

Chinese Bulletin of Botany ›› 2022, Vol. 57 ›› Issue (4): 422-433.DOI: 10.11983/CBB21147 cstr: 32102.14.CBB21147
• EXPERIMENTAL COMMUNICATIONS • Previous Articles Next Articles
Li Yue1, Hu Desheng1, Tan Jinfang2, Mei Hao1, Wang Yi1, Li Hui1, Li Fang1,*( ), Han Yanlai1,*(
), Han Yanlai1,*( )
)
Received:2021-08-27
Revised:2022-01-13
Online:2022-07-01
Published:2022-07-14
Contact:
Li Fang,Han Yanlai
Li Yue, Hu Desheng, Tan Jinfang, Mei Hao, Wang Yi, Li Hui, Li Fang, Han Yanlai. Chaetomium uniseriatum Promotes Maize Growth by Accelerating Straw Degradation and Regulating the Expression of Hormone Responsive Genes[J]. Chinese Bulletin of Botany, 2022, 57(4): 422-433.
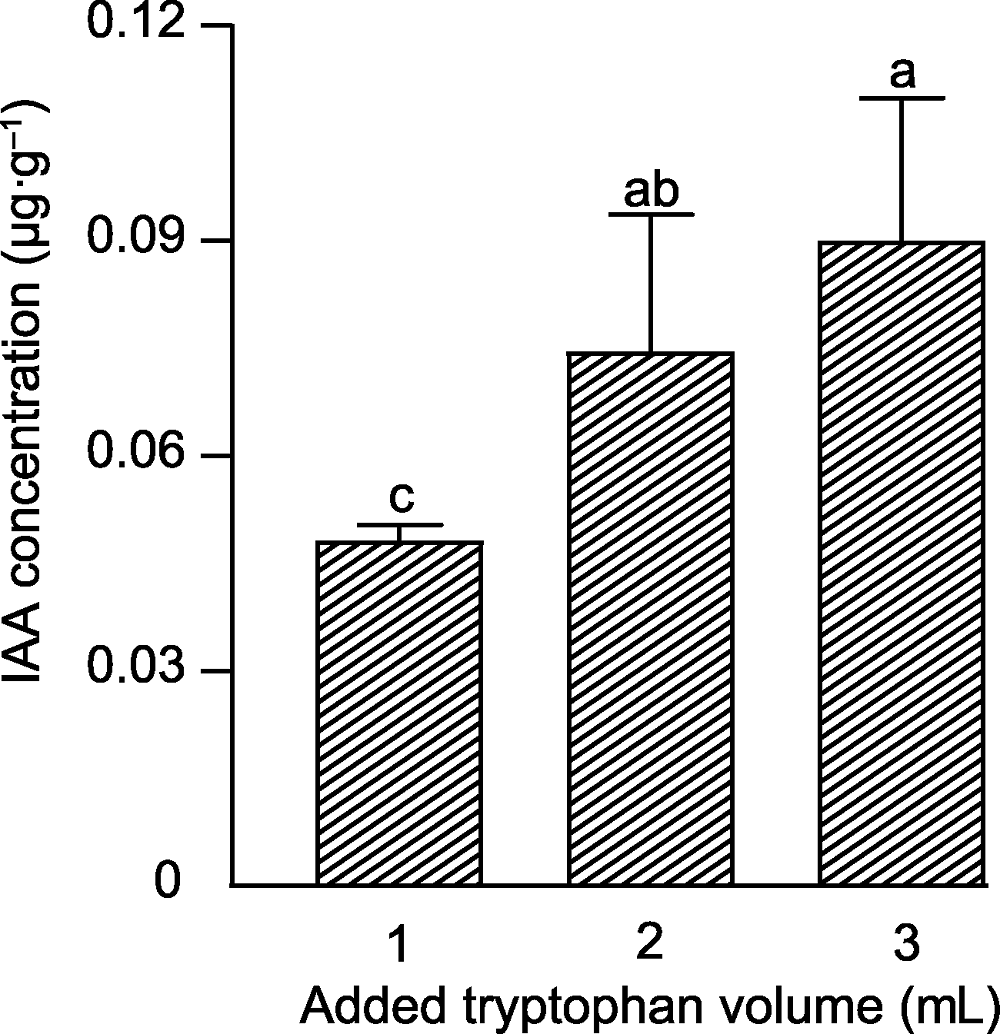
Figure 1 The concentration of indole-3-acetic acid (IAA) produced by Chaetomium uniseriatum with different concentrations of tryptophan Different lowercase letters indicate significant differences at 5% level (n=4).
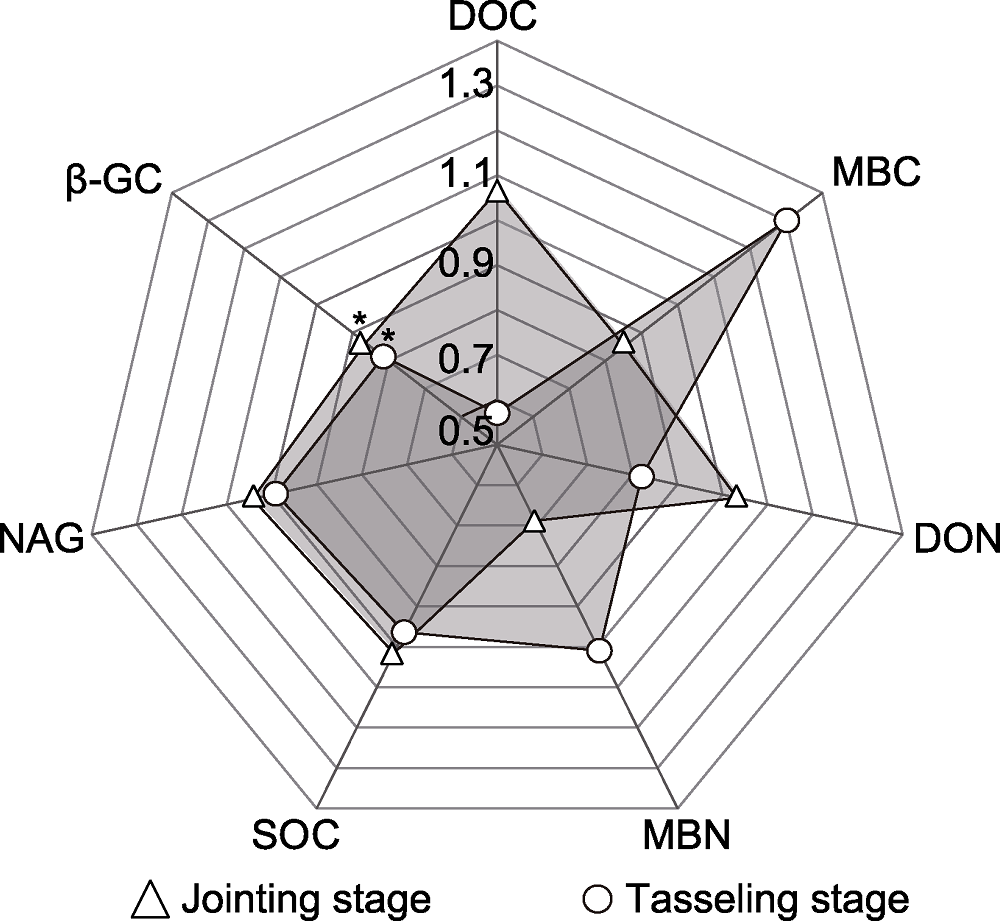
Figure 2 Fold change of soil properties relative to the control in different periods The values in the figure are the change fold of soil properties relative to the control. * indicated significant differences at 5% level. DOC: Dissolved organic carbon; MBC: Microbial biomass carbon; DON: Dissolved organic nitrogen; MBN: Microbial biomass nitrogen; NAG: N-acetyl-β,D-glucosaminidase; β-GC: β-glucosidase; SOC: Soil organic carbon
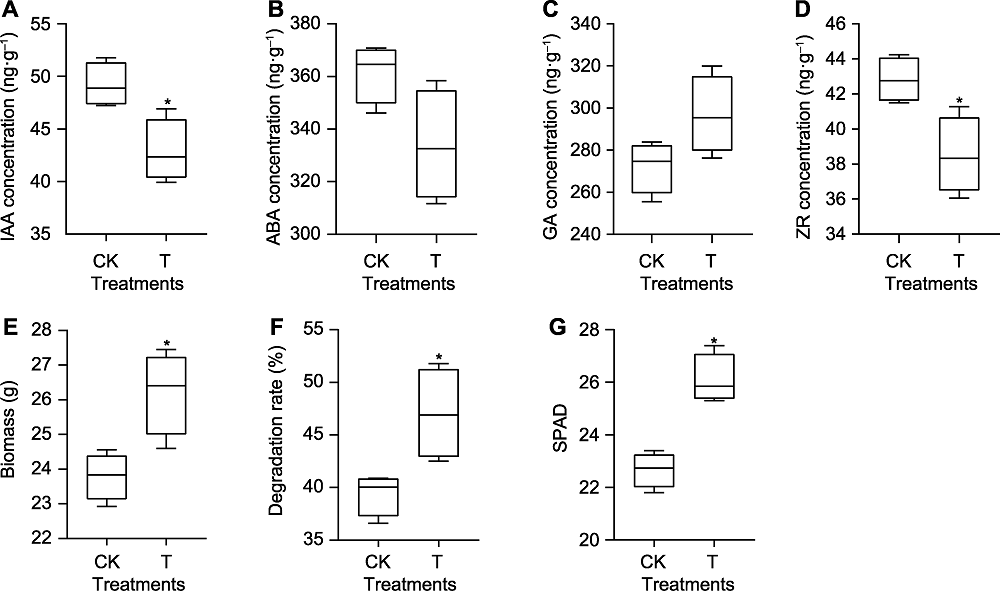
Figure 3 Effects of Chaetomium uniseriatum inoculation on maize physiological characteristics (A) Auxin (IAA) content in maize roots; (B) Abscisic acid (ABA) content in maize roots; (C) Gibberellin (GA) content in maize roots; (D) Zeatin (ZR) content in maize roots; (E) Aboveground biomass of maize; (F) Degradation rate of straw in net bag; (G) SPAD value of maize leaves. * indicated significant differences at 5% level (n=4). CK represents the control treatment; T represents the inoculation treatment.
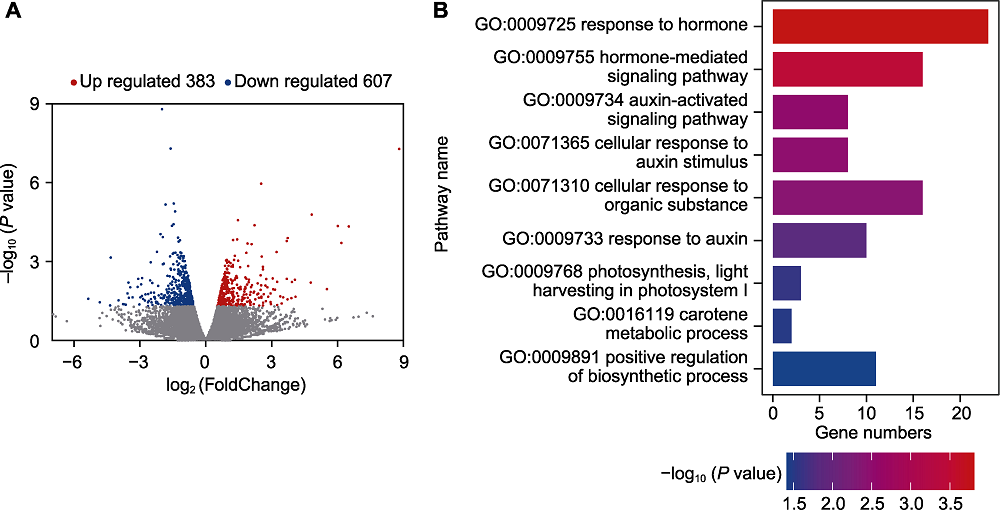
Figure 4 Analysis of differentially expressed genes in maize after inoculation with Chaetomium uniseriatum (A) The number of up-regulated/down-regulated genes; (B) The results of GO annotation on differentially expressed genes
| Gene | log2(FoldChange) | P value | Gene description |
|---|---|---|---|
| Zm00001d032724* | -1.541 | 0.000 | Disease resistance RPP13-like protein 4 |
| Zm00001d035172* | -0.901 | 0.041 | Disease resistance protein RPM1 |
| Zm00001d030888 | -0.327 | 0.227 | Probable disease resistance protein |
| Zm00001d054090 | -1.297 | 0.259 | Protein enhanced disease resistance 2 |
| Zm00001d041343 | -0.278 | 0.307 | Probable disease resistance protein |
| Zm00001d043197 | -0.549 | 0.349 | Probable disease resistance protein |
| Zm00001d052992 | -0.251 | 0.368 | Disease resistance protein RPM1 |
| Zm00001d021491 | -0.267 | 0.370 | Disease resistance RPP13-like protein 4 |
| Zm00001d041358 | -1.208 | 0.394 | NBS-LRR disease resistance protein-like |
| Zm00001d043233 | -0.239 | 0.407 | Disease resistance gene analog PIC21 |
| Zm00001d031711 | -0.529 | 0.435 | Disease resistance gene analog PIC15 |
| Zm00001d032510 | -0.632 | 0.473 | Putative disease resistance RPP13-like protein 1 |
| Zm00001d006873 | -0.329 | 0.517 | Disease resistance response protein-like; protein |
| Zm00001d007776 | -0.298 | 0.519 | Disease resistance protein RGA2 |
| Zm00001d024681 | -0.599 | 0.547 | Disease resistance protein RPM1 |
| Zm00001d017954 | -0.179 | 0.554 | Disease resistance protein RPM1 |
| Zm00001d049121 | -0.296 | 0.582 | Disease resistance protein RPM1 |
| Zm00001d035973 | -0.159 | 0.601 | Protein enhanced disease resistance 2 |
| Zm00001d048663 | -0.180 | 0.611 | Disease resistance protein RGA2 |
| Zm00001d024977 | -0.376 | 0.623 | Disease resistance protein RPM1 |
| Zm00001d014654 | -0.130 | 0.651 | Disease resistance protein RPM1 |
| Zm00001d034555 | -0.179 | 0.653 | Disease resistance protein RPM1 |
| Zm00001d006755 | -0.260 | 0.658 | Disease resistance RPP13-like protein 4 |
| Zm00001d021564 | -0.936 | 0.681 | Disease resistance protein RPM1 |
| Zm00001d014876 | -0.104 | 0.726 | Disease resistance RPP13-like protein 4 |
| Zm00001d037648 | -0.090 | 0.753 | Disease resistance protein RPP13 |
| Zm00001d024975 | -0.111 | 0.756 | Disease resistance protein RPM1 |
| Zm00001d048639 | -0.147 | 0.762 | Disease resistance protein RPM1 |
| Zm00001d007935 | -0.593 | 0.781 | Disease resistance response protein 206 |
| Zm00001d007630 | -0.051 | 0.844 | Disease resistance protein RPS2 |
| Zm00001d023923 | -0.056 | 0.872 | Disease resistance protein RPM1 |
| Zm00001d045512 | -0.177 | 0.886 | Putative disease resistance RPP13-like protein 3 |
| Zm00001d044172 | -0.022 | 0.903 | SGT1 disease resistance protein homolog1 |
| Zm00001d045335 | -0.027 | 0.916 | Putative disease resistance RPP13-like protein 1 |
| Zm00001d052389 | -0.094 | 0.923 | Disease resistance protein RPM1 |
| Zm00001d048637 | -0.062 | 0.950 | Disease resistance RPP13-like protein 4 |
| Zm00001d048635 | -0.282 | 0.971 | Disease resistance protein RPM1 |
| Zm00001d032166 | -0.016 | 0.975 | Protein enhanced disease resistance 2 |
| Zm00001d053244 | -0.002 | 1.000 | Disease resistance protein (TIR-NBS class) |
| Zm00001d048613 | -0.031 | 1.000 | Disease resistance protein RGA2 |
Table 1 Expression of genes associated with disease resistance
| Gene | log2(FoldChange) | P value | Gene description |
|---|---|---|---|
| Zm00001d032724* | -1.541 | 0.000 | Disease resistance RPP13-like protein 4 |
| Zm00001d035172* | -0.901 | 0.041 | Disease resistance protein RPM1 |
| Zm00001d030888 | -0.327 | 0.227 | Probable disease resistance protein |
| Zm00001d054090 | -1.297 | 0.259 | Protein enhanced disease resistance 2 |
| Zm00001d041343 | -0.278 | 0.307 | Probable disease resistance protein |
| Zm00001d043197 | -0.549 | 0.349 | Probable disease resistance protein |
| Zm00001d052992 | -0.251 | 0.368 | Disease resistance protein RPM1 |
| Zm00001d021491 | -0.267 | 0.370 | Disease resistance RPP13-like protein 4 |
| Zm00001d041358 | -1.208 | 0.394 | NBS-LRR disease resistance protein-like |
| Zm00001d043233 | -0.239 | 0.407 | Disease resistance gene analog PIC21 |
| Zm00001d031711 | -0.529 | 0.435 | Disease resistance gene analog PIC15 |
| Zm00001d032510 | -0.632 | 0.473 | Putative disease resistance RPP13-like protein 1 |
| Zm00001d006873 | -0.329 | 0.517 | Disease resistance response protein-like; protein |
| Zm00001d007776 | -0.298 | 0.519 | Disease resistance protein RGA2 |
| Zm00001d024681 | -0.599 | 0.547 | Disease resistance protein RPM1 |
| Zm00001d017954 | -0.179 | 0.554 | Disease resistance protein RPM1 |
| Zm00001d049121 | -0.296 | 0.582 | Disease resistance protein RPM1 |
| Zm00001d035973 | -0.159 | 0.601 | Protein enhanced disease resistance 2 |
| Zm00001d048663 | -0.180 | 0.611 | Disease resistance protein RGA2 |
| Zm00001d024977 | -0.376 | 0.623 | Disease resistance protein RPM1 |
| Zm00001d014654 | -0.130 | 0.651 | Disease resistance protein RPM1 |
| Zm00001d034555 | -0.179 | 0.653 | Disease resistance protein RPM1 |
| Zm00001d006755 | -0.260 | 0.658 | Disease resistance RPP13-like protein 4 |
| Zm00001d021564 | -0.936 | 0.681 | Disease resistance protein RPM1 |
| Zm00001d014876 | -0.104 | 0.726 | Disease resistance RPP13-like protein 4 |
| Zm00001d037648 | -0.090 | 0.753 | Disease resistance protein RPP13 |
| Zm00001d024975 | -0.111 | 0.756 | Disease resistance protein RPM1 |
| Zm00001d048639 | -0.147 | 0.762 | Disease resistance protein RPM1 |
| Zm00001d007935 | -0.593 | 0.781 | Disease resistance response protein 206 |
| Zm00001d007630 | -0.051 | 0.844 | Disease resistance protein RPS2 |
| Zm00001d023923 | -0.056 | 0.872 | Disease resistance protein RPM1 |
| Zm00001d045512 | -0.177 | 0.886 | Putative disease resistance RPP13-like protein 3 |
| Zm00001d044172 | -0.022 | 0.903 | SGT1 disease resistance protein homolog1 |
| Zm00001d045335 | -0.027 | 0.916 | Putative disease resistance RPP13-like protein 1 |
| Zm00001d052389 | -0.094 | 0.923 | Disease resistance protein RPM1 |
| Zm00001d048637 | -0.062 | 0.950 | Disease resistance RPP13-like protein 4 |
| Zm00001d048635 | -0.282 | 0.971 | Disease resistance protein RPM1 |
| Zm00001d032166 | -0.016 | 0.975 | Protein enhanced disease resistance 2 |
| Zm00001d053244 | -0.002 | 1.000 | Disease resistance protein (TIR-NBS class) |
| Zm00001d048613 | -0.031 | 1.000 | Disease resistance protein RGA2 |
| [1] | 鲍士旦 (2000). 土壤农化分析(第3版). 北京: 中国农业出版社. pp. 25-38. |
| [2] | 郭栋, 杜媚, 周宝元, 刘颖慧, 赵明 (2019). 玉米SAUR基因家族的鉴定与生物信息学分析. 植物遗传资源学报 20, 90-99. |
| [3] | 何芳芳, 王海军, 王雪莹 (2020). 纤维素酶的研究进展. 造纸科学与技术 39(4), 1-8. |
| [4] | 滕青云, 乐丽娜, 宋冰倩, 陈英 (2020). 植物TIR1/AFBs基因家族研究进展. 农业工程 10, 93-97. |
| [5] |
Abdel-Azeem AM, Gherbawy YA, Sabry AM (2016). Enzyme profiles and genotyping of Chaetomium globosum isolates from various substrates. Plant Biosyst 150, 420-428.
DOI URL |
| [6] |
Allen HR, Ptashnyk M (2020). Mathematical modelling of auxin transport in plant tissues: flux meets signaling and growth. Bull Math Biol 82, 17.
DOI URL |
| [7] |
Anders S, Huber W (2010). Differential expression analysis for sequence count data. Genome Biol 11, R106.
DOI URL |
| [8] |
Archer SK, Shirokikh NE, Preiss T (2014). Selective and flexible depletion of problematic sequences from RNA-seq libraries at the cDNA stage. BMC Genomics 15, 401.
DOI URL |
| [9] |
Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, Harris MA, Hill DP, Issel-Tarver L, Kasarskis A, Lewis S, Matese JC, Richardson JE, Ringwald M, Rubin GM, Sherlock G (2000). Gene ontology: tool for the unification of biology. Nat Genet 25, 25-29.
DOI PMID |
| [10] |
Berendsen RL, Pieterse CMJ, Bakker PAHM (2012). The rhizosphere microbiome and plant health. Trends Plant Sci 17, 478-486.
DOI PMID |
| [11] |
Berhane M, Xu M, Liang ZY, Shi JL, Wei GH, Tian XH (2020). Effects of long-term straw return on soil organic carbon storage and sequestration rate in North China upland crops: a meta-analysis. Glob Change Biol 26, 2686-2701.
DOI URL |
| [12] |
Cañizares R, Benitez E, Ogunseitan OA (2011). Molecular analyses of β-glucosidase diversity and function in soil. Eur J Soil Biol 47, 1-8.
DOI URL |
| [13] |
Chen YN, Chen YR, Li YP, Wu YX, Zhu FZ, Zeng GM, Zhang JC, Li H (2018). Application of Fenton pretreatment on the degradation of rice straw by mixed culture of Phanerochaete chrysosporium and Aspergillus niger. Ind Crop Prod 112, 290-295.
DOI URL |
| [14] |
Fattorini L, Falasca G, Kevers C, Mainero Rocca L, Zadra C, Altamura MM (2009). Adventitious rooting is enhanced by methyl jasmonate in tobacco thin cell layers. Planta 231, 155-168.
DOI PMID |
| [15] |
Glickmann E, Dessaux Y (1995). A critical examination of the specificity of the salkowski reagent for indolic compounds produced by phytopathogenic bacteria. Appl Environ Microbiol 61, 793-796.
DOI URL |
| [16] |
Guilfoyle TJ, Ulmasov T, Hagen G (1998). The ARF family of transcription factors and their role in plant hormone-responsive transcription. Cell Mol Life Sci 54, 619-627.
PMID |
| [17] |
Jaroszuk-Ściseł J, Kurek E, Trytek M (2014). Efficiency of indoleacetic acid, gibberellic acid and ethylene synthesized in vitro by Fusarium culmorum strains with different effects on cereal growth. Biologia 69, 281-292.
DOI URL |
| [18] |
Jiang C, Song JZ, Zhang JZ, Yang Q (2017). New production process of the antifungal chaetoglobosin A using cornstalks. Braz J Microbiol 48, 410-418.
DOI URL |
| [19] |
Jiang XZ, Du JW, He RN, Zhang ZY, Qi F, Huang JZ, Qin L (2020). Improved production of majority cellulases in Trichoderma reesei by integration of cbh1gene grom Chaetomium thermophilum. Front Microbiol 11, 1633.
DOI URL |
| [20] |
Kanehisa M, Araki M, Goto S, Hattori M, Hirakawa M, Itoh M, Katayama T, Kawashima S, Okuda S, Tokimatsu T, Yamanishi Y (2008). KEGG for linking genomes to life and the environment. Nucleic Acids Res 36, D480-D484.
DOI PMID |
| [21] |
Kang SM, Khan AL, Waqas M, You YH, Kim JH, Kim JG, Hamayun M, Lee IJ (2014). Plant growth-promoting rhizobacteria reduce adverse effects of salinity and osmotic stress by regulating phytohormones and antioxidants in Cucumis sativus. J Plant Interact 9, 673-682.
DOI URL |
| [22] |
Kazan K, Manners JM (2009). Linking development to defense: auxin in plant-pathogen interactions. Trends Plant Sci 14, 373-382.
DOI URL |
| [23] | Khan AL, Shinwari ZK, Kim YH, Waqas M, Hamayun M, Kamran M, Lee IJ (2012). Role of endophyte Chaetomium globosum lk4 in growth of Capsicum annuum by producion of gibberellins and indole acetic acid. Pak J Bot 44, 1601-1607. |
| [24] |
Laothanachareon T, Bunterngsook B, Suwannarangsee S, Eurwilaichitr L, Champreda V (2015). Synergistic action of recombinant accessory hemicellulolytic and pectinolytic enzymes to Trichoderma reesei cellulase on rice straw degradation. Bioresour Technol 198, 682-690.
DOI URL |
| [25] |
Leyser O (2005). Auxin distribution and plant pattern formation: how many angels can dance on the point of PIN? Cell 121, 819-822.
DOI URL |
| [26] |
Li F, Zhang SQ, Wang Y, Li Y, Li PP, Chen L, Jie XL, Hu DS, Feng B, Yue K, Han YL (2020). Rare fungus, Mortierella capitata, promotes crop growth by stimulating primary metabolisms related genes and reshaping rhizosphere bacterial community. Soil Biol Biochem 151, 108017.
DOI URL |
| [27] |
Li ZX, Zhang XR, Zhao YJ, Li YJ, Zhang GF, Peng ZH, Zhang JR (2018). Enhancing auxin accumulation in maize root tips improves root growth and dwarfs plant height. Plant Biotechnol J 16, 86-99.
DOI URL |
| [28] |
Liu G, Yu HY, Ma J, Xu H, Wu QY, Yang JH, Zhuang YQ (2015). Effects of straw incorporation along with microbial inoculant on methane and nitrous oxide emissions from rice fields. Sci Total Environ 518-519, 209-216.
DOI URL |
| [29] |
Liu HW, Brettell LE, Qiu ZG, Singh BK (2020). Microbiome-mediated stress resistance in plants. Trends Plant Sci 25, 733-743.
DOI URL |
| [30] |
Liu X, Zhou F, Hu GQ, Shao S, He HB, Zhang W, Zhang XD, Li LJ (2019). Dynamic contribution of microbial residues to soil organic matter accumulation influenced by maize straw mulching. Geoderma 333, 35-42.
DOI URL |
| [31] |
Manirakiza E, Ziadi N, St Luce M, Hamel C, Antoun H, Karam A (2019). Nitrogen mineralization and microbial biomass carbon and nitrogen in response to co-application of biochar and paper mill biosolids. Appl Soil Ecol 142, 90-98.
DOI |
| [32] |
Millevoi S, Vagner S (2010). Molecular mechanisms of eukaryotic pre-mRNA 3′ end processing regulation. Nucleic Acids Res 38, 2757-2774.
DOI URL |
| [33] |
Narukawa-Nara M, Nakamura A, Kikuzato K, Kakei Y, Sato A, Mitani Y, Yamasaki-Kokudo Y, Ishii T, Hayashi KI, Asami T, Ogura T, Yoshida S, Fujioka S, Kamakura T, Kawatsu T, Tachikawa M, Soeno K, Shimada Y (2016). Aminooxy-naphthylpropionic acid and its derivatives are inhibitors of auxin biosynthesis targeting L-tryptophan aminotransferase: structure-activity relationships. Plant J 87, 245-257.
DOI URL |
| [34] |
Ortiz J, Soto J, Fuentes A, Herrera H, Meneses C, Arriagada C (2019). The endophytic fungus Chaetomium cupreum regulates expression of genes involved in the tolerance to metals and plant growth promotion in eucalyptus globulus roots. Microorganisms 7, 490.
DOI URL |
| [35] |
Park JH, Choi GJ, Jang KS, Lim HK, Kim HT, Cho KY, Kim JC (2005). Antifungal activity against plant pathogenic fungi of chaetoviridins isolated from Chaetomium globosum. FEMS Microbiol Lett 252, 309-313.
DOI URL |
| [36] |
Pedraza-Zapata DC, Sánchez-Garibello AM, Quevedo-Hidalgo B, Moreno-Sarmiento N, Gutiérrez-Rojas I (2017). Promising cellulolytic fungi isolates for rice straw degradation. J Microbiol 55, 711-719.
DOI PMID |
| [37] |
Raza W, Mei XL, Wei Z, Ling N, Yuan J, Wang JC, Huang QW, Shen QR (2017). Profiling of soil volatile organic compounds after long-term application of inorganic, organic and organic-inorganic mixed fertilizers and their effect on plant growth. Sci Total Environ 607-608, 326-338.
DOI URL |
| [38] |
Saiya-Cork KR, Sinsabaugh RL, Zak DR (2002). The effects of long term nitrogen deposition on extracellular enzyme activity in an Acer saccharum forest soil. Soil Biol Biochem 34, 1309-1315.
DOI URL |
| [39] |
Sato M, Winter JM, Kishimoto S, Noguchi H, Tang Y, Watanabe K (2016). Combinatorial generation of chemical diversity by redox enzymes in chaetoviridin biosynthesis. Org Lett 18, 1446-1449
DOI URL |
| [40] |
Shanthiyaa V, Saravanakumar D, Rajendran L, Karthikeyan G, Prabakar K, Raguchander T (2013). Use of Chaetomium globosum for biocontrol of potato late blight disease. Crop Prot 52, 33-38.
DOI URL |
| [41] |
Siegel-Hertz K, Edel-Hermann V, Chapelle E, Terrat S, Raaijmakers JM, Steinberg C (2018). Comparative microbiome analysis of a Fusarium wilt suppressive soil and a Fusarium wilt conducive soil from the Châteaurenard region. Front Microbiol 9, 568.
DOI PMID |
| [42] |
Singh A, Sharma S (2002). Composting of a crop residue through treatment with microorganisms and subsequent vermicomposting. Bioresour Technol 85, 107-111.
DOI URL |
| [43] |
Timmusk S, Wagner EGH (1999). The plant-growth-promoting rhizobacterium Paenibacillus polymyxa induces changes in Arabidopsis thaliana gene expression: a possible connection between biotic and abiotic stress responses. Mol Plant Microbe Interact 12, 951-959.
DOI URL |
| [44] |
Uddling J, Gelang-Alfredsson J, Piikki K, Pleijel H (2007). Evaluating the relationship between leaf chlorophyll concentration and SPAD-502 chlorophyll meter readings. Photosynth Res 91, 37-46.
DOI URL |
| [45] |
Vance ED, Brookes PC, Jenkinson DS (1987). Anextraction method for measuring soil microbial biomass C. Soil Biol Biochem 19, 703-707.
DOI URL |
| [46] |
Wang B, Funakoshi D, Dalpé Y, Hamel C (2002). Phosphorus-32 absorption and translocation to host plants by arbuscular mycorrhizal fungi at low root-zone temperature. Mycorrhiza 12, 93-96.
PMID |
| [47] |
Wang R, Zhang HC, Sun LG, Qi GF, Chen S, Zhao XY (2017). Microbial community composition is related to soil biological and chemical properties and bacterial wilt outbreak. Sci Rep 7, 343.
DOI PMID |
| [48] |
Wu JS, Joergensen RG, Pommerening B, Chaussod R (1990). Measurement of soil microbial biomass c by fumigation extraction—an automated procedure. Soil Biol Biochem 22, 1167-1169.
DOI URL |
| [49] |
Xu J, Han HF, Ning TY, Li ZJ, Lal R (2019). Long-term effects of tillage and straw management on soil organic carbon, crop yield, and yield stability in a wheat-maize system. Field Crop Res 233, 33-40.
DOI URL |
| [50] |
Yang HS, Zhou JJ, Weih M, Li YF, Zhai SL, Zhang Q, Chen WP, Liu J, Liu L, Hu SJ (2020). Mycorrhizal nitrogen uptake of wheat is increased by earthworm activity only under no-till and straw removal conditions. Appl Soil Ecol 155, 103672.
DOI URL |
| [51] |
Yang J, Kloepper JW, Ryu CM (2009). Rhizosphere bacteria help plants tolerate abiotic stress. Trends Plant Sci 14, 1-4.
DOI URL |
| [52] |
Yun SI, Jeong CS, Chung DK, Choi HS (2001). Purification and some properties of a β-glucosidase from Trichoderma harzianum type C-4. Biosci Biotechnol Biochem 65, 2028-2032.
DOI URL |
| [53] |
Zhang Y, Yu XX, Zhang WJ, Lang DY, Zhang XJ, Cui GC, Zhang XH (2019). Interactions between endophytes and plants: beneficial effect of endophytes to ameliorate biotic and abiotic stresses in plants. J Plant Biol 62, 1-13.
DOI |
| [54] |
Zhao XL, Yuan GY, Wang HY, Lu DJ, Chen XQ, Zhou JM (2019). Effects of full straw incorporation on soil fertility and crop yield in rice-wheat rotation for silty clay loamy cropland. Agronomy 9, 133.
DOI URL |
| [55] |
Zhao XY, He XS, Xi BD, Gao RT, Tan WB, Zhang H, Li D (2016). The evolution of water extractable organic matter and its association with microbial community dynamics during municipal solid waste composting. Waste Manage 56, 79-87.
DOI URL |
| [1] | Jing Zhang Li JunPan Han XU Yi-De LI Hai ShengHe. Comparison of plant biomass in conifer and broadleaved mixed artificial forests in south subtropical area and analyses of the influential factors [J]. Chin J Plant Ecol, 2025, 49(化学计量与功能性状): 0-0. |
| [2] | Liu Xupeng, Wang Min, Han Shou'an, Zhu Xuehui, Wang Yanmeng, Pan Mingqi, Zhang Wen. Research Progress on Factors and Molecular Mechanisms Regulating Plant Organ Abscission [J]. Chinese Bulletin of Botany, 2025, 60(3): 472-482. |
| [3] | Xiangge Zhang, Chen Chen, Shan Cheng, Chunxin Li, Yajing Zhu, Xinran Xu, Huiwei Wang. Identification and Analysis of Tuber-specific Expression Genes in Cyperus esculentus [J]. Chinese Bulletin of Botany, 2025, 60(1): 33-48. |
| [4] | MA Lu-Hua, MENG Xian-Chao, WANG Gui-Qiang, MA Zi-Feng, LI Yi-Kang, LI Yue-Mei, ZHOU Hua-Kun, ZHANG Fa-Wei, LIN Li. Effects of moss crust inoculation on soil properties and microbial communities in alpine meadow in Sanjiangyuan, China [J]. Chin J Plant Ecol, 2025, 49(1): 173-188. |
| [5] | Tingxin Chen, Min Fu, Na Li, Leilei Yang, Lingfei Li, Chunmei Zhong. Identification and Expression Analysis of DNA Methyltransferase in Begonia masoniana [J]. Chinese Bulletin of Botany, 2024, 59(5): 726-737. |
| [6] | Qingqing Du, Siyuan Ren, Nicole Tsz Shun Yuan, Yan Zhu. Factors affecting the productivity of sapling and adult trees in the warm temperate deciduous broad-leaved forest of Donglingshan, Beijing [J]. Biodiv Sci, 2024, 32(12): 24284-. |
| [7] | JIANG Hai-Gang, ZENG Yun-Hong, TANG Hua-Xin, LIU Wei, LI Jie-Lin, HE Guo-Hua, QIN Hai-Yan, WANG Li-Chao, Victor RESCO de DIOS, YAO Yin-An. Rhythmic regulation of carbon fixation and water dissipation in three mosses [J]. Chin J Plant Ecol, 2023, 47(7): 988-997. |
| [8] | BAI Xue, LI Yu-Jing, JING Xiu-Qing, ZHAO Xiao-Dong, CHANG Sha-Sha, JING Tao-Yu, LIU Jin-Ru, ZHAO Peng-Yu. Response mechanisms of millet and its rhizosphere soil microbial communities to chromium stress [J]. Chin J Plant Ecol, 2023, 47(3): 418-433. |
| [9] | SUN Cai-Li, QIU Mo-Sheng, HUANG Chao-Xiang, WANG Yi-Wei. Characteristics of soil extracellular enzyme activities and their stoichiometry during rocky desertification in southwestern Guizhou, China [J]. Chin J Plant Ecol, 2022, 46(7): 834-845. |
| [10] | Yanyan Meng, Nan Zhang, Yan Xiong. Novel Links in the Plant Target of Rapamycin Signaling Networks [J]. Chinese Bulletin of Botany, 2022, 57(1): 1-11. |
| [11] | MAO Jin, DUO Ying, DENG Jun, CHENG Jie, CHENG Ji-Min, PENG Chang-Hui, GUO Liang. Influences of warming and snow reduction in winter on soil nutrients and bacterial communities composition in a typical grassland of the Loess Plateau [J]. Chin J Plant Ecol, 2021, 45(8): 891-902. |
| [12] | Aixia Wang, Jingjing Ma, Huidie Gong, Guoan Fan, Mao Wang, Hongmei Zhao, Junhui Cheng. Patterns and drivers of species richness of early spring annual ephemeral plants in northern Xinjiang [J]. Biodiv Sci, 2021, 29(6): 735-745. |
| [13] | Ting Wang, Zengqiang Xia, Jiangping Shu, Jiao Zhang, Meina Wang, Jianbing Chen, Kanglin Wang, Jianying Xiang, Yuehong Yan. Dating whole-genome duplication reveals the evolutionary retardation of Angiopteris [J]. Biodiv Sci, 2021, 29(6): 722-734. |
| [14] | Xiaoting Zhao, Kaitao Mao, Jiahui Xu, Chuan Zheng, Xiaofeng Luo, Kai Shu. Protein Phosphorylation and Its Regulatory Roles in Seed Dormancy and Germination [J]. Chinese Bulletin of Botany, 2021, 56(4): 488-499. |
| [15] | Qilu Yu, Jiangzhe Zhao, Xiaoxian Zhu, Kewei Zhang. Regulation of Rice Growth by Root-secreted Phytohormones [J]. Chinese Bulletin of Botany, 2021, 56(2): 175-182. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||