

植物学报 ›› 2019, Vol. 54 ›› Issue (5): 662-673.DOI: 10.11983/CBB19100 cstr: 32102.14.CBB19100
• 专题论坛 • 上一篇
收稿日期:2019-05-28
接受日期:2019-08-09
出版日期:2019-09-01
发布日期:2020-03-10
通讯作者:
周文彬
基金资助:Received:2019-05-28
Accepted:2019-08-09
Online:2019-09-01
Published:2020-03-10
Contact:
Wenbin Zhou
摘要: 自然界中, 植物通常面对多重联合胁迫。在全球气候变化日益加剧的背景下, 多重联合胁迫对植物生长发育及作物产量形成的不利影响日益显著。阐明植物响应和适应联合胁迫的生理与分子机制, 对人们理解植物对自然环境的适应机理, 及培育耐受联合胁迫的新品种有重要意义。研究表明, 植物响应联合胁迫的机制是特异的, 不能简单地从单一胁迫响应叠加来推断。植物遭受联合胁迫时, 各种生理、代谢和信号途径相互作用, 使得植物响应联合胁迫非常复杂。该文综述了植物响应联合胁迫的生理与分子机理的最新进展, 并阐述了植物响应联合胁迫的研究方法。
郭倩倩,周文彬. 植物响应联合胁迫机制的研究进展. 植物学报, 2019, 54(5): 662-673.
Qianqian Guo,Wenbin Zhou. Advances in the Mechanism Underlying Plant Response to Stress Combination. Chinese Bulletin of Botany, 2019, 54(5): 662-673.
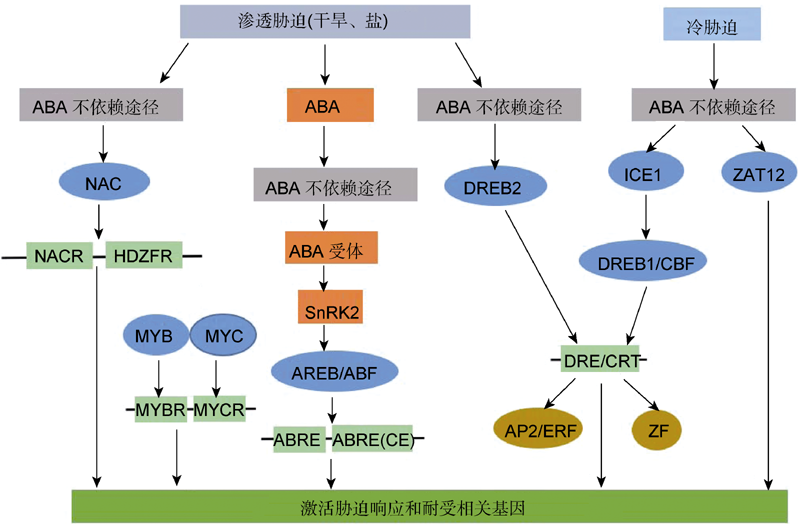
图1 植物响应非生物胁迫(干旱、盐和冷害)的ABA依赖与ABA不依赖途径的交互作用网络(改自Roychoudhury et al., 2013)
Figure 1 Cross-talk network in ABA-dependent and ABA-independent pathways during abiotic stress (drought, salinity and low temperature) (modified from Roychoudhury et al., 2013)
| 联合胁迫类型 | 植物 | 文献 | |
|---|---|---|---|
| 负向相互作用 | 干旱+盐 | 大麦(Hordeum vulgare) | |
| 干旱+热 | 小麦(Triticum aestivum), 大麦, 烟草(Nicotiana tabacum), 拟南芥(Arabidopsis thaliana), 高粱(Sorghum bicolor), 高羊茅(Festuca arundinacea), 棉花(Gossypium spp.), 柑橘(Citrus reticulata) | ||
| 干旱+冷害 | 甘蔗(Saccharum officinarum) | ||
| 干旱+UV辐射 | 拟南芥, 白三叶(Trifolium repens), 云杉(Picea asperata), 油菜(Brassica napus), 柳树(Salix babylonica), 杨树(Populus) | ||
| 干旱+高光 | 拟南芥 | ||
| 干旱+重金属 | 红枫(Acer rubrum) | ||
| 盐+高温 | 小麦 | ||
| 盐+臭氧 | 欧洲白桦(Betula pendula), 鹰嘴豆(Cicer arietinum) | ||
| 高温+臭氧 | 欧洲白桦, 杨树 | ||
| 高温+UV辐射 | 西芹(Apium graveolens) | ||
| 高温+高光 | 向日葵(Helianthus annuus) | ||
| 冷害+高光 | 盐藻(Populus tremula) | ||
| UV辐射+重金属 | 豌豆(Pisum sativum) | ||
| 正向相互作用 | 干旱+臭氧 | 苜蓿(Medicago truncatula), 欧洲白桦, 欧洲山毛榉(Fagus sylvatica) | |
| 干旱+高CO2 | 高粱 | ||
| 盐+高温 | 番茄(Solanum lycopersicon) | ||
| 盐+高CO2 | 莴苣(Lactuca sativa) | ||
| 盐+硼 | 玉米(Zea mays) | ||
| 臭氧+高CO2 | 大豆(Glycine max) | ||
| 高CO2+高光 | 莴苣 |
表1 联合胁迫之间的相互作用
Table 1 Interactions of combined stresses
| 联合胁迫类型 | 植物 | 文献 | |
|---|---|---|---|
| 负向相互作用 | 干旱+盐 | 大麦(Hordeum vulgare) | |
| 干旱+热 | 小麦(Triticum aestivum), 大麦, 烟草(Nicotiana tabacum), 拟南芥(Arabidopsis thaliana), 高粱(Sorghum bicolor), 高羊茅(Festuca arundinacea), 棉花(Gossypium spp.), 柑橘(Citrus reticulata) | ||
| 干旱+冷害 | 甘蔗(Saccharum officinarum) | ||
| 干旱+UV辐射 | 拟南芥, 白三叶(Trifolium repens), 云杉(Picea asperata), 油菜(Brassica napus), 柳树(Salix babylonica), 杨树(Populus) | ||
| 干旱+高光 | 拟南芥 | ||
| 干旱+重金属 | 红枫(Acer rubrum) | ||
| 盐+高温 | 小麦 | ||
| 盐+臭氧 | 欧洲白桦(Betula pendula), 鹰嘴豆(Cicer arietinum) | ||
| 高温+臭氧 | 欧洲白桦, 杨树 | ||
| 高温+UV辐射 | 西芹(Apium graveolens) | ||
| 高温+高光 | 向日葵(Helianthus annuus) | ||
| 冷害+高光 | 盐藻(Populus tremula) | ||
| UV辐射+重金属 | 豌豆(Pisum sativum) | ||
| 正向相互作用 | 干旱+臭氧 | 苜蓿(Medicago truncatula), 欧洲白桦, 欧洲山毛榉(Fagus sylvatica) | |
| 干旱+高CO2 | 高粱 | ||
| 盐+高温 | 番茄(Solanum lycopersicon) | ||
| 盐+高CO2 | 莴苣(Lactuca sativa) | ||
| 盐+硼 | 玉米(Zea mays) | ||
| 臭氧+高CO2 | 大豆(Glycine max) | ||
| 高CO2+高光 | 莴苣 |
| 1 | 杜康兮, 沈文辉, 董爱武 (2018). 表观遗传调控植物响应非生物胁迫的研究进展. 植物学报 53, 581-593. |
| 2 | Achuoa EA, Prinsenb E, Höfte M (2006). Influence of drought, salt stress and abscisic acid on the resistance of tomato to Botrytis cinerea and Oidium neolycopersici. Plant Pathol 55, 178-186. |
| 3 | Ahmed IM, Dai HX, Zheng WT, Cao FB, Zhang GP, Sun DF, Wu FB (2013). Genotypic differences in physiological characteristics in the tolerance to drought and salinity combined stress between Tibetan wild and cultivated barley. Plant Physiol Biochem 63, 49-60. |
| 4 | Ainsworth EA, Rogers A, Leakey ADB (2008). Targets for crop biotechnology in a future high-CO2 and high-O3 world. Plant Physiol 147, 13-19. |
| 5 | Allakhverdiev SI, Hayashi H, Nishiyama Y, Ivanov AG, Aliev JA, Klimov VV, Murata N, Carpentier R (2003). Glycinebetaine protects the D1/D2/Cytb559 complex of photosystem II against photo-induced and heat-induced inactivation. J Plant Physiol 160, 41-49. |
| 6 | Atkinson NJ, Lilley CJ, Urwin PE (2013). Identification of genes involved in the response of Arabidopsis to simultaneous biotic and abiotic stresses. Plant Physiol 162, 2028-2041. |
| 7 | Averyanov AA, Lapikova VP, Nikolaev ON, Stepanov AI (2000). Active oxygen-associated control of rice blast disease by riboflavin and roseoflavin. Biochemistry 11, 1292-1298. |
| 8 | Bai YL, Kissoudis C, Yan Z, Visser RGF, van der Linden G (2018). Plant behaviour under combined stress: tomato responses to combined salinity and pathogen stress. Plant J 93, 781-793. |
| 9 | Bandurska H, Niedziela J, Chadzinikolau T (2013). Separate and combined responses to water deficit and UV-B radiation. Plant Sci 213, 98-105. |
| 10 | Berens ML, Wolinska KW, Spaepen S, Ziegler J, Nobori T, Nair A, Krüler V, Winkelmüller TM, Wang YM, Mine A, Becker D, Garrido-Oter R, Schulze-Lefert P, Tsuda K (2019). Balancing trade-offs between biotic and abiotic stress responses through leaf age-dependent variation in stress hormone cross-talk. Proc Natl Acad Sci USA 116, 2364-2373. |
| 11 | Booker FL, Fiscus EL (2005). The role of ozone flux and antioxidants in the suppression of ozone injury by elevated CO2 in soybean. J Exp Bot 56, 2139-2151. |
| 12 | Brouder SM, Volenec JJ (2008). Impact of climate change on crop nutrient and water use efficiencies. Physiol Plant 133, 705-724. |
| 13 | Carmo-Silva AE, Gore MA, Andrade-Sanchez P, French AN, Hunsaker DJ, Salvucci ME (2012). Decreased CO2 availability and inactivation of Rubisco limit photosynthesis in cotton plants under heat and drought stress in the field. Environ Exp Bot 83, 1-11. |
| 14 | Chaves MM, Maroco JP, Pereira JS (2003). Understanding plant responses to drought—from genes to the whole plant. Funct Plant Biol 30, 239-264. |
| 15 | Chen TH, Murata N (2008). Glycinebetaine: an effective protectant against abiotic stress in plants. Trends Plant Sci 13, 499-505. |
| 16 | Coolen S, Proietti S, Hickman R, Davila Olivas NH, Huang PP, Van Verk MC, Van Pelt JA, Wittenberg AHJ, De Vos M, Prins M, Van Loon JJA, Aarts MGM, Dicke M, Pieterse CMJ, Van Wees SCM (2016). Transcriptome dynamics of Arabidopsis during sequential biotic and abiotic stresses. Plant J 86, 249-267. |
| 17 | Craufurd PQ, Peacock JM (1993). Effect of heat and drought stress on sorghum (Sorghum bicolor). II. Grain yield. Exp Agric 29, 77-86. |
| 18 | Cuin TA, Shabala S (2005). Exogenously supplied compatible solutes rapidly ameliorate NaCl-induced potassium efflux from barley roots. Plant Cell Physiol 46, 1924-1933. |
| 19 | de Silva NDG, Cholewa E, Ryser P (2012). Effects of combined drought and heavy metal stresses on xylem structure and hydraulic conductivity in red maple ( Acer rubrum L.). J Exp Bot 63, 5957-5966. |
| 20 | Duan BL, Ran F, Zhang XL, Zhang YB, Korpelainen H, Li CY (2011). Long-term acclimation of mesophyll conductance, carbon isotope discrimination and growth in two contrasting Picea asperata populations exposed to drought and enhanced UV-B radiation for three years. Agric Forest Meteor 151, 116-126. |
| 21 | Fan J, Hill L, Crooks C, Doerner P, Lamb C (2009). Abscisic acid has a key role in modulating diverse plant- pathogen interactions. Plant Physiol 150, 1750-1761. |
| 22 | Figueroa P, Browse J (2012). The Arabidopsis JAZ2 promoter contains a G-box and thymidine-rich module that are necessary and sufficient for jasmonate-dependent activation by MYC transcription factors and repression by JAZ proteins. Plant Cell Physiol 53, 330-343. |
| 23 | Gao QM, Venugopal S, Navarre D, Kachroo A (2011). Low oleic acid-derived repression of jasmonic acid-inducible defense responses requires the WRKY50 and WRKY51 proteins. Plant Physiol 155, 464-476. |
| 24 | Garg AK, Kim JK, Owens TG, Ranwala AP, Do Choi Y, Kochian LV, Wu RJ (2002). Trehalose accumulation in rice plants confers high tolerance levels to different abiotic stresses. Proc Natl Acad Sci USA 99, 15898-15903. |
| 25 | Ghandi A, Adi M, Lilia F, Linoy A, Rena G (2016). Tomato yellow leaf curl virus infection mitigates the heat stress response of plants grown at high temperatures. Sci Rep 6, 19715. |
| 26 | Giraud E, Ho LHM, Clifton R, Carroll A, Estavillo G, Tan YF, Howell KA, Ivanova A, Pogson BJ, Millar AH, Whelan J (2008). The absence of ALTERNATIVE OXIDASE1a in Arabidopsis results in acute sensitivity to combined light and drought stress. Plant Physiol 147, 595-610. |
| 27 | Gupta A, Hisano H, Hojo Y, Matsuura T, Ikeda Y, Mori IC, Senthil-Kumar M (2017). Global profiling of phytohormone dynamics during combined drought and pathogen stress in Arabidopsis thaliana reveals ABA and JA as major regulators. Sci Rep 7, 4017. |
| 28 | Haghjou MM, Shariati M, Smirnoff N (2009). The effect of acute high light and low temperature stresses on the ascorbate-glutathione cycle and superoxide dismutase activity in two Dunaliella salina strains. Physiol Plant 135, 272-280. |
| 29 | Han SH, Park YJ, Park CM (2019). Light primes the thermally induced detoxification of reactive oxygen species during thermotolerance development in Arabidopsis. Plant Cell Physiol 60, 230-241. |
| 30 | Hartikainen K, Nerg AM, Kivimäenpää M, Kontunen-Soppela S, Mäenpää M, Oksanen E, Rousi M, Holopainen T (2009). Emissions of volatile organic compounds and leaf structural characteristics of European aspen (Populus tremula) grown under elevated ozone and temperature. Tree Physiol 29, 1163-1173. |
| 31 | Hewezi T, Léger M, Gentzbittel L (2008). A comprehensive analysis of the combined effects of high light and high temperature stresses on gene expression in sunflower. Ann Bot 102, 127-140. |
| 32 | Hofmann RW, Campbell BD, Fountain DF (2003). Sensitivity of white clover to UV-B radiation depends on water availability, plant productivity and duration of stress. Glob Chang Biol 9, 473-477. |
| 33 | Hubbard M, Germida JJ, Vujanovic V (2014). Fungal endophytes enhance wheat heat and drought tolerance in terms of grain yield and second-generation seed viability. J Appl Microbiol 116, 109-122. |
| 34 | IPCC ( 2014). Summary for policymakers. In: Climate change 2014: Impacts, Adaptation, and Vulnerability. Summaries, Frequently Asked Questions, and Cross-Chapter Boxes. A Contribution of Working Group II to the Fifth Assessment Report of the IPCC. Cambridge and New York: Cam- bridge University Press. pp. 190. |
| 35 | Iyer NJ, Tang YH, Mahalingam R (2013). Physiological, biochemical and molecular responses to a combination of drought and ozone in Medicago truncatula. Plant Cell Environ 36, 706-720. |
| 36 | Jiang YW, Huang BR (2001). Drought and heat stress injury to two cool-season turfgrasses in relation to antioxidant metabolism and lipid peroxidation. Crop Sci 41, 436-442. |
| 37 | Kasurinen A, Biasi C, Holopainen T, Rousi M, Mäenpää M, Oksanen E (2012). Interactive effects of elevated ozone and temperature on carbon allocation of silver birch (Betula pendula) genotypes in an open-air field exposure. Tree Physiol 32, 737-751. |
| 38 | Kazan K, Manners JM (2012). JAZ repressors and the orchestration of phytohormone crosstalk. Trends Plant Sci 17, 22-31. |
| 39 | Keleş Y, Öncel I (2002). Response of antioxidative defence system to temperature and water stress combinations in wheat seedlings. Plant Sci 163, 783-790. |
| 40 | Koussevitzky S, Suzuki N, Huntington S, Armijo L, Sha W, Cortes D, Shulaev V, Mittler R (2008). Ascorbate peroxidase 1 plays a key role in the response of Arabidopsis thaliana to stress combination. J Biol Chem 283, 34197-34203. |
| 41 | Kumar D, Datta R, Hazra S, Sultana A, Mukhopadhyay R, Chattopadhyay S (2015). Transcriptomic profiling of Arabidopsis thaliana mutant pad2.1 in response to combined cold and osmotic stress. PLoS One 10, e0122690. |
| 42 | Liu JZ, Feng LL, Gu XT, Deng X, Qiu Q, Li Q, Zhang YY, Wang MY, Deng YW, Wang ET, He YK, Bäurle I, Li JM, Cao XF, He ZH (2019). An H3K27me3 demethylase- HSFA2 regulatory loop orchestrates transgenerational thermomemory in Arabidopsis. Cell Res 29, 379-390. |
| 43 | Lobell DB, Cassman KG, Field CB (2009). Crop yield gaps: their importance, magnitudes, and causes. Annu Rev Environ Resour 34, 179-204. |
| 44 | Löw M, Herbinger K, Nunn AJ, Häberle KH, Leuchner M, Heerdt C, Werner H, Wipfler P, Pretzsch H, Tausz M, Matyssek R (2006). Extraordinary drought of 2003 overrules ozone impact on adult beech trees ( Fagus sylvatica). Trees 20, 539-548. |
| 45 | Luck J, Spackman M, Freeman A, Trebicki P, Griffiths W, Finlay K, Chakraborty S (2011). Climate change and diseases of food crops. Plant Pathol 60, 113-121. |
| 46 | Lunn JE (2007). Gene families and evolution of trehalose metabolism in plants. Funct Plant Biol 34, 550-563. |
| 47 | Madgwick JW, West JS, White RP, Semenov MA, Townsend JA, Turner JA, Fitt BDL (2011). Impacts of climate change on wheat anthesis and fusarium ear blight in the UK. Eur J Plant Pathol 130, 117-131. |
| 48 | Mahrookashani A, Siebert S, Hüging H, Ewert F (2017). Independent and combined effects of high temperature and drought stress around anthesis on wheat. J Agron Crop Sci 203, 453-463. |
| 49 | Martínez-Ballesta MDC, Bastías E, Carvajal M (2008). Combined effect of boron and salinity on water transport: the role of aquaporins. Plant Signal Behav 3, 844-845. |
| 50 | Mittler R, Blumwald E (2010). Genetic engineering for modern agriculture: challenges and perspectives. Annu Rev Plant Biol 61, 443-462. |
| 51 | Obata T, Witt S, Lisec J, Palacios-Rojas N, Florez-Sarasa I, Yousfi S, Araus JL, Cairns JE, Fernie AR (2015). Metabolite profiles of maize leaves in drought, heat, and combined stress field trials reveal the relationship between metabolism and grain yield. Plant Physiol 169, 2665-2683. |
| 52 | Ottman MJ, Kimball BA, Pinter PJ, Wall GW, Vanderlip RL, Leavitt SW, LaMorte RL, Matthias AD, Brooks TJ (2001). Elevated CO2 increases sorghum biomass under drought conditions. New Phytol 150, 261-273. |
| 53 | Pääkkönen E, Vahala J, Pohjola M, Holopainen T, Kärenlampi L (1998). Physiological, stomatal and ultrastructural ozone responses in birch ( Betula pendula Roth.) are modified by water stress. Plant Cell Environ 21, 671-684. |
| 54 | Pandey P, Ramegowda V, Senthil-Kumar M (2015). Shared and unique responses of plants to multiple individual stresses and stress combinations: physiological and molecular mechanisms. Front Plant Sci 6, 723. |
| 55 | Parent B, Bonneau J, Maphosa L, Kovalchuk A, Langridge P, Fleury D (2017). Quantifying wheat sensitivities to environmental constraints to dissect genotype × environ- ment interactions in the field. Plant Physiol 174, 1669-1682. |
| 56 | Pérez-López U, Miranda-Apodaca J, Muñoz-Rueda A, Mena-Petite A (2013). Lettuce production and antioxidant capacity are differentially modified by salt stress and light intensity under ambient and elevated CO2. J Plant Physiol 170, 1517-1525. |
| 57 | Poulson ME, Boeger MRT, Donahue RA (2006). Response of photosynthesis to high light and drought for Arabidopsis thaliana grown under a UV-B enhanced light regime. Photosynth Res 90, 79-90. |
| 58 | Prasad PVV, Pisipati SR, Momčilović I, Ristic Z (2011). Independent and combined effects of high temperature and drought stress during grain filling on plant yield and chloroplast EF-Tu expression in spring wheat. J Agron Crop Sci 197, 430-441. |
| 59 | Prasch CM, Sonnewald U (2013). Simultaneous application of heat, drought, and virus to Arabidopsis plants reveals significant shifts in signaling networks. Plant Physiol 162, 1849-1866. |
| 60 | Ramegowda V, Senthil-Kumar M (2015). The interactive effects of simultaneous biotic and abiotic stresses on plants: mechanistic understanding from drought and pathogen combination. J Plant Physiol 176, 47-54. |
| 61 | Rasmussen S, Barah P, Suarez-Rodriguez MC, Bressendorff S, Friis P, Costantino P, Bones AM, Nielsen HB, Mundy J (2013). Transcriptome responses to combinations of stresses in Arabidopsis. Plant Physiol 161, 1783-1794. |
| 62 | Rivero RM, Mestre TC, Mittler R, Rubio F, Garcia- Sanchez F, Martinez V (2014). The combined effect of salinity and heat reveals a specific physiological, biochemical and molecular response in tomato plants. Plant Cell Environ 37, 1059-1073. |
| 63 | Rizhsky L, Davletova S, Liang HJ, Mittler R (2004). The zinc finger protein Zat12 is required for cytosolic ascorbate peroxidase 1 expression during oxidative stress in Arabidopsis. J Biol Chem 279, 11736-11743. |
| 64 | Rizhsky L, Liang HJ, Mittler R (2002). The combined effect of drought stress and heat shock on gene expression in tobacco. Plant Physiol 130, 1143-1151. |
| 65 | Rollins JA, Habte E, Templer SE, Colby T, Schmidt J, Von Korff M (2013). Leaf proteome alterations in the context of physiological and morphological responses to drought and heat stress in barley ( Hordeum vulgare L.). J Exp Bot 64, 3201-3212. |
| 66 | Roychoudhury A, Paul S, Basu S (2013). Cross-talk between abscisic acid-dependent and abscisic acid-independent pathways during abiotic stress. Plant Cell Rep 32, 985-1006. |
| 67 | Sales CRG, Ribeiro RV, Silveira JAG, Machado EC, Martins MO, Lagôa AMMA (2013). Superoxide dismutase and ascorbate peroxidase improve the recovery of photosynthesis in sugarcane plants subjected to water deficit and low substrate temperature. Plant Physiol Biochem 73, 326-336. |
| 68 | Salvucci ME, Crafts-Brandner SJ (2004). Relationship bet- ween the heat tolerance of photosynthesis and the thermal stability of Rubisco activase in plants from contrasting thermal environments. Plant Physiol 134, 1460-1470. |
| 69 | Sangtarash MH, Qaderi MM, Chinnappa CC, Reid DM (2009). Differential sensitivity of canola ( Brassica napus) seedlings to ultraviolet-B radiation, water stress and abscisic acid. Environ Exp Bot 66, 212-219. |
| 70 | Schenke D, Böettcher C, Scheel D (2011). Crosstalk between abiotic ultraviolet-B stress and biotic (flg22) stress signaling in Arabidopsis prevents flavonol accumulation in favor of pathogen defence compound production. Plant Cell Environ 34, 1849-1864. |
| 71 | Srivastava G, Kumar S, Dubey G, Mishra V, Prasad SM (2012). Nickel and ultraviolet-B stresses induce differential growth and photosynthetic responses in Pisum sativum L. seedlings. Biol Trace Elem Res 149, 86-96. |
| 72 | Suzuki N, Rivero RM, Shulaev V, Blumwald E, Mittler R (2014). Abiotic and biotic stress combinations. New Phytol 203, 32-43. |
| 73 | Turtola S, Rousi M, Pusenius J, Yamaji K, Heiska S, Tirkkonen V, Meier B, Julkunen-Tiitto R (2006). Genotypic variation in drought response of willows grown under ambient and enhanced UV-B radiation. Environ Exp Bot 56, 80-86. |
| 74 | Van Ittersum MK, Cassman KG, Grassini P, Wolf J, Tittonell P, Hochman Z (2013). Yield gap analysis with local to global relevance—a review. Field Crops Res 143, 4-17. |
| 75 | Vile D, Pervent M, Belluau M, Vasseur F, Bresson J, Muller B, Granier C, Simonneau T (2012). Arabidopsis growth under prolonged high temperature and water deficit: independent or interactive effects? Plant Cell Environ 35, 702-718. |
| 76 | Waller F (2005). The endophytic fungus Pirifomospora indica reprograms barley to salt-stress tolerance, disease resistance, and higher yield. Proc Natl Acad Sci USA 38, 13386-13391. |
| 77 | Walter MH (1989). The induction of phenylpropanoid biosynthetic enzymes by ultraviolet light or fungal elicitor in cultured parsley cells is overriden by a heat-shock treatment. Planta 177, 1-8. |
| 78 | Way DA, Oren R (2010). Differential responses to changes in growth temperature between trees from different functional groups and biomes: a review and synthesis of data. Tree Physiol 30, 669-688. |
| 79 | Welfare K, Yeo AR, Flowers TJ (2002). Effects of salinity and ozone, individually and in combination, on the growth and ion contents of two chickpea ( Cicer arietinum L.) varieties. Environ Pollut 120, 397-403. |
| 80 | Wen XG, Qiu NW, Lu QT, Lu CM (2005). Enhanced thermotolerance of photosystem II in salt-adapted plants of the halophyte Artemisia anethifolia. Planta 220, 486-497. |
| 81 | Zandalinas SI, Balfagón D, Arbona V, Gómez-Cadenas A, Inupakutika MA, Mittler R (2016a). ABA is required for the accumulation of APX1 and MBF1c during a combination of water deficit and heat stress. J Exp Bot 67, 5381-5390. |
| 82 | Zandalinas SI, Rivero RM, Martínez V, Gómez-Cadenas A, Arbona V (2016b). Tolerance of citrus plants to the combination of high temperatures and drought is associated to the increase in transpiration modulated by a reduction in abscisic acid levels. BMC Plant Biol 16, 105. |
| 83 | Zandalinas SI, Sales C, Beltrán J, Gómez-Cadenas A, Arbona V (2017). Activation of secondary metabolism in citrus plants is associated to sensitivity to combined drought and high temperatures. Front Plant Sci 7, 1954. |
| 84 | Zhang HN, Sonnewald U (2017). Differences and commonalities of plant responses to single and combined stresses. Plant J 90, 839-855. |
| 85 | Zinta G, AbdElgawad H, Domagalska MA, Vergauwen L, Knapen D, Nijs I, Janssens IA, Beemster GTS, Asard H (2014). Physiological, biochemical, and genome-wide transcriptional analysis reveals that elevated CO2 mitigates the impact of combined heat wave and drought stress in Arabidopsis thaliana at multiple organizational levels. Glob Chang Biol 20, 3670-3685. |
| [1] | 逯子佳, 王天瑞, 郑斯斯, 孟宏虎, 曹建国, Gregor Kozlowski, 宋以刚. 孑遗植物湖北枫杨的环境适应性遗传变异与遗传脆弱性[J]. , 2025, 49(濒危植物的保护与恢复): 0-. |
| [2] | 唐远翔, 熊仕臣, 朱洪锋, 张新生, 游成铭, 刘思凝, 谭波, 徐振锋. 长期氮添加对四川盆地西缘常绿阔叶林优势树种凋落叶产量及碳氮磷归还的影响[J]. 植物生态学报, 2025, 49(5): 720-731. |
| [3] | 卢晓强, 董姗姗, 马月, 徐徐, 邱凤, 臧明月, 万雅琼, 李孪鑫, 于赐刚, 刘燕. 前沿技术在生物多样性研究中的应用现状、挑战与展望[J]. 生物多样性, 2025, 33(4): 24440-. |
| [4] | 许庭旸, 刘雨辰, 王万鹏, 苏航, 苏昆龙, 吴振映, 吕明, 李福利, 王小山, 付春祥. 喷施不同植物生长调节剂对盐碱地小麦生长发育的影响[J]. 植物学报, 2025, 60(3): 354-362. |
| [5] | 夏琳凤, 李瑞, 王海政, 冯大领, 王春阳. 轮藻门植物基因组学研究进展[J]. 植物学报, 2025, 60(2): 271-282. |
| [6] | 王亚萍, 包文泉, 白玉娥. 单细胞转录组学在植物生长发育及胁迫响应中的应用进展[J]. 植物学报, 2025, 60(1): 101-113. |
| [7] | 陈文娜, 李良涛, 周璐, 姚纲. 太行山近期隆升促进太行花属(蔷薇科)谱系分化[J]. 植物学报, 2024, 59(5): 763-773. |
| [8] | 商慧颖, 翟云雁, 鬲晓敏, 刘帅, 王尚林, 周涛, 柏国清. 基于广泛靶向代谢组学揭示不同时期漆树生漆中代谢物变化规律[J]. 植物学报, 2024, 59(1): 66-74. |
| [9] | 杜志烨, 李明玉, 陈稷, 黄进. 植物胁迫相关蛋白功能研究进展[J]. 植物学报, 2024, 59(1): 110-121. |
| [10] | 曾鑫海, 陈锐, 师宇, 盖超越, 范凯, 李兆伟. 植物SPL转录因子的生物功能研究进展[J]. 植物学报, 2023, 58(6): 982-997. |
| [11] | 王露露, 杨智, 杨永. 利用标本组学推进植物超级DNA条形码研究[J]. 植物学报, 2023, 58(5): 831-842. |
| [12] | 景昭阳, 程可光, 舒恒, 马永鹏, 刘平丽. 全基因组重测序方法在濒危植物保护中的应用[J]. 生物多样性, 2023, 31(5): 22679-. |
| [13] | 许亚楠, 闫家榕, 孙鑫, 王晓梅, 刘玉凤, 孙周平, 齐明芳, 李天来, 王峰. 红光和远红光在调控植物生长发育及应答非生物胁迫中的作用[J]. 植物学报, 2023, 58(4): 622-637. |
| [14] | 任晓童, 张冉冉, 魏绍巍, 罗晓峰, 徐佳慧, 舒凯. 种子际微生物研究展望[J]. 植物学报, 2023, 58(3): 499-509. |
| [15] | 严语萍, 俞晓琦, 任德勇, 钱前. 水稻穗粒数遗传机制与育种利用[J]. 植物学报, 2023, 58(3): 359-372. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
