

植物学报 ›› 2023, Vol. 58 ›› Issue (3): 373-384.DOI: 10.11983/CBB22063 cstr: 32102.14.CBB22063
收稿日期:2022-04-05
接受日期:2022-07-25
出版日期:2023-05-01
发布日期:2023-05-17
通讯作者:
*E-mail: maojuan@scau.edu.cn
基金资助:
Shuyao Zhou, Jianming Li, Juan Mao( )
)
Received:2022-04-05
Accepted:2022-07-25
Online:2023-05-01
Published:2023-05-17
Contact:
*E-mail: maojuan@scau.edu.cn
摘要: 植物激素油菜素甾醇和生长素在植物生长发育过程中发挥重要作用, 且两者之间存在复杂的调控关系。为探究拟南芥(Arabidopsis thaliana)生长素早期响应基因GH3.17是否参与生长素和油菜素甾醇之间的调控, 利用转基因技术和CRISPR/Cas9基因编辑系统分别获得拟南芥GH3.17过表达和缺失突变植株, 并对其表型进行比较分析。结果显示, 与野生型相比, GH3.17缺失突变体的根长增加, 而GH3.17过表达植株呈现根长变短、叶片卷曲、叶柄缩短和株高变矮等典型的生长素缺陷表型。采用一定浓度的生长素或者油菜素甾醇处理植株, 结果显示过表达植株的根明显变长, 表明其对生长素以及油菜素甾醇的响应更为敏感。通过qRT-PCR分析过表达植株中生长素信号通路和油菜素甾醇合成通路相关基因的表达情况, 发现生长素信号通路基因Aux/IAA家族成员IAA12和IAA16的表达受到抑制, 而油菜素甾醇合成酶基因DWF4和CPD的表达量增高。综上, 推测活性生长素含量减少导致的生长素信号被抑制可以通过增加油菜素甾醇合成来进行一定的补偿。
周淑瑶, 李建明, 毛娟. AtGH3.17调控拟南芥生长素和油菜素甾醇的响应. 植物学报, 2023, 58(3): 373-384.
Shuyao Zhou, Jianming Li, Juan Mao. AtGH3.17-mediated Regulation of Auxin and Brassinosteroid Response in Arabidopsis thaliana. Chinese Bulletin of Botany, 2023, 58(3): 373-384.
| Primer name | Primer sequence (5′-3′) |
|---|---|
| pBRI1:gGH3.17-F | CAAACTCTTGAGAAGGTACCATGATA- CCAAGTTACGACCC |
| pBRI1:gGH3.17-R | GTGTCGACTCTAGAGGATCCAGAAT- CTAAACCAAGTGGTT |
| GH3.17-DT1-BsF | ATATATGGTCTCGATTGGATCTCATGTCCTAAGAGTGTTTTAGAGCTAGAAATAGC |
| GH3.17-DT1-BsR | ATTATTGGTCTCGAAACGCAGAGACTCGGTCTTGTCCAATCTCTTAGTCGACTCTAC |
| U6-26p-F | TGTCCCAGGATTAGAATGATTAGGC |
| U6-26p-R | AGCCCTCTTCTTTCGATCCATCAAC |
| CAS 9-F | ATCCAATCTTCGGCAACAT |
| CAS 9-R | TATCCAGGTCATCGTCGTA |
| GH3.17-ba-F | AGATGAGGGAAAAGGGATGT |
| GH3.17-ba-R | TCAAGATTCCTTCCCACGAC |
| GH3.17-qPCR-F | CGATGTATGCTTCCTCTGAGTG |
| GH3.17-qPCR-R | ATCTCTTCGTGGGATTTGTCG |
| IAA12-qPCR-F | TGGGTTACACAGGATGAACAG |
| IAA12-qPCR-R | AACCCTAAGCCCTGAACTTTC |
| IAA16-qPCR-F | TGAAGATAAAGATGGCGACTGG |
| IAA16-qPCR-R | AAGTCCGATTGCTTCTGATCC |
| IAA20-qPCR-F | GTACTCGAAACCTAAGCACGG |
| IAA20-qPCR-R | CACATATTCCGCATCCTCTACC |
| DWF4-qPCR-F | CATTGCTCTCGCTATCTTCTTC |
| DWF4-qPCR-R | GACTCTCCTAGTTCCTTCTTGG |
| CPD-qPCR-F | TCCTTGTGGGTCTAGTGTTTG |
| CPD-qPCR-R | TTGAACCATTGAAGCAGAAGAG |
| BES1-qPCR-F | CAGCCATTCTCTGCCTCTATG |
| BES1-qPCR-R | ACTCGGAGCTTTGACCAATC |
| Actin2-qPCR-F | GGTAACATTGTGCTCAGTGGTGG |
| Actin2-qPCR-R | AACGACCTTAATCTTCATGCTGC |
表1 引物序列
Table 1 Primers used in this study
| Primer name | Primer sequence (5′-3′) |
|---|---|
| pBRI1:gGH3.17-F | CAAACTCTTGAGAAGGTACCATGATA- CCAAGTTACGACCC |
| pBRI1:gGH3.17-R | GTGTCGACTCTAGAGGATCCAGAAT- CTAAACCAAGTGGTT |
| GH3.17-DT1-BsF | ATATATGGTCTCGATTGGATCTCATGTCCTAAGAGTGTTTTAGAGCTAGAAATAGC |
| GH3.17-DT1-BsR | ATTATTGGTCTCGAAACGCAGAGACTCGGTCTTGTCCAATCTCTTAGTCGACTCTAC |
| U6-26p-F | TGTCCCAGGATTAGAATGATTAGGC |
| U6-26p-R | AGCCCTCTTCTTTCGATCCATCAAC |
| CAS 9-F | ATCCAATCTTCGGCAACAT |
| CAS 9-R | TATCCAGGTCATCGTCGTA |
| GH3.17-ba-F | AGATGAGGGAAAAGGGATGT |
| GH3.17-ba-R | TCAAGATTCCTTCCCACGAC |
| GH3.17-qPCR-F | CGATGTATGCTTCCTCTGAGTG |
| GH3.17-qPCR-R | ATCTCTTCGTGGGATTTGTCG |
| IAA12-qPCR-F | TGGGTTACACAGGATGAACAG |
| IAA12-qPCR-R | AACCCTAAGCCCTGAACTTTC |
| IAA16-qPCR-F | TGAAGATAAAGATGGCGACTGG |
| IAA16-qPCR-R | AAGTCCGATTGCTTCTGATCC |
| IAA20-qPCR-F | GTACTCGAAACCTAAGCACGG |
| IAA20-qPCR-R | CACATATTCCGCATCCTCTACC |
| DWF4-qPCR-F | CATTGCTCTCGCTATCTTCTTC |
| DWF4-qPCR-R | GACTCTCCTAGTTCCTTCTTGG |
| CPD-qPCR-F | TCCTTGTGGGTCTAGTGTTTG |
| CPD-qPCR-R | TTGAACCATTGAAGCAGAAGAG |
| BES1-qPCR-F | CAGCCATTCTCTGCCTCTATG |
| BES1-qPCR-R | ACTCGGAGCTTTGACCAATC |
| Actin2-qPCR-F | GGTAACATTGTGCTCAGTGGTGG |
| Actin2-qPCR-R | AACGACCTTAATCTTCATGCTGC |
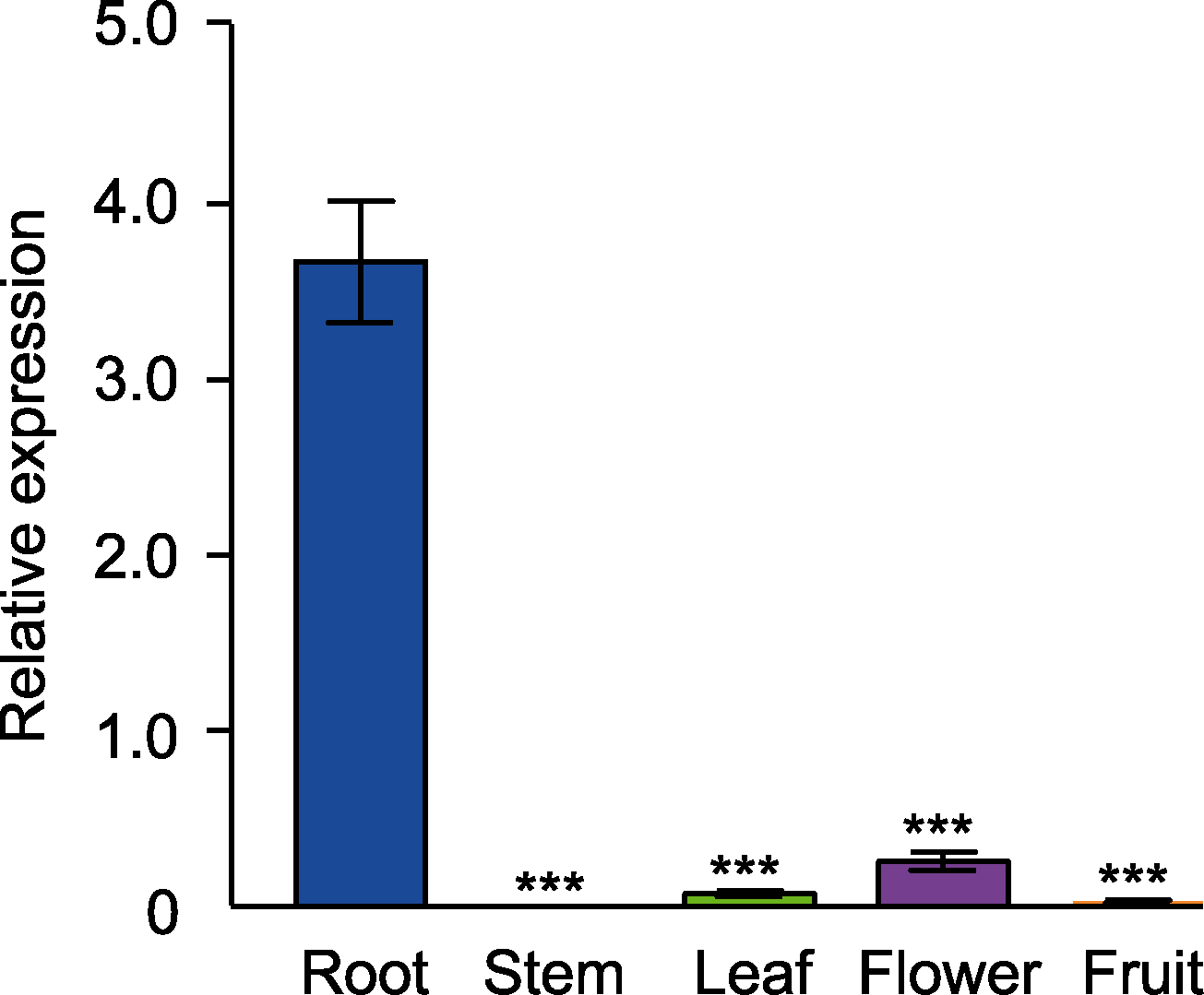
图1 拟南芥GH3.17在不同组织中的表达 误差线代表标准误差。***表示差异十分显著(P<0.001) (Student’s t-test)。
Figure 1 Tissue-specific expression of GH3.17 in Arabidopsis thaliana Error bars denote SEM. *** indicate significant differences at P<0.001 (Student’s t-test).
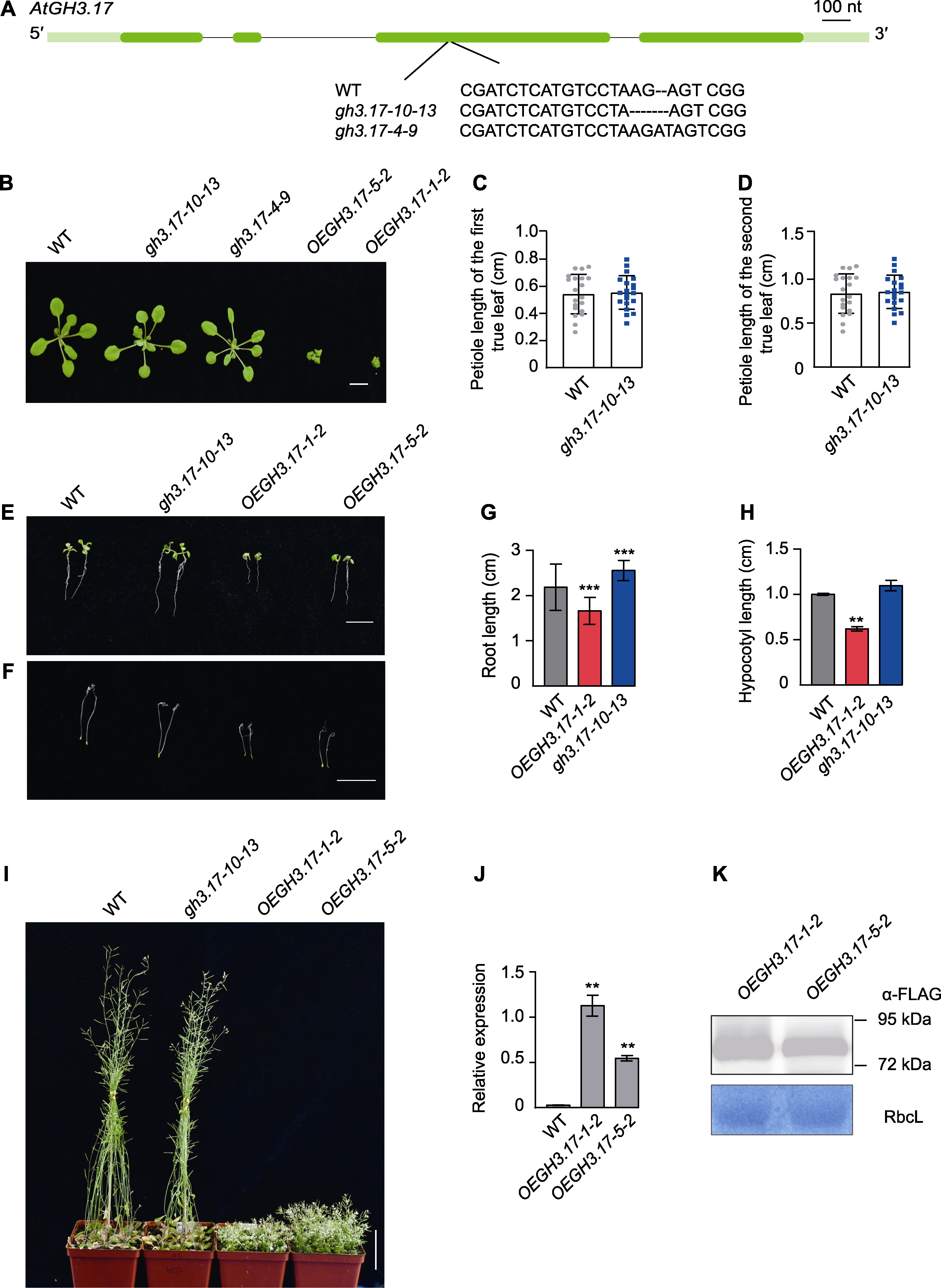
图2 拟南芥野生型(WT)、GH3.17基因缺失突变体和过表达植株的表型分析 (A) 利用CRISPR/Cas9技术编辑基因GH3.17获得gh3.17-10-13和gh3.17-4-9突变体(外显子用深绿色方框表示, 内含子用黑色线条表示, 5'和3'非翻译区(UTR)用浅绿色方框表示, 突变位点和序列如图所示); (B) 野生型、GH3.17基因缺失突变体和过表达植株在土壤中生长20天地上部分的表型(bar=1 cm); (C) 野生型和GH3.17基因缺失突变体第1对真叶的叶柄长度统计(n=20); (D) 野生型和GH3.17基因缺失突变体第2对真叶的叶柄长度统计(n=20); (E), (G) 野生型、GH3.17基因缺失突变体和过表达植株在1/2MS培养基中竖直培养7天的根长表型(bar=1 cm)和统计分析, 数值为3次独立实验的平均值(n=30); (F), (H) 野生型、GH3.17基因缺失突变体和过表达植株在1/2MS培养基中暗培养5天的下胚轴表型(bar=1 cm)和统计分析, 数值为3次独立实验的平均值(n=30); (I) 野生型、GH3.17基因缺失突变体和过表达植株在土壤中生长50天地上部分的表型(bar=5 cm); (J) qRT-PCR检测GH3.17基因在野生型和过表达植株中的表达情况; (K) 免疫印迹检测GH3.17过表达株系中GH3.17-Flag融合蛋白的表达情况。(C)、(D)、(G)和(H)中误差线代表标准差; (J)中误差线代表标准误差。**表示差异极显著(P<0.01); ***表示差异十分显著(P<0.001) (Student’s t-test)。
Figure 2 Phenotype of Arabidopsis thaliana wild type (WT), GH3.17 CRISPR mutant and overexpression seedlings (A) Generation of knockout alleles of GH3.17 (gh3.17-10-13 and gh3.17-4-9) by CRISPR/Cas9 (exons are represented by dark green boxes, introns by black lines and 5' and 3' untranslated region (UTR) by light green boxes; the mutation site and sequence are presented in the figure); (B) The aerial part phenotype of 20-day-old soil-grown WT, GH3.17 CRISPR mutant and overexpression lines under normal conditions (bar=1 cm); (C) Petiole length statistics of the first pair of true leaves of WT and GH3.17 CRISPR mutant (n=20); (D) Statistics of petiole length of the second pair of true leaves of WT and GH3.17 CRISPR mutant (n=20); (E), (G) The primary root length phenotype and statistics of 7-day-old WT, GH3.17 CRISPR mutant and overexpression seedlings vertically grown on 1/2MS medium under normal conditions (bar=1 cm) (the data was obtained through three replicates of 30 seedlings each); (F), (H) The hypocotyls phenotype and statistics of 5-day-old dark-grown WT, GH3.17 CRISPR mutant and overexpression seedlings grown on 1/2MS medium under normal conditions (bar=1 cm) (the data was obtained through three replicates of 30 seedlings each); (I) The above-ground phenotypes of 50-day-old soil-grown WT, GH3.17 CRISPR mutant and overexpression lines under normal conditions (bar=5 cm); (J) qRT-PCR analysis of GH3.17 transcriptional level in WT and GH3.17 overexpression plants; (K) Immunoblot analysis of GH3.17-Flag fusion protein in GH3.17 overexpression transgenic lines. Error bars in (C), (D), (G) and (H) denote SD; error bars in (J) denote SEM. ** indicate significant differences at P<0.01; *** indicate significant differences at P<0.001 (Student’s t-test).
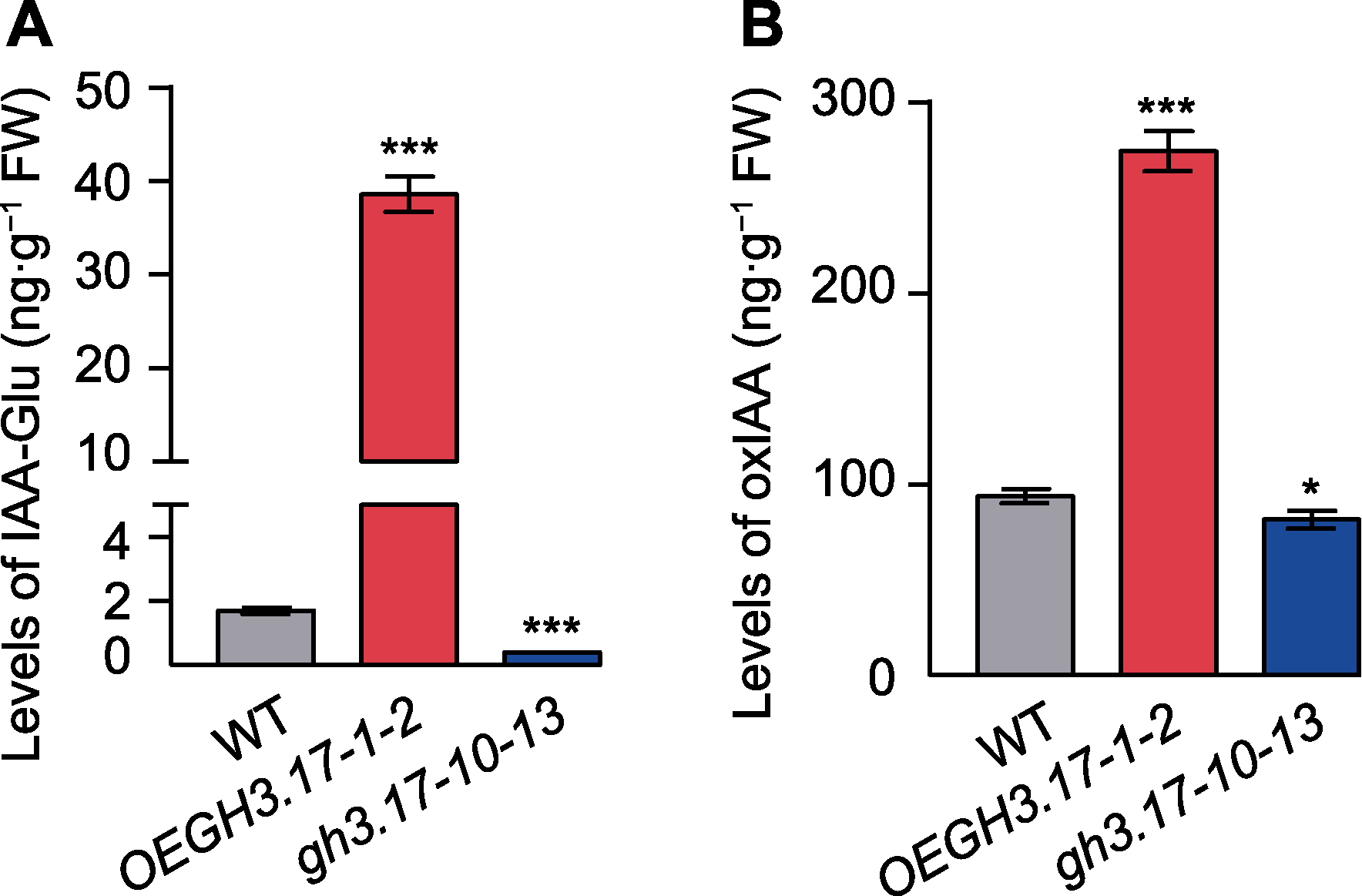
图3 拟南芥野生型(WT)、GH3.17过表达和CRISPR突变体中的IAA-Glu和oxIAA含量 (A) 野生型、GH3.17过表达和CRISPR突变体中IAA-Glu的含量; (B) 野生型、GH3.17过表达和CRISPR突变体中oxIAA的含量。对在1/2MS培养基中生长12天拟南芥植株的IAA-Glu和oxIAA含量进行测定。设3次生物学重复。误差线代表标准差。*表示差异显著(P<0.05); ***表示差异十分显著(P<0.001) (Student’s t-test)。
Figure 3 The IAA-Glu and oxIAA content of Arabidopsis tha- liana wild type (WT), GH3.17 overexpression and CRISPR mutant (A) The IAA-Glu content of WT, GH3.17 overexpression and CRISPR mutant; (B) The oxIAA content of WT, GH3.17 overexpression and CRISPR mutant. IAA level in 12-day-old Arabidopsis thaliana seedlings grown on 1/2MS solid medium is obtained from extraction of three independent tissue samples. Error bars indicate SD. * indicates significant difference at P<0.05; *** indicate significant differences at P<0.001 (Student’s t-test).
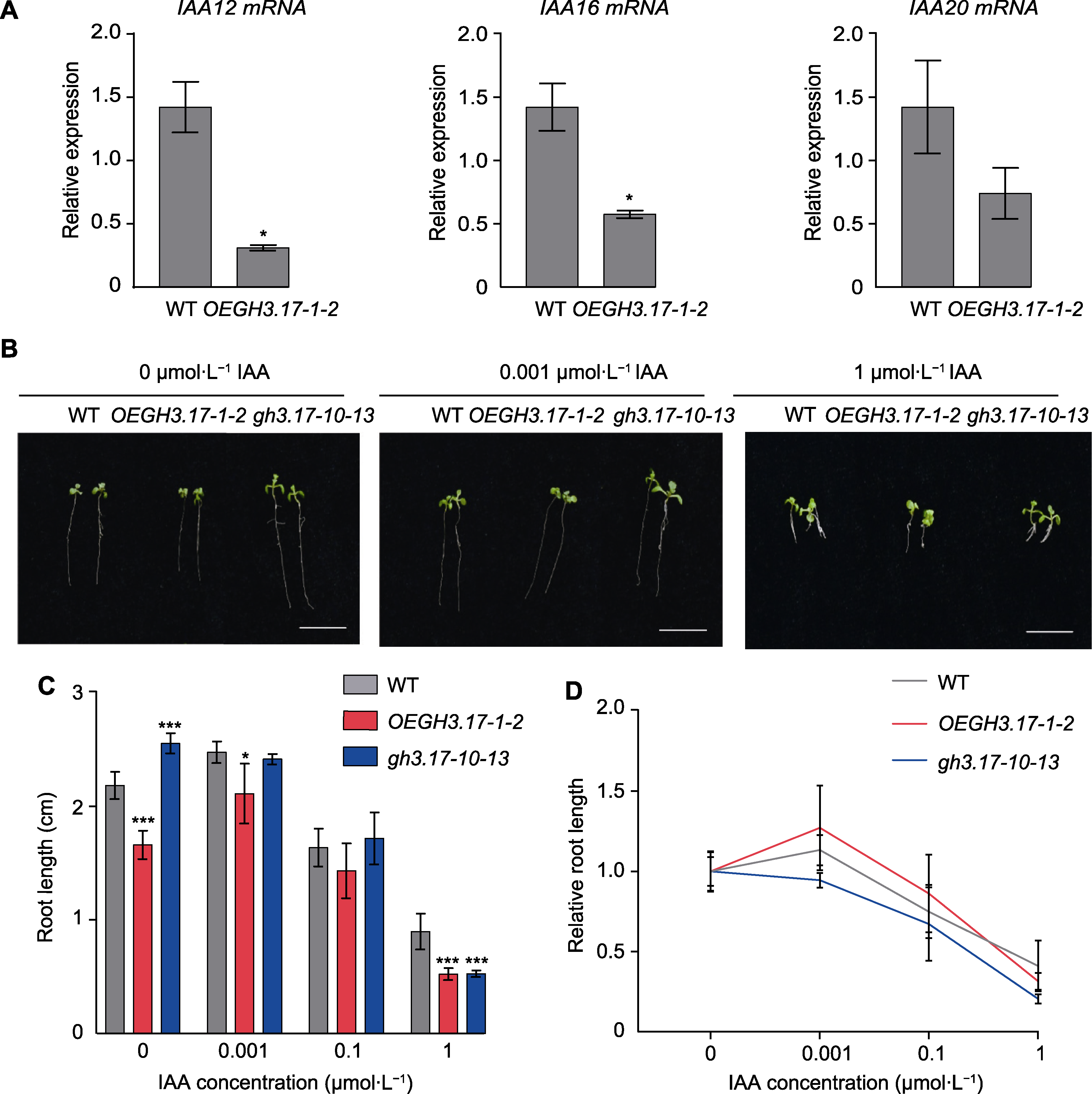
图4 拟南芥野生型(WT)、GH3.17 CRISPR突变体和GH3.17过表达植株对生长素的响应 (A) 野生型和GH3.17过表达植株OEGH3.17-1-2中IAA12、IAA16和IAA20 mRNA的相对表达量(数值为3次独立实验的平均值, 误差线代表标准误差); (B) 野生型、GH3.17 CRISPR突变体和过表达植株在含有不同浓度IAA的1/2MS固体培养基竖直生长7天的根长表型(bars=1 cm); (C) 野生型、GH3.17 CRISPR突变体和过表达植株在含有不同浓度IAA的1/2MS固体培养基竖直生长7天的根长统计分析(数值为3次独立实验的平均值(n=30), 误差线代表标准差); (D) 野生型、GH3.17 CRISPR突变体和过表达植株在不同浓度IAA生长条件下根的相对长度变化趋势(数值为3次独立实验的平均值(n=30), 误差线代表标准差)。*表示差异显著(P<0.05); ***表示差异十分显著(P<0.001) (Student’s t-test)。
Figure 4 Response of Arabidopsis thaliana wild type (WT), GH3.17 CRISPR mutant and overexpression seedlings to auxin (A) The relative expression level of IAA12, IAA16 and IAA20 in WT and OEGH3.17-1-2 overexpression plants (error bars represent SEM of three technical duplicates); (B) The root phenotype of WT, GH3.17 CRISPR mutant and overexpression seedling on 1/2MS medium with different concentrations of IAA for 7 days (bars=1 cm); (C) The statistical analysis of WT, GH3.17 CRISPR mutant and overexpression seedling root length grown on 1/2MS medium with different concentrations of IAA for 7 days (the data was obtained through three replicates of 30 seedlings each, error bars represent SD); (D) Root length curves of WT, GH3.17 CRISPR mutant and overexpression seedling showing their response to different concentrations of IAA (the data was obtained through three replicates of 30 seedlings each, error bars represent SD). * indicate significant differences at P<0.05; *** indicate significant differences at P<0.001 (Student’s t-test).
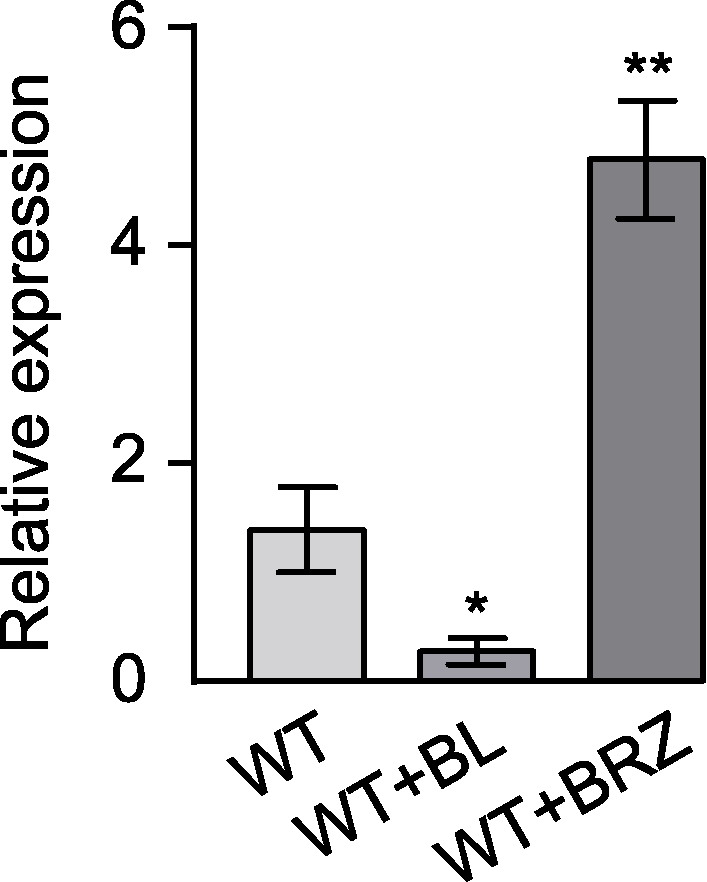
图5 油菜素内酯(BL)处理抑制拟南芥GH3.17基因的转录水平 数值为3次技术重复的平均值, 误差线代表标准误差。*表示差异显著(P<0.05); **表示差异极显著(P<0.01) (Student’s t-test)。WT: 野生型
Figure 5 The transcription level of Arabidopsis thaliana GH3.17 was inhibited by brassinolide (BL) treatment Error bars represent SEM of three technical duplicates. * indicates significant difference at P<0.05; ** indicates significant difference at P<0.01 (Student’s t-test). WT: Wild type
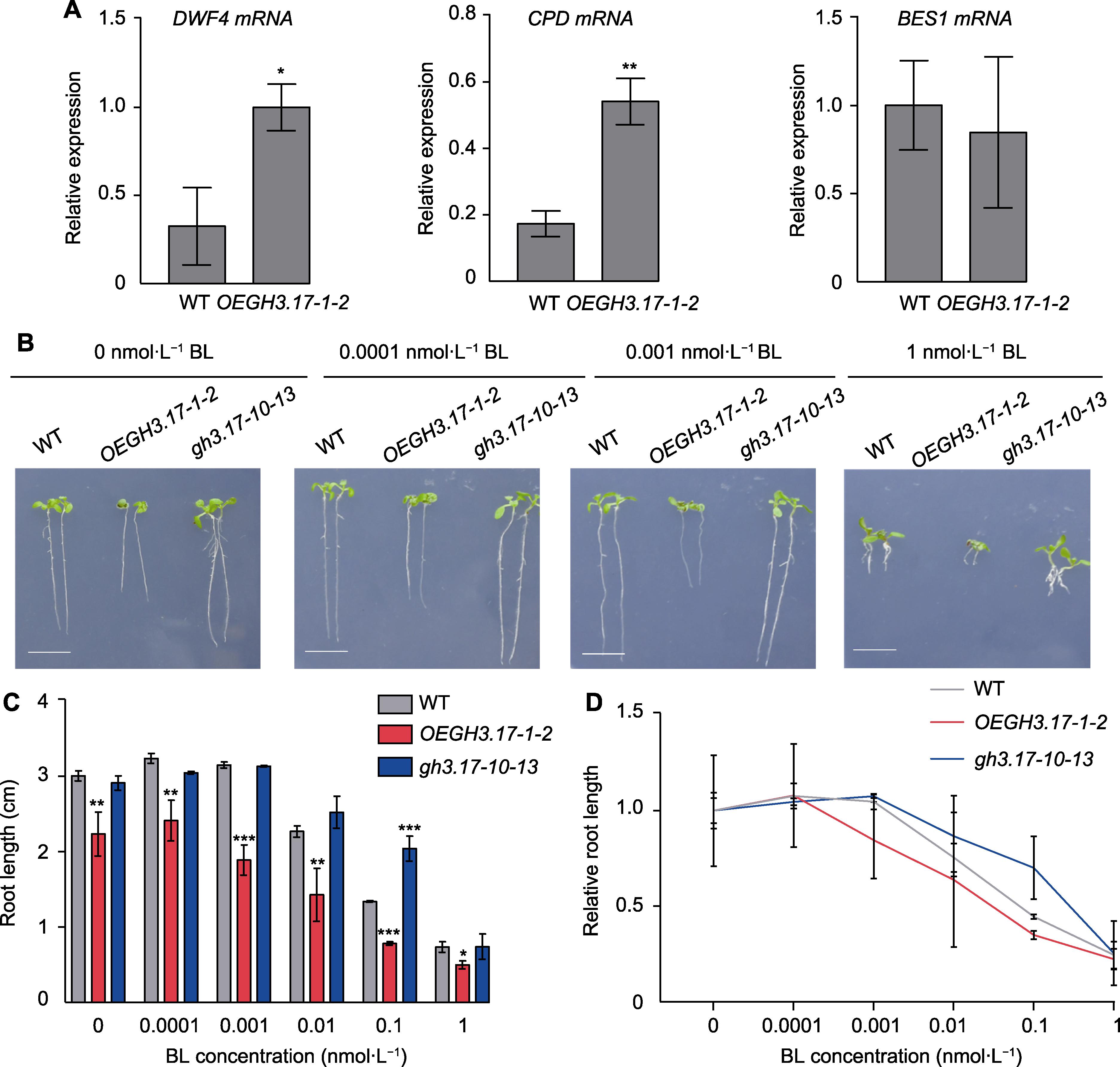
图6 拟南芥野生型(WT)、GH3.17过表达和CRISPR突变体株系对油菜素甾醇(BR)的响应 (A) qRT-PCR检测野生型和OEGH3.17-1-2过表达材料中DWF4、CPD和BES1的相对表达量(数值为3次独立实验的平均值, 误差线代表标准误差); (B) WT、OEGH3.17-1-2和gh3.17-10-13在含有不同浓度油菜素内酯(BL)的1/2MS固体培养基竖直生长9天的根长表型(bars=1 cm); (C) WT、OEGH3.17-1-2和gh3.17-10-13在含有不同浓度BL的1/2MS固体培养基竖直生长9天的根长统计分析(数值为3次独立实验的平均值(n=30), 误差线代表标准差); (D) WT、OEGH3.17-1-2和gh3.17-10-13在不同浓度梯度BL条件下的根系相对长度的变化趋势(数值为3次独立实验的平均值(n=30), 误差线代表标准差)。*表示差异显著(P<0.05); **表示差异极显著(P<0.01); ***表示差异十分显著(P<0.001) (Student’s t-test)。
Figure 6 Response of Arabidopsis thaliana wild type (WT), GH3.17 overexpression and CRISPR mutant seedlings to brassinosteroid (BR) (A) The relative expression level of DWF4, CPD and BES1 in WT and OEGH3.17-1-2 overexpression plants by qRT-PCR (error bars represent SEM of three technical duplicates); (B) The root phenotype of WT, OEGH3.17-1-2 and gh3.17-10-13 grown on 1/2MS solid medium with different concentrations of brassinolide (BL) for 9 days (bars=1 cm); (C) The statistical analysis of WT, OEGH3.17-1-2 and gh3.17-10-13 root length grown on 1/2MS solid medium with different concentrations of BL for 9 days (the data was obtained through three replicates of 30 seedlings each, error bars represent SD); (D) Root length curves of WT, OEGH3.17-1-2 and gh3.17-10-13 showing their response to different concentrations of BL (the data was obtained through three replicates of 30 seedlings each, error bars represent SD). * indicate significant differences at P<0.05; ** indicate significant differences at P<0.01; *** indicate significant differences at P<0.001 (Student’s t-test).
| [1] |
李艳艳, 齐艳华 (2022). 植物Aux/IAA基因家族生物学功能研究进展. 植物学报 57, 30-41.
DOI |
| [2] |
任鸿雁, 王莉, 马青秀, 吴光 (2015). 油菜素内酯生物合成途径的研究进展. 植物学报 50, 768-778.
DOI |
| [3] | 孙超, 黎家 (2017). 油菜素甾醇类激素的生物合成、代谢及信号转导. 植物生理学报 53, 291-307. |
| [4] | 王冰, 李家洋, 王永红 (2006). 生长素调控植物株型形成的研究进展. 植物学通报 23, 443-458. |
| [5] |
谢先荣, 曾栋昌, 谭健韬, 祝钦泷, 刘耀光 (2021). 基于CRISPR编辑系统的DNA片段删除技术. 植物学报 56, 44-49.
DOI |
| [6] |
Abel S, Theologis A (1996). Early genes and auxin action. Plant Physiol 111, 9-17.
DOI PMID |
| [7] |
Bao F, Shen JJ, Brady SR, Muday GK, Asami T, Yang ZB (2004). Brassinosteroids interact with auxin to promote lateral root development in Arabidopsis. Plant Physiol 134, 1624-1631.
DOI URL |
| [8] |
Chung Y, Maharjan PM, Lee O, Fujioka S, Jang S, Kim B, Takatsuto S, Tsujimoto M, Kim H, Cho S, Park T, Cho H, Hwang I, Choe S (2011). Auxin stimulates DWARF4 expression and brassinosteroid biosynthesis in Arabidopsis. Plant J 66, 564-578.
DOI URL |
| [9] |
Clouse SD, Sasse JM (1998). BRASSINOSTEROIDS: essential regulators of plant growth and development. Annu Rev Plant Physiol Plant Mol Biol 49, 427-451.
DOI URL |
| [10] | Di Mambro R, De Ruvo M, Pacifici E, Salvi E, Sozzani R, Benfey PN, Busch W, Novak O, Ljung K, Di Paola L, Marée AFM, Costantino P, Grieneisen VA, Sabatini S (2017). Auxin minimum triggers the developmental switch from cell division to cell differentiation in the Arabidopsis root. Proc Natl Acad Sci USA 114, E7641-E7649. |
| [11] |
Di Mambro R, Svolacchia N, Dello Ioio R, Pierdonati E, Salvi E, Pedrazzini E, Vitale A, Perilli S, Sozzani R, Benfey PN, Busch W, Costantino P, Sabatini S (2019). The lateral root cap acts as an auxin sink that controls meristem size. Curr Biol 29, 1199-1205.
DOI PMID |
| [12] |
Ding ZJ, Friml J (2010). Auxin regulates distal stem cell differentiation in Arabidopsis roots. Proc Natl Acad Sci USA 107, 12046-12051.
DOI URL |
| [13] | Du MM, Spalding EP, Gray WM (2020). Rapid auxin-mediated cell expansion. Annu Rev Plant Biol 71, 379-402. |
| [14] |
Favero DS, Le KN, Neff MM (2017). Brassinosteroid signaling converges with SUPPRESSOR OF PHYTOCHROME B4-#3 to influence the expression of SMALL AUXIN UP RNA genes and hypocotyl growth. Plant J 89, 1133-1145.
DOI URL |
| [15] |
Goda H, Sawa S, Asami T, Fujioka S, Shimada Y, Yoshida S (2004). Comprehensive comparison of auxin-regulated and brassinosteroid-regulated genes in Arabidopsis. Plant Physiol 134, 1555-1573.
DOI URL |
| [16] |
Hagen G, Guilfoyle T (2002). Auxin-responsive gene expression: genes, promoters and regulatory factors. Plant Mol Biol 49, 373-385.
PMID |
| [17] |
Hagen G, Guilfoyle TJ (1985). Rapid induction of selective transcription by auxins. Mol Cell Biol 5, 1197-1203.
DOI PMID |
| [18] |
Hirano K, Yoshida H, Aya K, Kawamura M, Hayashi M, Hobo T, Sato-Izawa K, Kitano H, Ueguchi-Tanaka M, Matsuoka M (2017). SMALL ORGAN SIZE 1 and SMALL ORGAN SIZE 2/DWARF AND LOW-TILLERING form a complex to integrate auxin and brassinosteroid signaling in rice. Mol Plant 10, 590-604.
DOI PMID |
| [19] |
Hsieh HL, Okamoto H, Wang ML, Ang LH, Matsui M, Goodman H, Deng XW (2000). FIN219, an auxin-regulated gene, defines a link between phytochrome A and the downstream regulator COP1 in light control of Arabidopsis development. Genes Dev 14, 1958-1970.
DOI URL |
| [20] |
Ibañes M, Fàbregas N, Chory J, Caño-Delgado AI (2009). Brassinosteroid signaling and auxin transport are required to establish the periodic pattern of Arabidopsis shoot vascular bundles. Proc Natl Acad Sci USA 106, 13630-13635.
DOI PMID |
| [21] |
Liu XL, Yang HX, Wang Y, Zhu ZH, Zhang W, Li JM (2020). Comparative transcriptomic analysis to identify b- rassinosteroid response genes. Plant Physiol 184, 1072-1082.
DOI URL |
| [22] |
Liu ZB, Ulmasov T, Shi X, Hagen G, Guilfoyle TJ (1994). Soybean GH3promoter contains multiple auxin-inducible elements. Plant Cell 6, 645-657.
PMID |
| [23] |
Luo J, Zhou JJ, Zhang JZ (2018). Aux/IAA gene family in plants: molecular structure, regulation, and function. Int J Mol Sci 19, 259.
DOI URL |
| [24] |
Mouchel CF, Osmont KS, Hardtke CS (2006). BRX mediates feedback between brassinosteroid levels and auxin signaling in root growth. Nature 443, 458-461.
DOI |
| [25] |
Nakamura A, Higuchi K, Goda H, Fujiwara MT, Sawa S, Koshiba T, Shimada Y, Yoshida S (2003). Brassinolide induces IAA5, IAA19, and DR5, a synthetic auxin response element in Arabidopsis, implying a cross talk point of brassinosteroid and auxin signaling. Plant Physiol 133, 1843-1853.
PMID |
| [26] |
Nakazawa M, Yabe N, Ichikawa T, Yamamoto YY, Yoshizumi T, Hasunuma K, Matsui M (2001). DFL1, an auxin-responsive GH3 gene homologue, negatively regulates shoot cell elongation and lateral root formation, and positively regulates the light response of hypocotyl length. Plant J 25, 213-221.
PMID |
| [27] |
Ohnishi T, Szatmari AM, Watanabe B, Fujita S, Bancos S, Koncz C, Lafos M, Shibata K, Yokota T, Sakata K, Szekeres M, Mizutani M (2006). C-23 hydroxylation by Arabidopsis CYP90C1 and CYP90D1 reveals a novel shortcut in brassinosteroid biosynthesis. Plant Cell 18, 3275-3288.
DOI URL |
| [28] |
Okushima Y, Overvoorde PJ, Arima K, Alonso JM, Chan A, Chang C, Ecker JR, Hughes B, Lui A, Nguyen D, Onodera C, Quach H, Smith A, Yu GX, Theologis A (2005). Functional genomic analysis of the AUXIN RESPONSE FACTOR gene family members in Arabidopsis thaliana: unique and overlapping functions of ARF7 and ARF19. Plant Cell 17, 444-463.
DOI PMID |
| [29] |
Parry G, Calderon-Villalobos LI, Prigge M, Peret B, Dharmasiri S, Itoh H, Lechner E, Gray WM, Bennett M, Estelle M (2009). Complex regulation of the TIR1/AFB family of auxin receptors. Proc Natl Acad Sci USA 106, 22540-22545.
DOI PMID |
| [30] |
Sakamoto T, Morinaka Y, Inukai Y, Kitano H, Fujioka S (2013). Auxin signal transcription factor regulates expression of the brassinosteroid receptor gene in rice. Plant J 73, 676-688.
DOI URL |
| [31] |
Staswick PE, Serban B, Rowe M, Tiryaki I, Maldonado MT, Maldonado MC, Suza W (2005). Characterization of an Arabidopsis enzyme family that conjugates amino acids to indole-3-acetic acid. Plant Cell 17, 616-627.
DOI PMID |
| [32] |
Suzuki H, Fujioka S, Takatsuto S, Yokota T, Murofushi N, Sakurai A (1994). Biosynthesis of brassinolide from teasterone via typhasterol and castasterone in cultured cells of Catharanthus roseus. J Plant Growth Regul 13, 21-26.
DOI URL |
| [33] |
Tan X, Calderon-Villalobos LIA, Sharon M, Zheng CX, Robinson CV, Estelle M, Zheng N (2007). Mechanism of auxin perception by the TIR1 ubiquitin ligase. Nature 446, 640-645.
DOI |
| [34] |
Ulmasov T, Hagen G, Guilfoyle TJ (1997a). ARF1, a transcription factor that binds to auxin response elements. Science 276, 1865-1868.
DOI URL |
| [35] |
Ulmasov T, Liu ZB, Hagen G, Guilfoyle TJ (1995). Composite structure of auxin response elements. Plant Cell 7, 1611-1623.
PMID |
| [36] | Ulmasov T, Murfett J, Hagen G, Guilfoyle TJ (1997b). Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell 9, 1963-1971. |
| [37] |
Wang ZY, Bai MY, Oh E, Zhu JY (2012). Brassinosteroid signaling network and regulation of photomorphogenesis. Annu Rev Genet 46, 701-724.
DOI URL |
| [38] |
Weijers D, Benkova E, Jäger KE, Schlereth A, Hamann T, Kientz M, Wilmoth JC, Reed JW, Jürgens G (2005). Developmental specificity of auxin response by pairs of ARF and Aux/IAA transcriptional regulators. EMBO J 24, 1874-1885.
DOI PMID |
| [39] |
Woodward AW, Bartel B (2005). Auxin: regulation, action, and interaction. Ann Bot 95, 707-735.
DOI URL |
| [40] | Yoshimitsu Y, Tanaka K, Fukuda W, Asami T, Yoshida S, Hayashi KI, Kamiya Y, Jikumaru Y, Shigeta T, Nakamura Y, Matsuo T, Okamoto S (2011). Transcription of DWARF4 plays a crucial role in auxin-regulated root elongation in addition to brassinosteroid homeostasis in Arabidopsis thaliana. PLoS One 6, e23851. |
| [41] |
Zhang C, Zhang L, Wang D, Ma H, Liu B, Shi Z, Ma X, Chen Y, Chen Q (2018). Evolutionary history of the Glycoside Hydrolase 3 (GH3) family based on the sequenced genomes of 48 plants and identification of jasmonic acid- related GH3 proteins in Solanum tuberosum. Int J Mol Sci 19, 1850.
DOI URL |
| [42] |
Zheng ZY, Guo YX, Novák O, Chen W, Ljung K, Noel JP, Chory J (2016). Local auxin metabolism regulates environment-induced hypocotyl elongation. Nat Plants 2, 16025.
DOI PMID |
| [1] | 周玉滢, 陈辉, 刘斯穆. 植物非典型Aux/IAA蛋白应答生长素研究进展[J]. 植物学报, 2024, 59(4): 651-658. |
| [2] | 孔祥培, 张蒙悦, 丁兆军. 柳暗花明:胞外生长素信号感受的新突破[J]. 植物学报, 2023, 58(6): 861-865. |
| [3] | 园园, 恩和巴雅尔, 齐艳华. 植物GH3基因家族生物学功能研究进展[J]. 植物学报, 2023, 58(5): 770-782. |
| [4] | 叶青, 闫晓燕, 陈慧泽, 冯金林, 韩榕. 氮掺杂石墨烯量子点对拟南芥主根生长方向的影响[J]. 植物学报, 2022, 57(5): 623-634. |
| [5] | 李彬琪, 闫佳慧, 李豪, 辛伟, 田云鹤, 杨贞标, 唐文鑫. 黄瓜卷须缠绕过程中小G蛋白活性变化[J]. 植物学报, 2022, 57(3): 299-307. |
| [6] | 贾利霞, 齐艳华. 生长素代谢、运输及信号转导调控水稻粒型研究进展[J]. 植物学报, 2022, 57(3): 263-275. |
| [7] | 李艳艳, 齐艳华. 植物Aux/IAA基因家族生物学功能研究进展[J]. 植物学报, 2022, 57(1): 30-41. |
| [8] | 王静文, 王兴军, 马长乐, 李膨呈. 植物核糖体应激响应机制研究进展[J]. 植物学报, 2022, 57(1): 80-89. |
| [9] | 林雨晴, 齐艳华. 生长素输出载体PIN家族研究进展[J]. 植物学报, 2021, 56(2): 151-165. |
| [10] | 黄荣峰, 徐通达. 生长素通过MAPK介导的超长链脂肪酸合成调控侧根发育[J]. 植物学报, 2021, 56(1): 6-9. |
| [11] | 姚玉婷,马家琦,冯晓莉,潘建伟,王超. 磷酸肌醇激酶FAB1调控拟南芥根毛伸长[J]. 植物学报, 2020, 55(2): 126-136. |
| [12] | 贺祯媚,李东明,齐艳华. 植物ABCB亚家族生物学功能研究进展[J]. 植物学报, 2019, 54(6): 688-698. |
| [13] | 张淑辉,王红,王文茹,吴雪莲,肖元松,彭福田. 蔗糖对桃幼苗生长发育及其SnRK1酶活性的影响[J]. 植物学报, 2019, 54(6): 744-752. |
| [14] | 胡孔琴, 丁兆军. 非TIR1受体依赖型激活生长素信号的新机制[J]. 植物学报, 2019, 54(3): 293-295. |
| [15] | 栗露露,殷文超,牛梅,孟文静,张晓星,童红宁. 油菜素甾醇调控水稻盐胁迫应答的作用研究[J]. 植物学报, 2019, 54(2): 185-193. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||