenyls and their biodegradation.Process Biochem 40, 1999-2013.
DOI URL |
| [22] |
Christian M, Hannah WB, Lüthen H, Jones AM (2008). Identification of auxins by a chemical genomics approach.J Exp Bot 59, 2757-2767.
DOI URL PMID |
| [23] |
Donnelly PK, Hegde RS, Fletcher JS (1994). Growth of PCB-degrading bacteria on compounds from photosynthetic plants.Chemosphere 28, 981-988.
DOI URL |
| [24] |
Fletcher JS, Hegde RS (1995). Release of phenols by perennial plant roots and their potential importance in bioremediation.Chemosphere 31, 3009-3016.
DOI URL |
| [25] | Huesemann MH, Hausmann TS, Fortman TJ, Thom RM, Cullinan V (2009). In situ phytoremediation of PAH- and PCB-contaminated marine sediments with eelgrass(Zostera marina). Ecol Eng 35, 1395-1404. |
| [26] |
Jefferson RA, Kavanagh TA, Bevan MW (1987). GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants.EMBO J 6, 3901-3907.
DOI URL PMID |
| [27] |
Liu JY, Schnoor JL (2008). Uptake and translocation of lesser-chlorinated polychlorinated biphenyls (PCBs) in whole hybrid poplar plants after hydroponic exposure.Chemosphere 73, 1608-1616.
DOI URL PMID |
| [28] |
Martinez A, Erdman NR, Rodenburg ZL, Eastling PM, Hornbuckle KC (2012). Spatial distribution of chlordanes and PCB congeners in soil in Cedar Rapids, lowa, USA.Environ Pollut 161, 222-228.
DOI URL |
| [29] |
Schulz H (1887). Zur Lehre von der Arzneiwirkung.Archiv für Pathologische Anatomie und Physiologie und für Klinische Medicin 108, 423-445.
DOI URL |
| [30] |
Solorzano-Ochoa G, de la Rosa DA, Maiz-Larralde P, Gullett BK, Tabor DG, Touati A, Wyrzykowska-Ceradini B, Fiedler H, Abel T, Carroll WF (2012). Open burning of household waste: effect of experimental condition on combustion quality and emission of PCDD, PCDF and PCB.Chemosphere 87, 1003-1008.
DOI URL PMID |
| [31] |
Sorin C, Bussell JD, Camus I, Ljung K, Kowalczyk M, Geiss G, McKhann H, Garcion C, Vaucheret H, Sandberg G, Bellini C (2005). Auxin and light control of adventitious rooting in Arabidopsis require ARGONAU- TE1.Plant Cell 17, 1343-1359.
DOI URL |
| [32] | Southam CM, Erlich J (1943). Effects of extract of western red-cedar heartwood on certain wood-decaying fungi in culture.Phytopathology 33, 517-524. |
| [33] |
Stebbing ARD (1982). Hormesis—The stimulation of growth by low levels of inhibitors.Sci Total Environ 22, 213-234.
DOI URL |
| [34] |
Surpin M, Rojas-Pierce M, Carter C, Hicks GR, Vasquez J, Raikhel NV (2005). The power of chemical genomics to study the link between endomembrane system components and the gravitropic response.Proc Natl Acad Sci USA 102, 4902-4907.
DOI URL PMID |
| [35] |
Ulmasov T, Murfett J, Hagen G, Guilfoyle TJ (1997). Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements.Plant Cell 9, 1963-1971.
DOI URL |
| [36] |
Verbeke P, Clark BFC, Rattan SIS (2000). Modulating cellular aging in vitro: hormetic effects of repeated mild heat stress on protein oxidation and glycation. Exp Gerontol 35, 787-794.
DOI URL PMID |
| [37] |
Xu J, Yin HX, Liu XJ, Li X (2010). Salt affects plant Cd- stress responses by modulating growth and Cd accumulation.Planta 231, 449-459.
DOI URL PMID |
| [38] |
Zeeb BA, Amphlett JS, Rutter A, Reimer KJ (2006). Potential for phytoremediation of polychlorinated biphenyl-(PCB)-contaminated soil.Int J Phytoremediat 8, 199-221.
DOI URL PMID |
| [39] |
Zhang Y, Luo XJ, Mo L, Wu JP, Mai BX, Peng YH (2015). Bioaccumulation and translocation of polyhalogenated compounds in rice (Oryza sativa L.) planted in paddy soil collected from an electronic waste recycling site, South China. Chemosphere 137, 25-32.
DOI URL PMID |
| [1] | Ziyun Wang, Yanwen Lv, Yu Xiao, Chao Wu, Xinsheng Hu. Advances in Regulation and Evolutionary Mechanisms of Plant Gene Expression [J]. Chinese Bulletin of Botany, 2025, 60(4): 1-0. |
| [2] | Liu Xupeng, Wang Min, Han Shou'an, Zhu Xuehui, Wang Yanmeng, Pan Mingqi, Zhang Wen. Research Progress on Factors and Molecular Mechanisms Regulating Plant Organ Abscission [J]. Chinese Bulletin of Botany, 2025, 60(3): 472-482. |
| [3] | Tingxin Chen, Min Fu, Na Li, Leilei Yang, Lingfei Li, Chunmei Zhong. Identification and Expression Analysis of DNA Methyltransferase in Begonia masoniana [J]. Chinese Bulletin of Botany, 2024, 59(5): 726-737. |
| [4] | Laipeng Zhao, Baike Wang, Tao Yang, Ning Li, Haitao Yang, Juan Wang, Huizhuan Yan. Investigation of the Regulation of Drought Tolerance by the SlHVA22l Gene in Tomato [J]. Chinese Bulletin of Botany, 2024, 59(4): 558-573. |
| [5] | Zhengyong Duan, Min Ding, Yuzhuo Wang, Yibing Ding, Ling Chen, Ruiyun Wang, Zhijun Qiao. Genome-wide Identification and Expression Analysis of SBP Genes in Panicum miliaceum [J]. Chinese Bulletin of Botany, 2024, 59(2): 231-244. |
| [6] | Hanqian Zhao, Jiayi Song, Jie Yang, Yongjing Zhao, Wennian Xia, Weizhuo Gu, Zhongyi Wang, Nan Yang, Huizhen Hu. Identification of XTH Family Genes in Antirrhinum majus and Screening of Genes Involoved in Sclerotinia sclerotiorum Resistance and Stamen Petalization [J]. Chinese Bulletin of Botany, 2024, 59(2): 188-203. |
| [7] | Nan Wu, Lei Qin, Kan Cui, Haiou Li, Zhongsong Liu, Shitou Xia. Cloning of Brassica napus EXA1 Gene and Its Regulation on Plant Disease Resistance [J]. Chinese Bulletin of Botany, 2023, 58(3): 385-393. |
| [8] | Feifei Wang, Zhenxiang Zhou, Yi Hong, Yangyang Gu, Chao Lü, Baojian Guo, Juan Zhu, Rugen Xu. Identification of the NF-YC Genes in Hordeum vulgare and Expression Analysis Under Salt Stress [J]. Chinese Bulletin of Botany, 2023, 58(1): 140-149. |
| [9] | Li Yue, Hu Desheng, Tan Jinfang, Mei Hao, Wang Yi, Li Hui, Li Fang, Han Yanlai. Chaetomium uniseriatum Promotes Maize Growth by Accelerating Straw Degradation and Regulating the Expression of Hormone Responsive Genes [J]. Chinese Bulletin of Botany, 2022, 57(4): 422-433. |
| [10] | Yanyan Meng, Nan Zhang, Yan Xiong. Novel Links in the Plant Target of Rapamycin Signaling Networks [J]. Chinese Bulletin of Botany, 2022, 57(1): 1-11. |
| [11] | Xiaoting Zhao, Kaitao Mao, Jiahui Xu, Chuan Zheng, Xiaofeng Luo, Kai Shu. Protein Phosphorylation and Its Regulatory Roles in Seed Dormancy and Germination [J]. Chinese Bulletin of Botany, 2021, 56(4): 488-499. |
| [12] | Kai Fan, Fangting Ye, Zhijun Mao, Xinfeng Pan, Zhaowei Li, Wenxiong Lin. Comparative Genomics of the Small Heat Shock Protein Family in Angiosperms [J]. Chinese Bulletin of Botany, 2021, 56(3): 245-261. |
| [13] | Qilu Yu, Jiangzhe Zhao, Xiaoxian Zhu, Kewei Zhang. Regulation of Rice Growth by Root-secreted Phytohormones [J]. Chinese Bulletin of Botany, 2021, 56(2): 175-182. |
| [14] | Lulu Xie, Qingqing Cui, Chunjuan Dong, Qingmao Shang. Recent Advances in Molecular Mechanisms of Plant Graft Healing Process [J]. Chinese Bulletin of Botany, 2020, 55(5): 634-643. |
| [15] | Ruifeng Yao,Daoxin Xie. New Insight into Strigolactone Signaling [J]. Chinese Bulletin of Botany, 2020, 55(4): 397-402. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||