

植物学报 ›› 2023, Vol. 58 ›› Issue (5): 750-759.DOI: 10.11983/CBB22106 cstr: 32102.14.CBB22106
收稿日期:2022-05-24
接受日期:2022-11-09
出版日期:2023-09-01
发布日期:2023-09-21
通讯作者:
*E-mail: yubo423@163.com
基金资助:
Liu Xiaofei, Sun Yingbo, Huang Lili, Yang Yuchai, Zhu Genfa, Yu Bo( )
)
Received:2022-05-24
Accepted:2022-11-09
Online:2023-09-01
Published:2023-09-21
Contact:
*E-mail: yubo423@163.com
摘要: 该研究建立了黑鹅绒海芋(Alocasia reginula)体细胞胚胎发生途径的植株再生体系。通过叶柄诱导获得胚性愈伤组织, 以此建立胚性细胞悬浮培养系, 并实现了高频率的植株再生。叶柄外植体在添加2.0 mg·L-1 2,4-D和1.0 mg·L-1 TDZ的MS培养基上诱导胚性愈伤组织的效率最高(达81.3%)。将胚性愈伤组织破碎成细胞团, 转移至添加2.0 mg·L-1 2,4-D和1.0 mg·L-1 TDZ的MS液体培养基中进行悬浮培养, 2周继代1次, 悬浮培养12周后获得大量细胞团。将悬浮培养28周的细胞团转移至不含植物生长调节剂的1/2MS固体培养基上进行分化培养, 平均每个细胞团可再生2.3-2.5棵植株。 利用光学显微镜和扫描电镜观察体细胞胚的萌发和形成。再生苗移栽至温室4个月后, 成活率为95.3%。通过流式细胞术对随机选取的50株成活植株进行检测, 未发现染色体倍性变异, 核DNA含量为10.94 pg·(2C)-1, 基因组大小为5 290.12 Mb·C-1。植株移栽到温室直至自然开花, 表型无明显变异。研究结果可为黑鹅绒海芋种苗商业化生产和生物技术育种提供良好的技术支持。
刘小飞, 孙映波, 黄丽丽, 杨钰钗, 朱根发, 于波. 黑鹅绒海芋体细胞胚发生和植株再生. 植物学报, 2023, 58(5): 750-759.
Liu Xiaofei, Sun Yingbo, Huang Lili, Yang Yuchai, Zhu Genfa, Yu Bo. Efficient Plant Regeneration via Somatic Embryogenesis in Alocasia reginula cv. ‘Black Velvet’. Chinese Bulletin of Botany, 2023, 58(5): 750-759.
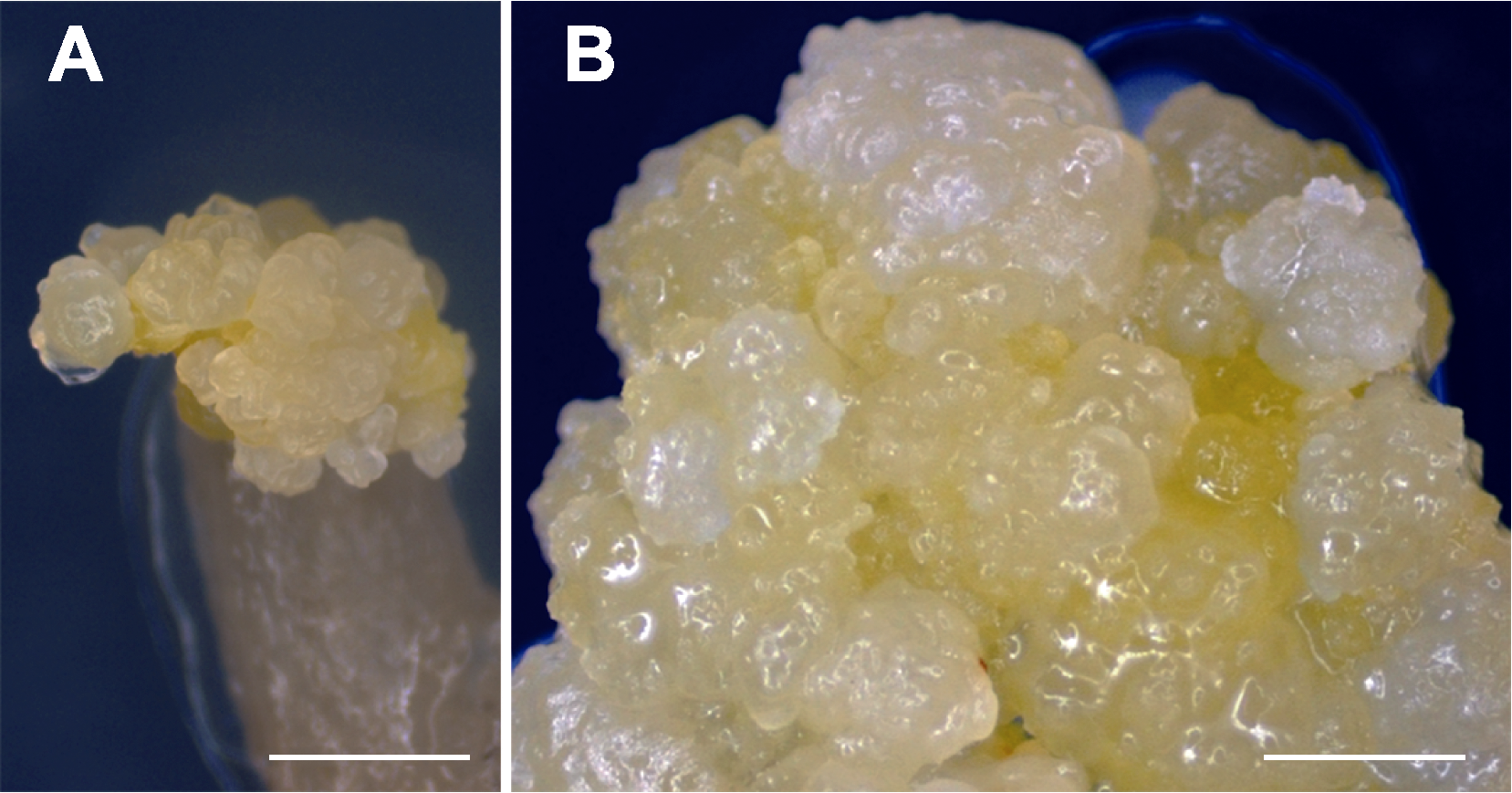
图1 黑鹅绒海芋胚性愈伤组织的诱导 (A) 培养8周后, 在MS+2.0 mg·L-1 2,4-D+1.0 mg·L-1 TDZ固体培养基上, 叶柄外植体的伤口处产生胚性愈伤组织(bar=3 mm); (B) 愈伤组织呈淡黄色致密颗粒(bar=2 mm)。
Figure 1 Induction of embryogenic calli from Alocasia reginula (A) Embryogenic calli at the wound site of petiole explants on MS solid medium containing 2.0 mg·L-1 2,4-D and 1.0 mg·L-1 TDZ after 8 weeks of incubation (bar=3 mm); (B) Calli were visible as light yellow dense grains (bar=2 mm).
| 2,4-D (mg·L-1) | TDZ (mg·L-1) | ||||
|---|---|---|---|---|---|
| 0 | 0.5 | 1.0 | 2.0 | 4.0 | |
| 0 | 0 | 0 | 0 | 0 | 0 |
| 0.5 | 0 | 14.7±2.3 f | 45.3±6.1 c | 38.7±4.6 cd | 12.0±4.0 f |
| 1.0 | 0 | 37.3±2.3 d | 58.7±2.3 b | 32.0±4.0 d | 10.7±4.6 fg |
| 2.0 | 0 | 38.7±4.6 cd | 81.3±6.1 a | 22.7±4.6 e | 4.0±4.0 gh |
| 4.0 | 0 | 10.6±4.6 fg | 33.3±6.1 d | 13.3±2.3 f | 2.7±2.3 h |
表1 黑鹅绒海芋叶柄片段在添加2,4-D和TDZ的MS培养基上培养8周后胚性愈伤组织的百分率
Table 1 Percentage of embryogenic calli formed on MS medium with 2,4-D and TDZ from petiole segments of Alocasia reginula after 8 weeks of culture
| 2,4-D (mg·L-1) | TDZ (mg·L-1) | ||||
|---|---|---|---|---|---|
| 0 | 0.5 | 1.0 | 2.0 | 4.0 | |
| 0 | 0 | 0 | 0 | 0 | 0 |
| 0.5 | 0 | 14.7±2.3 f | 45.3±6.1 c | 38.7±4.6 cd | 12.0±4.0 f |
| 1.0 | 0 | 37.3±2.3 d | 58.7±2.3 b | 32.0±4.0 d | 10.7±4.6 fg |
| 2.0 | 0 | 38.7±4.6 cd | 81.3±6.1 a | 22.7±4.6 e | 4.0±4.0 gh |
| 4.0 | 0 | 10.6±4.6 fg | 33.3±6.1 d | 13.3±2.3 f | 2.7±2.3 h |
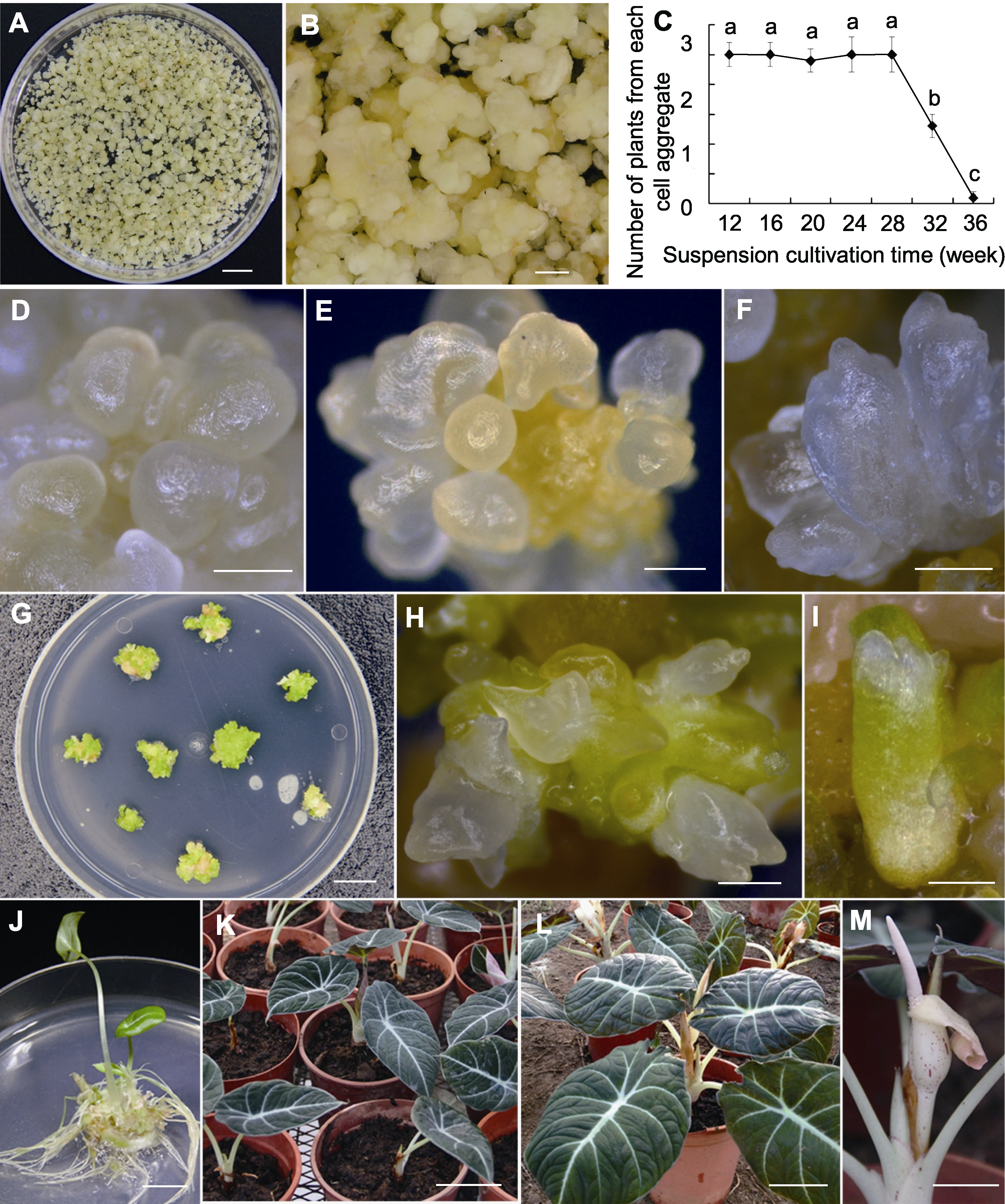
图2 黑鹅绒海芋胚性愈伤组织的悬浮培养、悬浮培养时间对其胚性细胞团再生植株平均数量的影响及植株再生 (A) 在MS液体培养基中悬浮培养获得的胚性细胞团(bar=5 mm); (B) 呈淡黄色的胚性细胞团(bar=1 mm); (C) 悬浮培养时间对胚性细胞团再生植株平均数量的影响(不同小写字母表示平均值在0.05水平差异显著); (D) 细胞团转移至MS培养基上形成圆球形胚(bar=0.5 mm); (E) 梨形胚(bar=0.5 mm); (F) 盾形胚(bar=0.5 mm); (G), (H) 子叶型胚(bar=10 mm, bar=1 mm); (I) 完整的子叶型胚(bar=1 mm); (J) 体细胞胚长出嫩叶和根, 形成完整的植株(bar=1 cm); (K) 移栽至温室3个月后, 叶片呈现出该物种特有的黑绿色黑鹅绒光泽(bar=5 cm); (L) 移栽至温室10个月后, 新生叶片面积明显增大(bar=5 cm); (M) 从植株顶端抽出佛焰苞花序(bar=4 cm)。
Figure 2 Suspension culture of embryogenic calli, the effect of suspension cultivation time on average number of plants originating from each cell aggregate, and plant regeneration of Alocasia reginula (A) Cell aggregates obtained by suspension cultivation in liquid MS medium (bar=5 mm); (B) Light yellow cell aggregates (bar= 1 mm). (C) Effect of suspension cultivation time on average number of plants originating from each cell aggregate (means with different lowercase letters are significantly different at the 0.05 level); (D) Cell aggregates were transferred onto MS medium to form spherical embryos (bar=0.5 mm); (E) Pear-shaped embryos (bar=0.5 mm); (F) Shield-shaped embryos (bar=0.5 mm); (G), (H) Cotyledonary embryos (bar=10 mm, bar=1 mm); (I) Integral cotyledonary embryo (bar=1 mm); (J) The separated integral plantlet sprouted young leaves and roots (bar=1 cm); (K) After 3 months of transplantation, leaves showed the unique blackish green velvet luster (bar=5 cm); (L) After 10 months of transplantation, the area of young leaves increased significantly (bar=5 cm); (M) Spadices sprouted from plant top (bar=4 cm).
| Medium strength | Plant regeneration rate (%) | Average number of rege- nerated plants of each cell aggregate |
|---|---|---|
| 1/8MS | 56.4±3.9 d | 0.5±0.1 d |
| 1/4MS | 70.3±5.7 c | 1.1±0.1 c |
| 1/2MS | 100±0.0 a | 2.5±0.2 a |
| MS | 83.0±5.5 b | 1.6±0.2 b |
表2 MS培养基强度对黑鹅绒海芋植株再生的影响
Table 2 Influence of MS medium strength on plant regeneration of Alocasia reginula
| Medium strength | Plant regeneration rate (%) | Average number of rege- nerated plants of each cell aggregate |
|---|---|---|
| 1/8MS | 56.4±3.9 d | 0.5±0.1 d |
| 1/4MS | 70.3±5.7 c | 1.1±0.1 c |
| 1/2MS | 100±0.0 a | 2.5±0.2 a |
| MS | 83.0±5.5 b | 1.6±0.2 b |
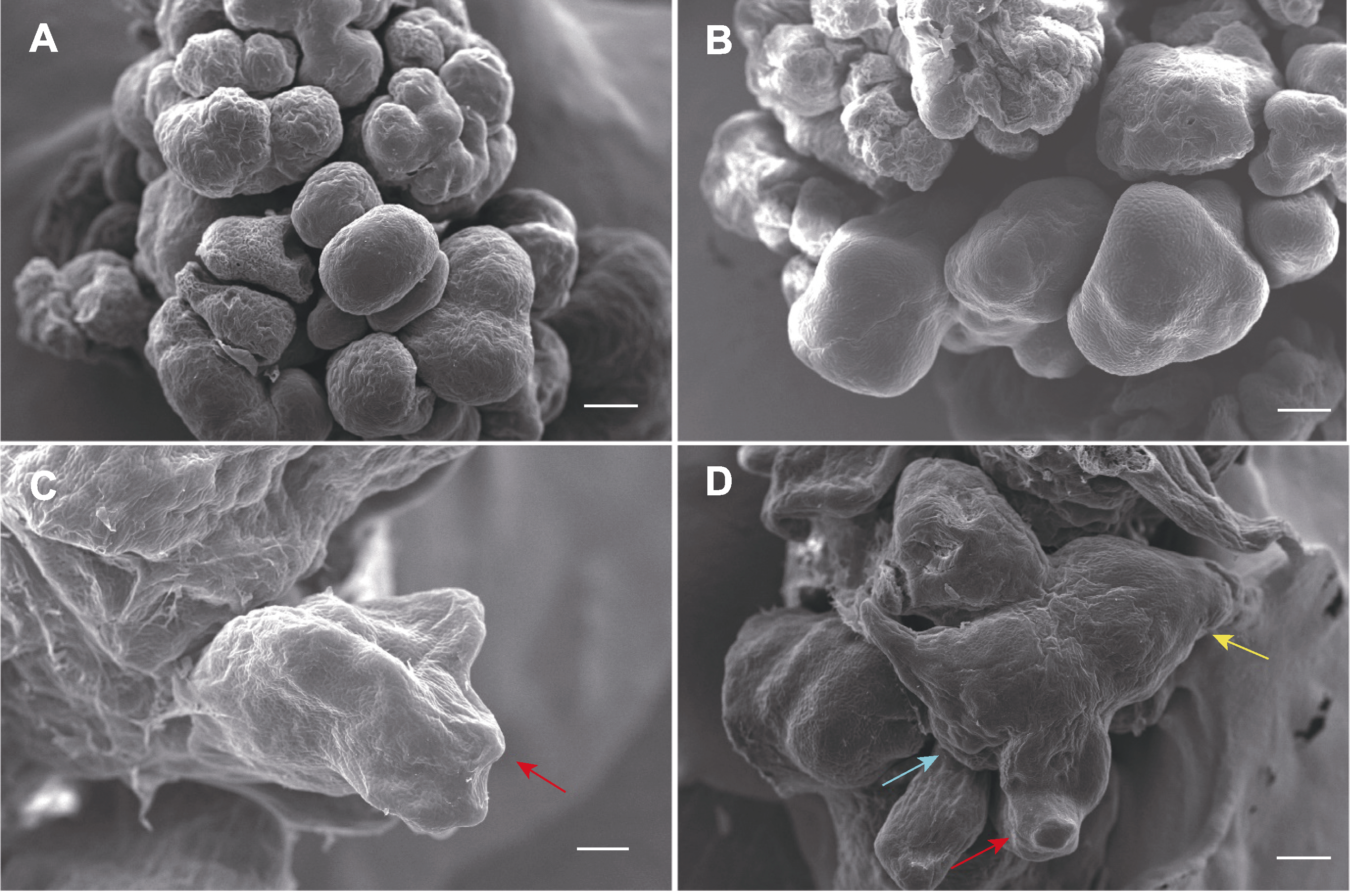
图3 体视显微镜图像(A, B)和扫描电镜图像(C, D)展示体细胞胚的发育阶段 (A) 球形胚(bar=250 μm); (B) 梨形胚(bar=250 m); (C) 具胚芽鞘的盾状胚(红色箭头) (bar=200 μm); (D) 具胚芽鞘(红色箭头)、芽点(蓝色箭头)和根尖(黄色箭头)的完整子叶(bar=250 μm)。
Figure 3 Stereo microscopy (A, B) and scanning electron microscope (SEM) (C, D) images showing developmental stages of somatic embryos (A) Spherical embryos (bar=250 μm); (B) Pear-shaped embryos (bar=250 μm); (C) Shield-shaped embryo with coleoptiles (red arrow) (bar=200 μm); (D) Complete cotyledonary embryo with coleoptiles (red arrow), shoot tip (blue arrow) and root tip (yellow arrow) (bar=250 μm).
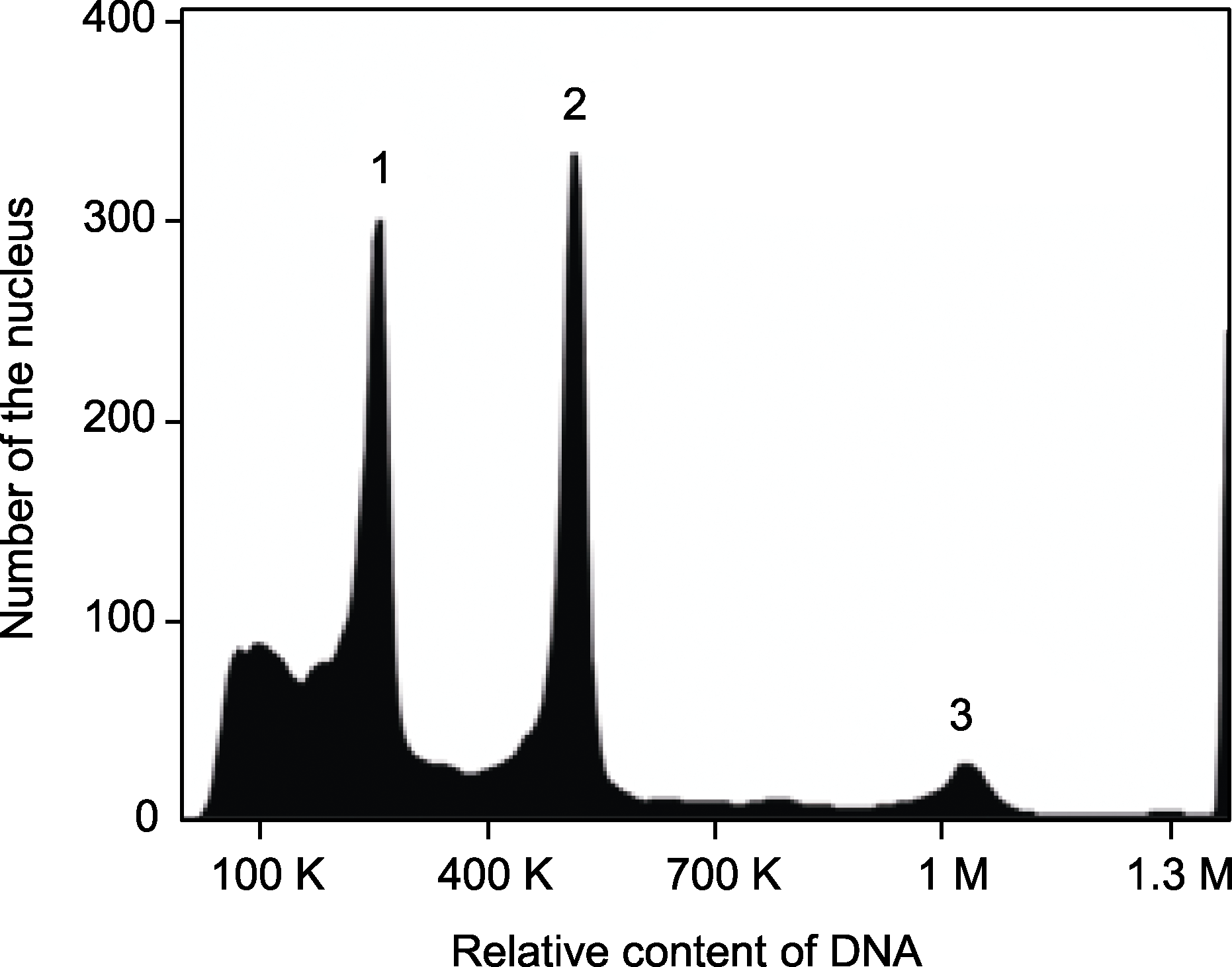
图4 黑鹅绒海芋再生植株的流式细胞术分析 1: 玉米的G0/G1期细胞核; 2: 黑鹅绒海芋再生植株的G0/G1期细胞核; 3: 黑鹅绒海芋再生植株的G2期细胞核。
Figure 4 Flow cytometry analysis of regenerated plant of Alocasia reginula 1: Nuclei at G0/G1 phase of Zea mays; 2: Nuclei at G0/G1 phase from regenerated plant of A. reginula; 3: Nuclei at G2 phase from regenerated plant of A. reginula.
| [1] | 陈妍月, 龚恒佩, 汪红, 钟晓明, 黄真 (2020). 不同地区海芋与混淆品尖尾芋的生药鉴定与显微定量分析研究. 浙江中医药大学学报 44, 89-98. |
| [2] | 范梦琳, 莫志贤 (2019). 尖尾芋的化学成分及抗肿瘤作用研究进展. 中国临床药理学杂志 35, 597-600. |
| [3] | 李艳敏, 蒋卉, 符真珠, 张晶, 袁欣, 王慧娟, 高杰, 董晓宇, 王利民, 张和臣 (2021). 芍药花药愈伤组织诱导及体细胞胚发生. 植物学报 56, 443-450. |
| [4] | 廖飞雄, 王恒明, 邹春萍 (2005). 黑鹅绒观音莲的组织培养和快速繁殖. 植物生理学通讯 41, 63. |
| [5] | 秦鹏, 罗燕羽, 黄绍力, 刘伟光 (2020). 黑叶观音莲组培快繁技术体系研究. 安徽农业科学 48(2), 117-118, 126. |
| [6] | 许雯婷, 刘慧春, 张加强, 周江华, 朱开元 (2021). 体胚发生的分子调控机制及其在花卉中的研究进展. 植物生理学报 57, 1625-1632. |
| [7] | 许智宏, 张宪省, 苏英华, 胡玉欣, 徐麟, 王佳伟 (2019). 植物细胞全能性和再生. 中国科学: 生命科学 49, 1282-1300. |
| [8] |
燕丽萍, 李丽, 刘翠兰, 吴德军, 王因花, 任飞, 赵梁军 (2016). 绒毛白蜡体胚诱导和植株再生. 植物学报 51, 807-816.
DOI |
| [9] | 杨建芬, 郭彭斐, 张兵划, 张寿洲 (2015). 绿绒海芋(Alocasia micholitziana)的组织培养及其植株再生的研究. 热带作物学报 36, 1451-1455. |
| [10] | 张润龙, 王小斌, 邵灵梅, 李丹青, 夏宜平, 张佳平 (2021). 芍药和牡丹的组织培养及遗传转化体系构建. 植物生理学报 57, 235-247. |
| [11] | 张新英, 林秀莲, 赖钟雄 (2012). 海芋种子的离体培养与快速繁殖. 福建农林大学学报(自然科学版) 41, 481-485. |
| [12] | 邹春好 (2021). 基于体细胞胚胎发生的苹果遗传转化体系的建立. 硕士论文. 泰安: 山东农业大学. pp. 1-4. |
| [13] |
Adelberg J, Toler J (2004). Comparison of agar and an agitated, thin-film, liquid system for micropropagation of ornamental elephant ears. HortScience 39, 1088-1092.
DOI URL |
| [14] |
Arumuganathan K, Earle ED (1991). Estimation of nuclear DNA content of plants by flow cytometry. Plant Mol Bio Rep 9, 229-233.
DOI URL |
| [15] |
Bao Y, Liu GF, Shi XP, Xing W, Ning GG, Liu J, Bao MZ (2012). Primary and repetitive secondary somatic embryogenesis in Rosa hybrida ‘Samantha’. Plant Cell Tissue Organ Cult 109, 411-418.
DOI URL |
| [16] |
Bhatt A, Stanly C, Keng CL (2013). In vitro propagation of five Alocasia species. Hortic Bras 31, 210-215.
DOI URL |
| [17] | Chan LK, Chong YT (2010). Establishment of a rapid in vitro propagation system for Alocasia longiloba Miq. ‘Watsoniana’. Propag Ornam Plants 10, 24-28. |
| [18] |
Chu SS, Jacobs DF, Liao DD, Liang LL, Wu DM, Chen PJ, Lai C, Zhong FD, Zeng SC (2018). Effects of landscape plant species and concentration of sewage sludge com-post on plant growth, nutrient uptake, and heavy metal removal. Environ Sci Poll Res 25, 35184-35199.
DOI |
| [19] |
Conde P, Loureiro J, Santos C (2004). Somatic embryo-genesis and plant regeneration from leaves of Ulmus minor Mill. Plant Cell Rep 22, 632-639.
PMID |
| [20] |
Deo PC, Taylor M, Harding RM, Tyagi AP, Becker DK (2010). Initiation of embryogenic cell suspensions of taro (Colocasia esculenta var. esculenta) and plant regenera-tion. Plant Cell Tissue Organ Cult 100, 283-291.
DOI URL |
| [21] |
Du DX, Jin RC, Guo JJ, Zhang FD (2019). Infection of embryonic callus with Agrobacterium enables high-speed transformation of maize. Int J Mol Sci 20, 279.
DOI URL |
| [22] |
Fang ST, Lin CY, Zhang QB, Wang L, Lin P, Zhang J, Wang XJ (2012). Anticancer potential of aqueous extract of Alocasia macrorrhiza against hepatic cancer in vitro and in vivo. J Ethnopharmacol 141, 947-956.
DOI URL |
| [23] | Galbraith DW (2009). Simultaneous flow cytometric quanti-fication of plant nuclear DNA contents over the full range of described angiosperm 2C values. Cytometry A 75, 692-698. |
| [24] |
Gill R, Saxena PK (1992). Direct somatic embryogenesis and regeneration of plants from seedling explants of peanut (Arachis hypogaea): promotive role of thidiazuron. Can J Bot 70, 1186-1192.
DOI URL |
| [25] |
Gill R, Saxena PK (1993). Somatic embryogenesis in Nico-tiana tabacum L: induction by thidiazuron of direct embryo differentiation from cultured leaf discs. Plant Cell Rep 12, 154-159.
DOI PMID |
| [26] | Hang DT, Savage GP (2021). Oxalate and calcium flows at the terminal ileum of pigs following the consumption of test diets containing fresh or ensiled taro petioles. J Fisheries Livest Prod 9(5), 1-4. |
| [27] |
Ivanova A, Velcheva M, Denchev P, Atanassov A, van Onckelen H (1994). Endogenous hormone levels during direct somatic embryogenesis in Medicago falcata. Physiol Plant 92, 85-89.
DOI URL |
| [28] | Jo EA, Murthy HN, Hahn EJ, Paek KY (2008a). Micropro-pagation of Alocasia amazonica using semisolid and liq-uid cultures. In Vitro Cell Dev Biol Plant 44, 26-32. |
| [29] |
Jo EA, Tewari RK, Hahn EJ, Paek KY (2008b). Effect of photoperiod and light intensity on in vitro propagation of Alocasia amazonica. Plant Biotechnol Rep 2, 207-212.
DOI URL |
| [30] |
Jo EA, Tewari RK, Hahn EJ, Paek KY (2009). In vitro suc-rose concentration affects growth and acclimatization of Alocasia amazonica Plantlets. Plant Cell Tissue Organ Cult 96, 307-315.
DOI URL |
| [31] |
Kato M (1996). Somatic embryogenesis from immature leaves of in vitro grown tea shoots. Plant Cell Rep 15, 920-923.
DOI PMID |
| [32] |
Kim SW, In DS, Choi PS, Liu JR (2004). Plant regeneration from immature zygotic embryo-derived embryogenic calluses and cell suspension cultures of Catharanthus roseus. Plant Cell Tissue Organ Cult 76, 131-135.
DOI URL |
| [33] |
Kim SW, In DS, Tea KH, Liu JR (2005). Somatic embryo-genesis and plant regeneration in leaf and petiole explant cultures and cell suspension cultures of Pinellia tripartita. Plant Cell Tissue Organ Cult 80, 267-270.
DOI URL |
| [34] |
Konieczny R, Sliwinska E, Pilarska M, Tuleja M (2012). Morphohistological and flow cytometric analyses of somatic embryogenesis in Trifolium nigrescens Viv. Plant Cell Tissue Organ Cult 109, 131-141.
DOI URL |
| [35] |
Li ZJ, Traore A, Maximova S, Guiltinan MJ (1998). So-matic embryogenesis and plant regeneration from floral explants of cacao (Theobroma cacao L.) using thidiazuron. In Vitro Cell Dev Biol Plant 34, 293-299.
DOI URL |
| [36] | Liu QC, Zhai H, Wang Y, Zhang DP (2001). Efficient plant regeneration from embryogenic suspension cultures of sweetpotato. In Vitro Cell Dev Biol Plant 37, 564-567. |
| [37] |
Lysák MA, Doležel J (1998). Estimation of nuclear DNA content in Sesleria (Poaceae). Caryologia 51, 123-132.
DOI URL |
| [38] |
Ma R, Guo YD, Pulli S (2003). Somatic embryogenesis and fertile green plant regeneration from suspension cell-derived protoplasts of rye (Secale cereale L.). Plant Cell Rep 22, 320-327.
PMID |
| [39] |
Michalczuk L, Cooke TJ, Cohen JD (1992a). Auxin levels at different stages of carrot somatic embryogenesis. Phytochemistry 31, 1097-1103.
DOI URL |
| [40] |
Michalczuk L, Druart P (1999). Indole-3-acetic acid me-tabolism in hormone-autotrophic, embryogenic callus of Inmil® cherry rootstock (Prunus incisa × serrula ‘GM 9’) and in hormone-dependent, nonembryogenic calli of Prunus incisa × serrula and Prunus domestica. Physiol Plant 107, 426-432.
DOI URL |
| [41] |
Michalczuk L, Ribnicky DM, Cooke TJ, Cohen JD (1992b). Regulation of indole-3-acetic acid biosynthetic pathways in carrot cell cultures. Plant Physiol 100, 1346-1353.
DOI URL |
| [42] |
Murashige T, Skoog F (1962). A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15, 473-497.
DOI URL |
| [43] |
Murthy BNS, Murch SJ, Saxena PK (1998). Thidiazuron: a potent regulator of in vitro plant morphogenesis. In Vitro Cell Dev Biol Plant 34, 267-275.
DOI URL |
| [44] |
Nakano M, Mizunashi K, Tanaka S, Godo T, Nakata M, Saito H (2004). Somatic embryogenesis and plant re-generation from callus cultures of several species in the genus Tricyrtis. In Vitro Cell Dev Biol Plant 40, 274-278.
DOI URL |
| [45] | Nasrollahzadeh M, Mehdipour E, Maryami M (2018). Effi-cient catalytic reduction of nitroarenes and organic dyes in water by synthesized Ag/diatomite nanocomposite using Alocasia macrorrhiza leaf extract. J Mater Sci: Mater Elec-tron 29, 17054-17066. |
| [46] |
Nauheimer L, Boyce PC, Renner SS (2012). Giant taro and its relatives: a phylogeny of the large genus Alocasia (Araceae) sheds light on Miocene floristic exchange in the Malesian region. Mol Phylogenet Evol 63, 43-51.
DOI PMID |
| [47] |
Palayam M, Ganapathy J, Balu KE, Pennathur G, Krish-nasamy G (2018). Structural insights into a multifunc-tional inhibitor, ‘AMTIN’ from tubers of Alocasia macror-rhizos and its possible role in dengue protease (NS2B- NS3) inhibition. Int J Biol Macromol 113, 681-691.
DOI URL |
| [48] | Pallavi CM, Rekha R, Neelambika TM (2011). Indirect so-matic embryogenesis from petiole segment in strawberry cv. Sweet Charlie. Indian J Hortic 68, 24-27. |
| [49] |
Pasternak T, Miskolczi P, Ayaydin F, Mészáros T, Dudits D, Fehér A (2000). Exogenous auxin and cytokinin de-pendent activation of CDKs and cell division in leaf proto-plast-derived cells of alfalfa. Plant Growth Regul 32, 129-141.
DOI URL |
| [50] |
Prado MJ, Rodriguez E, Rey L, González MV, Santos C, Rey M (2010). Detection of somaclonal variants in so-matic embryogenesis-regenerated plants of Vitis vinifera by flow cytometry and microsatellite markers. Plant Cell Tissue Organ Cult 103, 49-59.
DOI URL |
| [51] |
Rahman MA, Solaiman M, Haque ME, Das AK (2011). Analgesic and anti-inflammatory activities of Alocasia in-dica (Roxb.) Schott. Orient Pharm Exp Med 11, 143-146.
DOI URL |
| [52] | Rajasekaran K (2013). Biolistic transformation of cotton embryogenic cell suspension cultures. In: Zhang BH, ed. Transgenic Cotton: Methods and Protocols Transgenic Cotton:Methods and Protocols. Totowa: Humana Press. pp. 59-70. |
| [53] |
Rajasekaran K, Hein MB, Davis GC, Carnes MG, Vasil IK (1987). Enogenous growth regulators in leaves and tissue cultures of Pennisetum purpureum Schum. J Plant Physiol 130, 13-25.
DOI URL |
| [54] | Thao NTP, Miyajima I, Ureshino K, Ozaki Y, Okubo H (2003a). Micropropagation of ornamental Alocasia. J Fac Agr Kyushu Univ 47, 277-282. |
| [55] |
Thao NTP, Ozaki Y, Okubo H (2003b). Callus induction and plantlet regeneration in ornamental Alocasia micholitziana. Plant Cell Tissue Organ Cult 73, 285-289.
DOI URL |
| [56] |
Thao NTP, Ureshino K, Miyajima I, Ozaki Y, Okubo H (2003c). Induction of tetraploids in ornamental Alocasia through colchicine and oryzalin treatments. Plant Cell Tissue Organ Cult 72, 19-25.
DOI URL |
| [57] |
Thomas JC, Katterman FR (1986). Cytokinin activity indu-ced by thidiazuron. Plant Physiol 81, 681-683.
DOI PMID |
| [58] |
Tien NQ, Ngoc P, Minh PH, van Kiem P, van Minh C, Kim YH (2004). New ceramide from Alocasia macrorrhiza. Arch Pharm Res 27, 1020-1022.
DOI URL |
| [59] | Viehmannová I, Cusimamani EF, Bechyne M, Vyvadilová M, Greplová M (2009). In vitro induction of polyploidy in yacon (Smallanthus sonchifolius). Plant Cell Tissue Or-gan Cult 97, 21-25. |
| [60] |
Visser C, Qureshi JA, Gill R, Saxena PK (1992). Mor-a)phoregulatory role of thidiazuron: substitution of auxin and cytokinin requirement for the induction of somatic em-bryogenesis in geranium hypocotyl cultures. Plant Physiol 99, 1704-1707.
DOI PMID |
| [61] |
Wei J, Li XR, Sun MX (2006). Establishment of a simple and efficient system for somatic embryo induction via ovule culture in Arabidopsis thaliana. Plant Cell Rep 25, 1275-1280.
DOI URL |
| [62] |
Wetzstein HY, Baker CM (1993). The relationship between somatic embryo morphology and conversion in peanut (Arachis hypogaea L.). Plant Sci 92, 81-89.
DOI URL |
| [63] | Xu WT, Zhang M, Wang C, Lou XZ, Han X, Zhang JH, Zhang YT, Tong ZK (2020). Somatic embryo induction and Agrobacterium-mediated transformation of embryonic callus tissue in Phoebe bournei, an endangered woody species in Lauraceae. Not Bot Horti Agrobot Cluj Napoca 48, 572-587. |
| [64] |
Yang JL, Seong ES, Kim MJ, Ghimire BK, Kang WH, Yu CY, Li CH (2010). Direct somatic embryogenesis from pericycle cells of broccoli (Brassica oleracea L. var. italica) root explants. Plant Cell Tissue Organ Cult 100, 49-58.
DOI URL |
| [65] |
Yu B, Liao FX, Liu JM, Sun YB, Huang LL (2016). Efficient regeneration and transformation of Spathiphyllum canni-folium. Plant Cell Tissue Organ Cult 127, 325-334.
DOI URL |
| [66] |
Zdravković-Korać S, Milojević J, Tubić L, Ćalić-Dragosavac D, Mitić N, Vinterhalter B (2010). Somatic em-bryogenesis and plant regeneration from root sections of Allium schoenoprasum L. Plant Cell Tissue Organ Cult 101, 237-244.
DOI URL |
| [67] |
Zhao JT, Cui J, Liu JX, Liao FX, Henny RJ, Chen JJ (2012). Direct somatic embryogenesis from leaf and petiole explants of Spathiphyllum cv. ‘Supreme’ and analysis of regenerants using flow cytometry. Plant Cell Tissue Organ Cult 110, 239-249.
DOI URL |
| [1] | 丁翔, 余元钧, 宋希强, 罗毅波. 具有泛化访花者的海芋特化传粉系统[J]. 生物多样性, 2024, 32(6): 24069-. |
| [2] | 李宇琛, 赵海霞, 姜希萍, 黄馨田, 刘亚玲, 吴振映, 赵彦, 付春祥. 根癌农杆菌介导的蒙古冰草稳定遗传转化体系建立[J]. 植物学报, 2024, 59(4): 600-612. |
| [3] | 逯锦春, 曹丽娜, 佟冠杰, 王鑫颖, 张利英, 喻锌, 李荟芳, 李彦慧. 大花银莲花愈伤组织诱导及再生体系的建立[J]. 植物学报, 2022, 57(2): 217-226. |
| [4] | 李孟悦, 刘柳, 刘艳, 张晓曼. 毛报春(Primula × pubescens)腋芽再生组织培养体系的建立[J]. 植物学报, 2021, 56(6): 732-739. |
| [5] | 熊雅倩, 邓显豹, 张会会, 杨东, 孙恒, 刘娟, 杨美. 莲的离体快速繁殖技术[J]. 植物学报, 2021, 56(5): 605-613. |
| [6] | 罗钱, 张燕莎, 欧静. 郁金樱愈伤组织诱导及植株再生[J]. 植物学报, 2021, 56(4): 451-461. |
| [7] | 刘建飞, 刘炎, 刘克俭, 池阳, 霍志发, 霍永洪, 由香玲. 长白落叶松体胚发生再生体系优化[J]. 植物学报, 2020, 55(5): 605-612. |
| [8] | 肖燕,王振兴,李东明,齐艳华,恩和巴雅尔. 羊草成熟胚诱导愈伤组织及植株再生系统的优化[J]. 植物学报, 2020, 55(2): 192-198. |
| [9] | 赖先军,张义正,古英洪,颜朗. 转昆虫抗冻蛋白基因增强甘薯抗冻能力[J]. 植物学报, 2020, 55(1): 9-20. |
| [10] | 张文婷,何燕红,舒宁,邢景景,刘宝骏,包满珠,刘国锋. 金黄花滇百合植株再生与离体快繁技术体系的建立[J]. 植物学报, 2019, 54(6): 773-778. |
| [11] | 郭佳,李衍素,贺超兴,闫妍,于贤昌. 南瓜高效再生体系的建立[J]. 植物学报, 2019, 54(4): 539-546. |
| [12] | 郑云凤, 张晓曼, 刘晓. 红宝石球花报春腋芽再生体系的建立[J]. 植物学报, 2018, 53(5): 686-692. |
| [13] | 李瑞雪, 李纪强, 蒲腾飞, 张晓丽, 赵喜亭, 李俊华, 李明军. 怀山药类原球茎的诱导形成与植株再生[J]. 植物学报, 2018, 53(3): 334-340. |
| [14] | 任如意, 薛巨坤, 国会艳, 魏继承. 北玄参毛状根诱导及其植株再生[J]. 植物学报, 2017, 52(6): 783-787. |
| [15] | 燕丽萍, 李丽, 刘翠兰, 吴德军, 王因花, 任飞, 赵梁军. 绒毛白蜡体胚诱导和植株再生[J]. 植物学报, 2016, 51(6): 807-816. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||