

植物学报 ›› 2023, Vol. 58 ›› Issue (1): 51-61.DOI: 10.11983/CBB22149 cstr: 32102.14.CBB22149
所属专题: 杂粮生物学专辑 (2023年58卷1期)
收稿日期:2022-07-12
接受日期:2023-02-09
出版日期:2023-01-01
发布日期:2023-02-10
通讯作者:
*E-mail: wujing@caas.cn
基金资助:
Yuan Li, Yujie Chang, Lanfen Wang, Shumin Wang, Jing Wu*( )
)
Received:2022-07-12
Accepted:2023-02-09
Online:2023-01-01
Published:2023-02-10
Contact:
*E-mail: wujing@caas.cn
摘要: 普通菜豆镰孢菌枯萎病是严重制约菜豆(Phaseolus vulgaris)产量的主要病害之一。采用下胚轴双孔注射法对601份普通菜豆种质资源进行枯萎病抗性鉴定, 共筛选出4份高抗材料。在此基础上, 基于分布在全基因组上的3 765 456个单核苷酸多态性(SNP)标记, 进行全基因组关联分析, 以P<1×10-5为阈值。结果检测到57个显著关联的SNP位点, 分布于1、2、6、8和11号染色体上; 共获得8个显著关联区域, 其中位于1号染色体上的区域1包含SNP最多(48个), 最显著SNP P值为2.18E-07。在8个显著关联区域中, 共检测到186个基因, 其中157个基因有注释信息, 编码过氧化物酶、抗病蛋白、转录因子和蛋白激酶等。结合KEGG富集分析和序列同源性比对, 鉴定出9个候选基因可能与抗性相关。
李园, 常玉洁, 王兰芬, 王述民, 武晶. 普通菜豆镰孢菌枯萎病抗性种质资源筛选及全基因组关联分析. 植物学报, 2023, 58(1): 51-61.
Yuan Li, Yujie Chang, Lanfen Wang, Shumin Wang, Jing Wu. Screening of Resistance Germplasm Resources and Genome-wide Association Study of Fusarium Wilt in Common Bean. Chinese Bulletin of Botany, 2023, 58(1): 51-61.

图1 普通菜豆不同抗性等级维管束病变程度 0、1、3、5、7和9代表病情分级标准。Bar=4 cm
Figure 1 Common bean vascular bundle lesions of different grades 0, 1, 3, 5, 7 and 9 represent the criteria for disease classification. Bar=4 cm
| Rating scale | System description |
|---|---|
| 0 | No symptom |
| 1 | <20% lesions or discoloration of hypocotyledonary axis |
| 3 | 20%-50% lesions or some discoloration of hypocotyledonary axis |
| 5 | 50%-100% lesions or heavy discoloration of hypocotyledonary axis |
| 7 | >100% lesions or severe discoloration of hypo- cotyledonary axis |
| 9 | Plants wilted and died |
表1 下胚轴双孔注射法接种普通菜豆枯萎病的病情分级标准
Table 1 Disease grading standard of common bean fusarium wilt inoculated by hypocotyl double-hole injection
| Rating scale | System description |
|---|---|
| 0 | No symptom |
| 1 | <20% lesions or discoloration of hypocotyledonary axis |
| 3 | 20%-50% lesions or some discoloration of hypocotyledonary axis |
| 5 | 50%-100% lesions or heavy discoloration of hypocotyledonary axis |
| 7 | >100% lesions or severe discoloration of hypo- cotyledonary axis |
| 9 | Plants wilted and died |
| Mean | Max | Min | Standard deviation | Skewness | Kurtosis |
|---|---|---|---|---|---|
| 0.65 | 1.00 | 0.18 | 0.19 | -0.10 | -0.62 |
表2 普通菜豆自然群体枯萎病抗性水平表型描述
Table 2 Phenotype description of fusarium wilt resistance levels in natural populations of common bean
| Mean | Max | Min | Standard deviation | Skewness | Kurtosis |
|---|---|---|---|---|---|
| 0.65 | 1.00 | 0.18 | 0.19 | -0.10 | -0.62 |
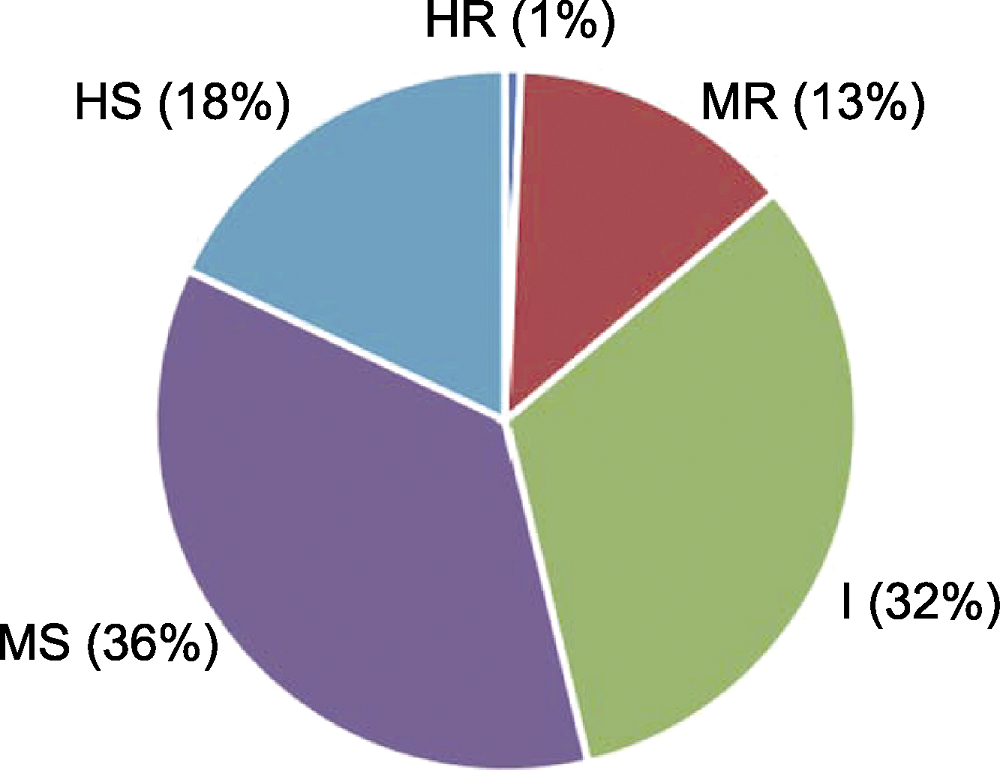
图3 普通菜豆种质资源枯萎病抗性等级分布 HR: 高抗; MR: 中抗; I: 中间型; MS: 中感; HS: 高感
Figure 3 Distribution of common bean germplasm resources for resistance to fusarium wilt HR: Highly resistant; MR: Medium resistant; I: Intermediate; MS: Medium susceptible; HS: Highly susceptible
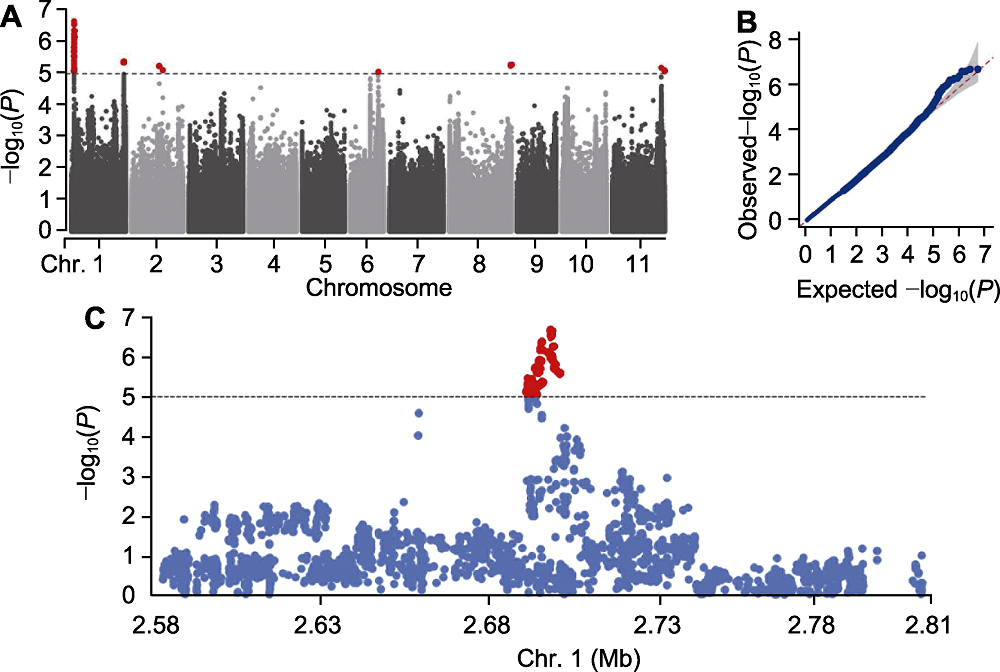
图4 普通菜豆枯萎病全基因组关联分析 (A) 曼哈顿图; (B) QQ图; (C) 区段1的局部曼哈顿图
Figure 4 Genome-wide association study of fusarium wilt in common bean (A) Manhattan plots; (B) QQ plots; (C) Local Manhattan plot of the Region 1
| Region | Chr. | Interval locationa | P-value |
|---|---|---|---|
| 1 | 1 | 2583702-2807552 | 2.18E-07 |
| 2 | 1 | 50017907-50231907 | 4.11E-06 |
| 3 | 2 | 26253984-26467984 | 5.72E-06 |
| 4 | 2 | 29642890-29856890 | 7.61E-06 |
| 5 | 6 | 26400983-26614983 | 8.82E-06 |
| 6 | 8 | 58863473-59077488 | 5.40E-06 |
| 7 | 11 | 46491343-46705343 | 6.48E-06 |
| 8 | 11 | 49598097-49814082 | 7.73E-06 |
表3 普通菜豆枯萎病全基因组关联分析位点
Table 3 Loci related with common bean fusarium wilt by genome-wide association study
| Region | Chr. | Interval locationa | P-value |
|---|---|---|---|
| 1 | 1 | 2583702-2807552 | 2.18E-07 |
| 2 | 1 | 50017907-50231907 | 4.11E-06 |
| 3 | 2 | 26253984-26467984 | 5.72E-06 |
| 4 | 2 | 29642890-29856890 | 7.61E-06 |
| 5 | 6 | 26400983-26614983 | 8.82E-06 |
| 6 | 8 | 58863473-59077488 | 5.40E-06 |
| 7 | 11 | 46491343-46705343 | 6.48E-06 |
| 8 | 11 | 49598097-49814082 | 7.73E-06 |
| Gene ID | Chr. | Start base-end basea (bp) | Predicted function |
|---|---|---|---|
| Phvul.001G240400 | 1 | 50026833-50031313 | RNI-like superfamily protein |
| Phvul.001G241500 | 1 | 50126351-50130393 | Transmembrane amino acid transporter family protein |
| Phvul.001G242200 | 1 | 50180058-50185697 | Cytochrome P450 superfamily protein |
| Phvul.002G133400 | 2 | 26437427-26440702 | NB-ARC domain-containing disease resistance protein |
| Phvul.002G133600 | 2 | 26447784-26451915 | NB-ARC domain-containing disease resistance protein |
| Phvul.002G154800 | 2 | 29644994-29651836 | PAS domain-containing protein tyrosine kinase family protein |
| Phvul.006G150900 | 6 | 26403392-26405713 | Protein kinase superfamily protein |
| Phvul.008G285400 | 8 | 59055451-59060614 | Heat shock protein 89.1 |
| Phvul.011G212100 | 11 | 49695779-49697294 | Leucine-rich repeat (LRR) family protein |
表4 候选基因及注释
Table 4 Candidate genes and annotation
| Gene ID | Chr. | Start base-end basea (bp) | Predicted function |
|---|---|---|---|
| Phvul.001G240400 | 1 | 50026833-50031313 | RNI-like superfamily protein |
| Phvul.001G241500 | 1 | 50126351-50130393 | Transmembrane amino acid transporter family protein |
| Phvul.001G242200 | 1 | 50180058-50185697 | Cytochrome P450 superfamily protein |
| Phvul.002G133400 | 2 | 26437427-26440702 | NB-ARC domain-containing disease resistance protein |
| Phvul.002G133600 | 2 | 26447784-26451915 | NB-ARC domain-containing disease resistance protein |
| Phvul.002G154800 | 2 | 29644994-29651836 | PAS domain-containing protein tyrosine kinase family protein |
| Phvul.006G150900 | 6 | 26403392-26405713 | Protein kinase superfamily protein |
| Phvul.008G285400 | 8 | 59055451-59060614 | Heat shock protein 89.1 |
| Phvul.011G212100 | 11 | 49695779-49697294 | Leucine-rich repeat (LRR) family protein |
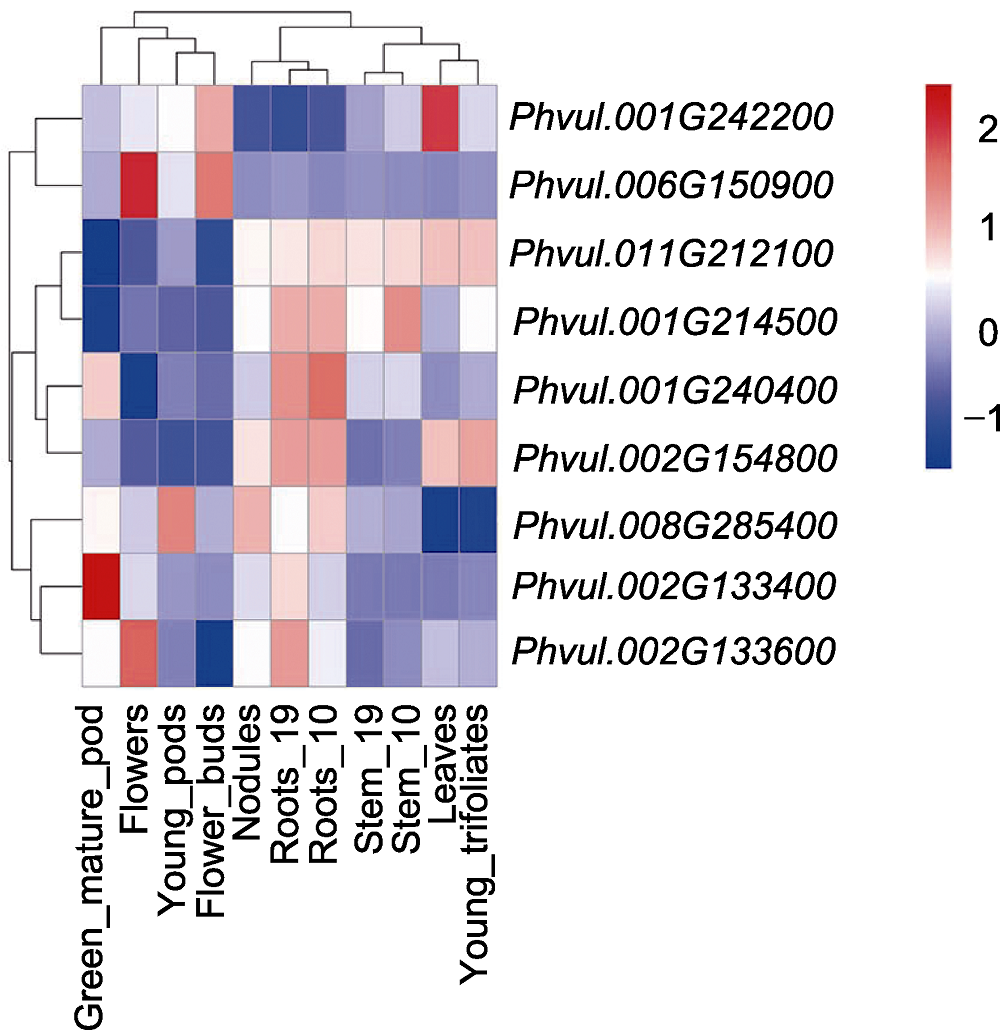
图5 9个抗性候选基因在普通菜豆不同组织中的表达模式 Stem_10为播种10天后于茎部取样, Stem_19为播种19天后于茎部取样, Roots_10为播种10天后于根部取样, Roots_19为播种19天后于根部取样。数据来源于 https://phytozome-next.jgi.doe.gov/info/Pvulgaris_v2_1。
Figure 5 Expression patterns of 9 resistance candidate ge- nes in common bean different tissues Stem_10 is to take samples from the stem 10 days after planting, Stem_19 is to take samples from the stem 19 days after planting, Roots_10 is to take samples from the root 10 days after planting, Roots_19 is to take samples from the root 19 days after planting. Data is from https://phytozome-next.jgi.doe.gov/info/Pvulgaris_v2_1.
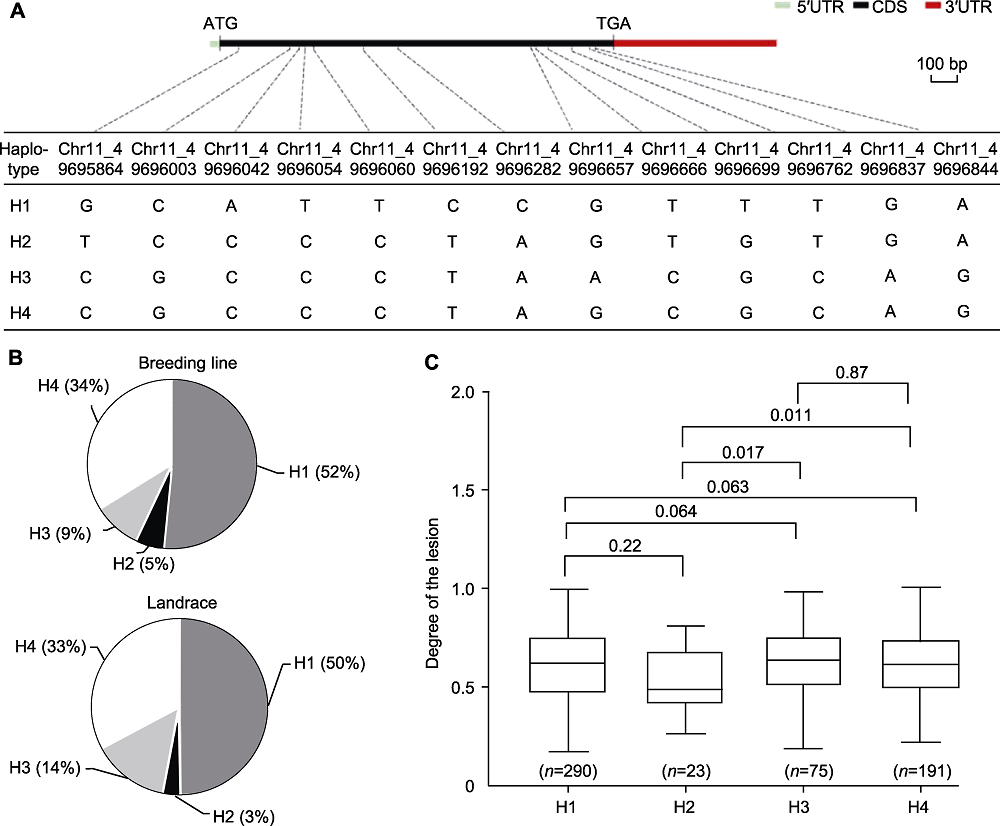
图6 Phvul.001G212100单倍型分析 (A) Phvul.001G212100基因结构和单倍型(线性基因结构显示5'UTR (青色矩形)、CDS区域(黑色矩形)和3'UTR (红色矩形); 黑色虚线表示SNP在基因中的位置); (B) 4个单倍型在育成品种和地方品种中的发生频率; (C) 单倍型间病变程度的差异显著性分析(箱线图上方数字为P-value值, P<0.05表示差异显著。n为种质资源份数。用双尾T检验进行显著性检验)。CDS: 编码序列; UTR: 非翻译区
Figure 6 Haplotype analysis of Phvul.001G212100 (A) The genome structure and haplotypes of the Phvul.001G212100 (the linear gene structure showing 5'UTR (cyan rectangle), CDS region (black rectangle), 3'UTR (red rectangle); the black dotted line indicate the position of the SNP in the gene); (B) Occurrence frequencies of 4 haplotypes in breeding lines and landraces; (C) Significant differences in the degree of lesions between haplotypes (the numbers above the boxplot are P-values, P<0.05 indicate significant differences. n represent the number of germplasm recourses. The significance was tested with two-tailed T-tests). CDS: Coding sequence; UTR: Untranslated region
| [1] | 程须珍 (2016). 普通菜豆生产技术. 北京: 北京教育出版社. pp. 83-84. |
| [2] | 薛仁风 (2012). 普通菜豆镰孢菌枯萎病抗病种质鉴定及抗病机理研究. 博士论文. 北京: 中国农业科学院. pp. 37-40. |
| [3] | 薛仁风, 丰明, 赵阳, 陈剑, 李韬, 葛维德 (2019). 普通菜豆生长素调节蛋白基因PvARP1的克隆及表达分析. 河南农业科学 48(9), 82-89. |
| [4] | 薛仁风, 朱振东, 王晓鸣, 王兰芬, 武小菲, 王述民 (2012). 普通菜豆镰孢菌枯萎病抗病相关基因PvCaM1的克隆及表达. 作物学报 38, 606-613. |
| [5] |
Alves-Santos FM, Cordeiro-Rodrigues L, Sayagués JM, Martín-Domínguez R, García-Benavides P, Crespo MC, Díaz-Mínguez JM, Eslava AP (2002). Pathogenicity and race characterization of Fusarium oxysporum f. sp. phaseoli isolates from Spain and Greece. Plant Pathol 51, 605-611.
DOI URL |
| [6] |
Bancoş S, Nomura T, Sato T, Molnár G, Bishop GJ, Koncz C, Yokota T, Nagy F, Szekeres M (2002). Regulation of transcript levels of the Arabidopsis cytochrome p450 genes involved in brassinosteroid biosynthesis. Plant Physiol 130, 504-513.
DOI URL |
| [7] |
Batista RO, Silva LC, Moura LM, Souza MH, Carneiro PCS, Filho JLSC, de Souza Carneiro JE (2017). Inheritance of resistance to fusarium wilt in common bean. Euphytica 213, 133.
DOI |
| [8] |
Belkhadir Y, Subramaniam R, Dangl JL (2004). Plant disease resistance protein signaling: NBS-LRR proteins and their partners. Curr Opin Plant Biol 7, 391-399.
DOI PMID |
| [9] |
Bent AF, Kunkel BN, Dahlbeck D, Brown KL, Schmidt R, Giraudat J, Leung J, Staskawicz BJ (1994). RPS2 of Arabidopsis thaliana: a leucine-rich repeat class of plant disease resistance genes. Science 265, 1856-1860.
DOI PMID |
| [10] |
Bolwell GP, Bozak K, Zimmerlin A (1994). Plant cytochrome P450. Phytochemistry 37, 1491-1506.
DOI PMID |
| [11] |
Boyes DC, Nam J, Dangl JL (1998). The Arabidopsis thaliana RPM1 disease resistance gene product is a peripheral plasma membrane protein that is degraded coincident with the hypersensitive response. Proc Natl Acad Sci USA 95, 15849-15854.
DOI PMID |
| [12] | Brick MA, Ogg JB, Schwartz HF, Byrne PF, Kelly JD (2006). Resistance to multiple races of Fusarium oxysporum f. sp. phaseoli in common bean. Annu Rep Bean Improv Coop 47, 131-132. |
| [13] |
Chen YC, Wong CL, Muzzi F, Vlaardingerbroek I, Kidd BN, Schenk PM (2014). Root defense analysis against Fusarium oxysporum reveals new regulators to confer resistance. Sci Rep 4, 5584.
DOI |
| [14] |
Cross H, Brick MA, Schwartz HF, Panella LW, Byrne PF (2000). Inheritance of resistance to Fusarium wilt in two common bean races. Crop Sci 40, 954-958.
DOI URL |
| [15] |
Deng Y, Chen H, Zhang C, Cai T, Zhang B, Zhou S, Fountain JC, Pan RL, Guo B, Zhuang WJ (2018). Evolution and characterisation of the AhRAF4 NB-ARC gene family induced by Aspergillus flavus inoculation and abiotic stresses in peanut. Plant Biol 20, 737-750.
DOI URL |
| [16] |
Fall AL, Byrne PF, Jung G, Coyne DP, Brick MA, Schwartz HF (2001). Detection and mapping of a major locus for Fusarium wilt resistance in common bean. Crop Sci 41, 1494-1498.
DOI URL |
| [17] |
Gao ZY, Chen YF, Randlett MD, Zhao XC, Findell JL, Kieber JJ, Schaller GE (2003). Localization of the Raf-like kinase CTR1 to the endoplasmic reticulum of Arabidopsis through participation in ethylene receptor signaling complexes. J Biol Chem 278, 34725-34732.
DOI URL |
| [18] |
Grant MR, Godiard L, Straube E, Ashfield T, Lewald J, Sattler A, Innes RW, Dangl JL (1995). Structure of the Arabidopsis RPM1 gene enabling dual specificity disease resistance. Science 269, 843-846.
DOI PMID |
| [19] | Harter LL (1929). A Fusarium disease of beans. Phytopathology 19, 82. |
| [20] |
Huang Y, Li H, Hutchison CE, Laskey J, Kieber JJ (2003). Biochemical and functional analysis of CTR1, a protein kinase that negatively regulates ethylene signaling in Arabidopsis. Plant J 33, 221-233.
DOI URL |
| [21] |
Kieber JJ, Rothenberg M, Roman G, Feldmann KA, Ecker JR (1993). CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the Raf family of protein kinases. Cell 72, 427-441.
DOI PMID |
| [22] | Leitão ST, Malosetti M, Song QJ, van Eeuwijk F, Rubiales D, Vaz Patto MC (2020). Natural variation in portuguese common bean germplasm reveals new sources of resistance against Fusarium oxysporum f. sp. phaseoli and resistance-associated candidate genes. Phytopath-ology 110, 633-647. |
| [23] |
Li YX, Wei KF (2020). Comparative functional genomics analysis of cytochrome P450 gene superfamily in wheat and maize. BMC Plant Biol 20, 93.
DOI PMID |
| [24] |
Manzo D, Ferriello F, Puopolo G, Zoina A, D'Esposito D, Tardella L, Ferrarini A, Ercolano MR (2016). Fusarium oxysporum f. sp. radicis-lycopersici induces distinct transcriptome reprogramming in resistant and susceptible isogenic tomato lines. BMC Plant Biol 16, 53.
DOI URL |
| [25] | Martin GB, Bogdanove AJ, Sessa G (2003). Understanding the functions of plant disease resistance proteins. Annu Rev Plant Biol 54, 23-61. |
| [26] |
McCarthy RL, Zhong RQ, Ye ZH (2009). MYB83 is a direct target of SND1 and acts redundantly with MYB46 in the regulation of secondary cell wall biosynthesis in Arabidopsis. Plant Cell Physiol 50, 1950-1964.
DOI PMID |
| [27] |
Mindrinos M, Katagiri F, Yu GL, Ausubel FM (1994). The A. thaliana disease resistance gene RPS2 encodes a protein containing a nucleotide-binding site and leucine- rich repeats. Cell 78, 1089-1099.
DOI PMID |
| [28] |
Mittler R, Vanderauwera S, Gollery M, van Breusegem F (2004). Reactive oxygen gene network of plants. Trends Plant Sci 9, 490-498.
DOI PMID |
| [29] |
Nakashita H, Yasuda M, Nitta T, Asami T, Fujioka S, Arai Y, Sekimata K, Takatsuto S, Yamaguchi I, Yoshida S (2003). Brassinosteroid functions in a broad range of disease resistance in tobacco and rice. Plant J 33, 887-898.
DOI PMID |
| [30] |
Nakayama N, Takemae A, Shoun H (1996). Cytochrome P450foxy, a catalytically self-sufficient fatty acid hydroxylase of the fungus Fusarium oxysporum. J Biochem 119, 435-440.
PMID |
| [31] |
Nie YB, Ji WQ (2019). Cloning and characterization of disease resistance protein RPM1 genes against powdery mildew in wheat line N9134. Cereal Res Commun 47, 473-483.
DOI |
| [32] |
Ribeiro RLD, Hagedorn DJ (1979). Inheritance and nature of resistance in beans to Fusarium oxysporum f. sp. phaseoli. Phytopathology 69, 859-861.
DOI URL |
| [33] |
Salgado MO, Schwartz HF, Brick MA (1995). Inheritance of resistance to a Colorado race of Fusarium oxysporum f. sp. phaseoli in common beans. Plant Dis 79, 279-281.
DOI URL |
| [34] |
Schmutz J, McClean PE, Mamidi S, Wu GA, Cannon SB, Grimwood J, Jenkins J, Shu SQ, Song QJ, Chavarro C, Torres-Torres M, Geffroy V, Moghaddam SM, Gao D, Abernathy B, Barry K, Blair M, Brick MA, Chovatia M, Gepts P, Goodstein DM, Gonzales M, Hellsten U, Hyten DL, Jia GF, Kelly JD, Kudrna D, Lee R, Richard MMS, Miklas PN, Osorno JM, Rodrigues J, Thareau V, Urrea CA, Wang M, Yu Y, Zhang M, Wing RA, Cregan PB, Rokhsar DS, Jackson SA (2014). A reference genome for common bean and genome-wide analysis of dual domestications. Nat Genet 46, 707-713.
DOI PMID |
| [35] |
Sun JH, Huang GZ, Fan FG, Wang SF, Zhang YY, Han YF, Zou YM, Lu DP (2017). Comparative study of Arabidopsis PBS1 and a wheat PBS1 homolog helps understand the mechanism of PBS1 functioning in innate immunity. Sci Rep 7, 5487.
DOI |
| [36] |
Swiderski MR, Innes RW (2001). The Arabidopsis PBS1 resistance gene encodes a member of a novel protein kinase subfamily. Plant J 26, 101-112.
DOI PMID |
| [37] |
Wang AJ, Shu XY, Jing X, Jiao CZ, Chen L, Zhang JF, Ma L, Jiang YQ, Yamamoto N, Li SC, Deng QM, Wang SQ, Zhu J, Liang YY, Zou T, Liu HN, Wang LZ, Huang YB, Li P, Zheng AP (2021). Identification of rice (Oryza sativa L.) genes involved in sheath blight resistance via a genome- wide association study. Plant Biotechnol J 19, 1553-1566.
DOI URL |
| [38] |
Whitham S, Dinesh-Kumar SP, Choi D, Hehl R, Corr C, Baker B (1994). The product of the tobacco mosaic virus resistance gene N: similarity to toll and the interleukin-1 receptor. Cell 78, 1101-1115.
DOI PMID |
| [39] |
Woo SL, Zoina A, Del Sorbo G, Lorito M, Nanni B, Scala F, Noviello C (1996). Characterization of Fusarium oxysporum f. sp. phaseoli by pathogenic races, VCGs, RFLPs, and RAPD. Phytopathology 86, 966-973.
DOI URL |
| [40] |
Wu J, Wang LF, Fu JJ, Chen JB, Wei SH, Zhang SL, Zhang J, Tang YS, Chen ML, Zhu JF, Lei L, Geng QH, Liu Cl, Wu L, Li XM, Wang Xl, Wang Q, Wang Zl, Xing Sl, Zhang HK, Blair MW, Wang SM (2020). Resequencing of 683 common bean genotypes identifies yield component trait associations across a north-south cline. Nat Genet 52, 118-125.
DOI PMID |
| [41] |
Xue RF, Wu J, Wang LF, Blair MW, Wang XM, Ge WD, Zhu Z, Wang SM (2014). Salicylic acid enhances resistance to Fusarium oxysporum f. sp. phaseoli in common beans (Phaseolus vulgaris L.). J Plant Growth Regul 33, 470-476.
DOI URL |
| [42] | Xue RF, Wu J, Zhu ZD, Wang LF, Wang XM, Wang SM, Blair MW (2015). Differentially expressed genes in resistant and susceptible common bean (Phaseolus vulgaris L.) genotypes in response to Fusarium oxysporum f. sp. phaseoli. PLoS One 10, e0127698. |
| [43] |
Xue RF, Wu XB, Wang YJ, Zhuang Y, Chen J, Wu J, Ge WD, Wang LF, Wang SM, Blair MW (2017). Hairy root transgene expression analysis of a secretory peroxidase (PvPOX1) from common bean infected by Fusarium wilt. Plant Sci 260, 1-7.
DOI PMID |
| [44] |
Yang DL, Yang YN, He ZH (2013). Roles of plant hormones and their interplay in rice immunity. Mol Plant 6, 675-685.
DOI URL |
| [45] |
Yuan SK, Zhou MG (2005). A major gene for resistance to carbendazim, in field isolates of Gibberella zeae. Can J Plant Pathol 27, 58-63.
DOI URL |
| [46] |
Zhong RQ, Ye ZH (2012). MYB46 and MYB83 bind to the SMRE sites and directly activate a suite of transcription factors and secondary wall biosynthetic genes. Plant Cell Physiol 53, 368-380.
DOI PMID |
| [1] | 崔娟, 于晓玉, 于跃娇, 梁铖玮, 孙健, 陈温福. 影响中国东北和日本粳稻食味品质差异的质构因素及其遗传基础解析[J]. 植物学报, 2025, 60(4): 1-0. |
| [2] | 武棒棒, 郝宇琼, 杨淑斌, 黄雨茜, 关攀锋, 郑兴卫, 赵佳佳, 乔玲, 李晓华, 刘维仲, 郑军. 山西小麦籽粒叶黄素含量变异及遗传特性分析[J]. 植物学报, 2023, 58(4): 535-547. |
| [3] | 张琦, 张文静, 袁宪凯, 李明, 赵强, 杜艳丽, 杜吉到. 褪黑素对盐胁迫下普通菜豆芽期核酸修复的调控机制[J]. 植物学报, 2023, 58(1): 108-121. |
| [4] | 李晓明, 王兰芬, 唐永生, 常玉洁, 张菊香, 王述民, 武晶. 普通菜豆抗菜豆象性状的全基因组关联分析[J]. 植物学报, 2023, 58(1): 77-89. |
| [5] | 金京波, 梁承志. 饲草基因组学研究进展[J]. 植物学报, 2022, 57(6): 732-741. |
| [6] | 宣伟, 徐国华. 植物适应土壤氮素环境的基因选择: 以水稻为例[J]. 植物学报, 2021, 56(1): 1-5. |
| [7] | 赵宇慧, 李秀秀, 陈倬, 鲁宏伟, 刘羽诚, 张志方, 梁承志. 生物信息学分析方法I: 全基因组关联分析概述[J]. 植物学报, 2020, 55(6): 715-732. |
| [8] | 汪鸿儒, 储成才. 组学技术揭示水稻杂种优势遗传机制[J]. 植物学报, 2017, 52(1): 4-9. |
| [9] | 杨行海, 农保选, 夏秀忠, 张宗琼, 曾宇, 刘开强, 邓国富, 李丹婷. 水稻糯性相关基因的全基因组关联分析[J]. 植物学报, 2016, 51(6): 737-742. |
| [10] | 厉新民, 林鸿宣. 全基因组关联分析实现水稻粒型自然变异的分子解析[J]. 植物学报, 2016, 51(4): 411-415. |
| [11] | 严玫, 张新友, 韩锁义, 黄冰艳, 董文召, 刘华, 孙子淇, 张忠信, 汤丰收. 花生重要农艺及产量性状的全基因组关联分析[J]. 植物学报, 2015, 50(4): 460-472. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||