

植物学报 ›› 2020, Vol. 55 ›› Issue (1): 9-20.DOI: 10.11983/CBB19133 cstr: 32102.14.CBB19133
收稿日期:2019-07-05
接受日期:2019-09-24
出版日期:2020-01-01
发布日期:2019-12-20
通讯作者:
颜朗
基金资助:
Xianjun Lai1,Yizheng Zhang2,Yinghong Gu2,Lang Yan1,*( )
)
Received:2019-07-05
Accepted:2019-09-24
Online:2020-01-01
Published:2019-12-20
Contact:
Lang Yan
摘要: 为明确昆虫抗冻蛋白基因转入甘薯(Ipomoea batatas)后是否能提升其抗冻能力, 进而为培育甘薯抗冻育种材料奠定基础, 将黄粉虫(Tenebrio molitor)抗冻蛋白基因TmAFP导入植物基因表达质粒, 经农杆菌介导的遗传转化获得抗冻甘薯新材料。以甘薯品种Huachano为受体材料建立甘薯植株高效再生体系, 并采用不同成分的体细胞胚成熟培养基培养胚性悬浮细胞。胚性愈伤组织对除草剂的敏感性测试结果表明, 转基因阳性植株筛选的最适培养基为MS+0.2 mg·L -12,4-D+0.8 mg·L -1GAP+100 mg·L -1Carb。将表达质粒分别转化Huachano后共获得7个胚性愈伤团并最终获得42株再生抗性植株, 其中转pSUIBEV3-AFP有23个株系, 转pCAMBIA-AFP有19个株系, 经PCR、Southern杂交和RT-PCR检测后证实TmAFP基因已整合至甘薯基因组中并获得表达。将转基因甘薯及对照植株在-1°C下处理15小时后转移至室温, 结果表明, 转基因甘薯植株的抗冻能力显著提升。
赖先军,张义正,古英洪,颜朗. 转昆虫抗冻蛋白基因增强甘薯抗冻能力. 植物学报, 2020, 55(1): 9-20.
Xianjun Lai,Yizheng Zhang,Yinghong Gu,Lang Yan. Transformation of Insect Derived Antifreeze Gene into Sweet Potato (Ipomoea batatas) and Enhanced Its Freeze-tolerance. Chinese Bulletin of Botany, 2020, 55(1): 9-20.

图1 甘薯遗传转化体系 (A), (B) 分别在MS+0.2 mg·L-1 2,4-D液体培养基中继代18和22周后的甘薯胚性悬浮细胞; (C) 在MS+0.2 mg·L-1 2,4-D固体培养基上培养8周后的甘薯胚性悬浮细胞; (D) 甘薯体细胞胚再生的小植株。(A), (B), (D) Bars=1 cm; (C) Bar=4 cm
Figure 1 Transformation system of sweet potato (A), (B) Sweet potato embryogenic suspension cells at 18 and 22 weeks cultivated in MS+0.2 mg·L-1 2,4-D liquid medium, respectively; (C) Sweet potato embryogenic suspension cells at 8 weeks cultivated in MS+0.2 mg·L-1 2,4-D solid medium; (D) Sweet potato seedlings regenerated from somatic embryo. (A), (B), (D) Bars=1 cm; (C) Bar=4 cm
| Treatment | Components (mg·L-1) | Number of seedlings in average | * | ** |
|---|---|---|---|---|
| 1 | MS | 44.8±5.22 | a | A |
| 2 | MS+ABA1.0 | 33.6±5.18 | b | AB |
| 3 | MS+GA31.0 | 30.2±4.73 | bc | B |
| 4 | MS+ABA1.0+GA31.0 | 23.2±6.29 | c | B |
| 5 | MS+ABA4.0+GA31.0 | 21.8±6.58 | c | B |
表1 不同体细胞胚成熟培养基对甘薯芽苗再生的影响
Table 1 Effect of different somatic embryo maturation medium on the regenerated seedlings
| Treatment | Components (mg·L-1) | Number of seedlings in average | * | ** |
|---|---|---|---|---|
| 1 | MS | 44.8±5.22 | a | A |
| 2 | MS+ABA1.0 | 33.6±5.18 | b | AB |
| 3 | MS+GA31.0 | 30.2±4.73 | bc | B |
| 4 | MS+ABA1.0+GA31.0 | 23.2±6.29 | c | B |
| 5 | MS+ABA4.0+GA31.0 | 21.8±6.58 | c | B |
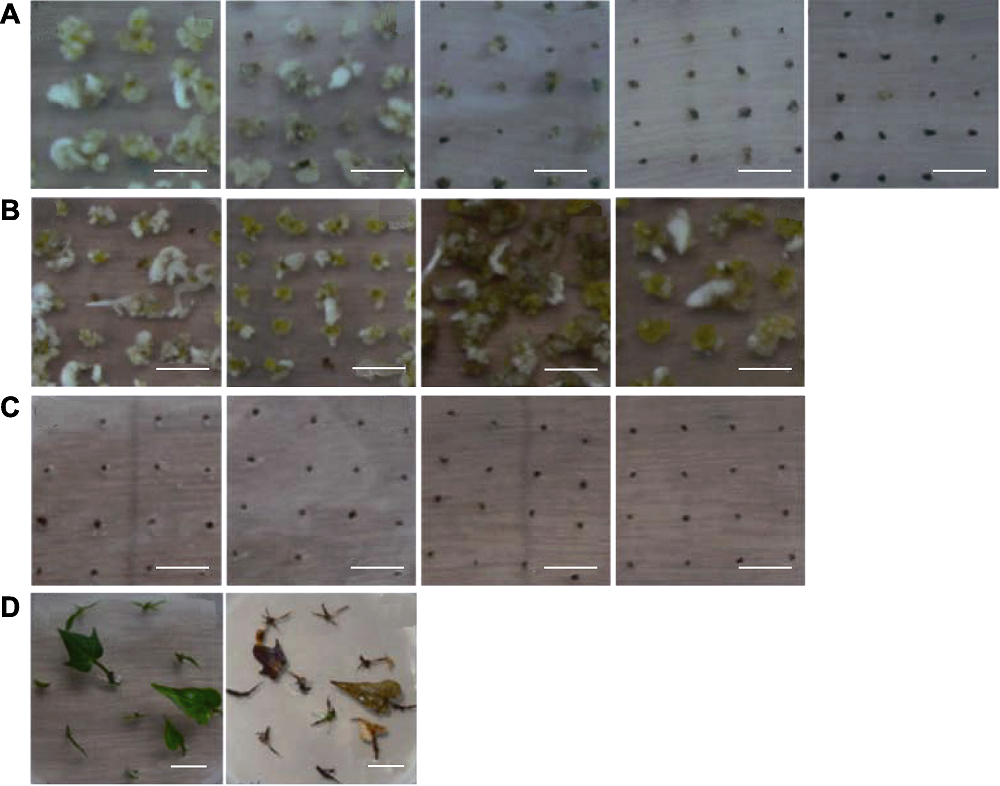
图2 不同浓度除草剂和抗生素对甘薯胚性愈伤组织和组培苗的影响 (A) 选择培养基上培养8周的甘薯胚性悬浮细胞。从左到右培养基依次为MS+0.2 mg·L-1 2,4-D分别添加0、0.2、0.4、0.6和0.8 mg·L-1 GAP; (B) 培养6周(从左至右第1, 2)和8周(3, 4)的胚性悬浮细胞, 培养基分别为MS+0.2 mg·L-1 2,4-D (1, 3)和MS+0.2 mg·L-1 2,4-D+100 mg·L-1 Carb (2, 4); (C) 培养8周的胚性悬浮细胞, 培养基为MS+0.2 mg·L-1 2,4-D+100 mg·L-1 Carb分别添加0.8、1.0、1.2和1.4 mg·L-1 GAP; (D) 分别为MS+0.2 mg·L-1 2,4-D+0.8 mg·L-1 GAP+100 mg·L-1 Carb培养基上接种0天和培养3周的Huachano茎尖。(A), (B), (C) Bars=1 cm; (D) Bar=2 cm
Figure 2 Effects of herbicide and antibiotic in different concentrations on embryogenic callus and regenerated seedlings of sweet potato (A) Sweet potato embryogenic suspension cells at 8 weeks cultivated in selective medium. The medium from left to right was MS+0.2 mg·L-1 2,4-D with 0, 0.2, 0.4, 0.6, 0.8 mg·L-1 GAP, respectively; (B) Embryogenic suspension cells at 6 weeks (the first and second from left to right) and 8 weeks (the third and fourth). The medium were MS+0.2 mg·L-1 2,4-D (the first and third) MS+0.2 mg·L-1 2,4-D+100 mg·L-1 Carb (the second and fourth); (C) Embryogenic suspension cells at 8 weeks, the medium are MS+0.2 mg·L-1 2,4-D+100 mg·L-1 Carb with 0.8, 1.0, 1.2, 1.4 mg·L-1 GAP, respectively; (D) Huachano stem tips cultivated 0 day and 3 weeks on medium of MS+0.2 mg·L-1 2,4-D+0.8 mg·L-1 GAP+100 mg·L-1 Carb, respectively. (A), (B), (C) Bars=1 cm; (D) Bar=2 cm
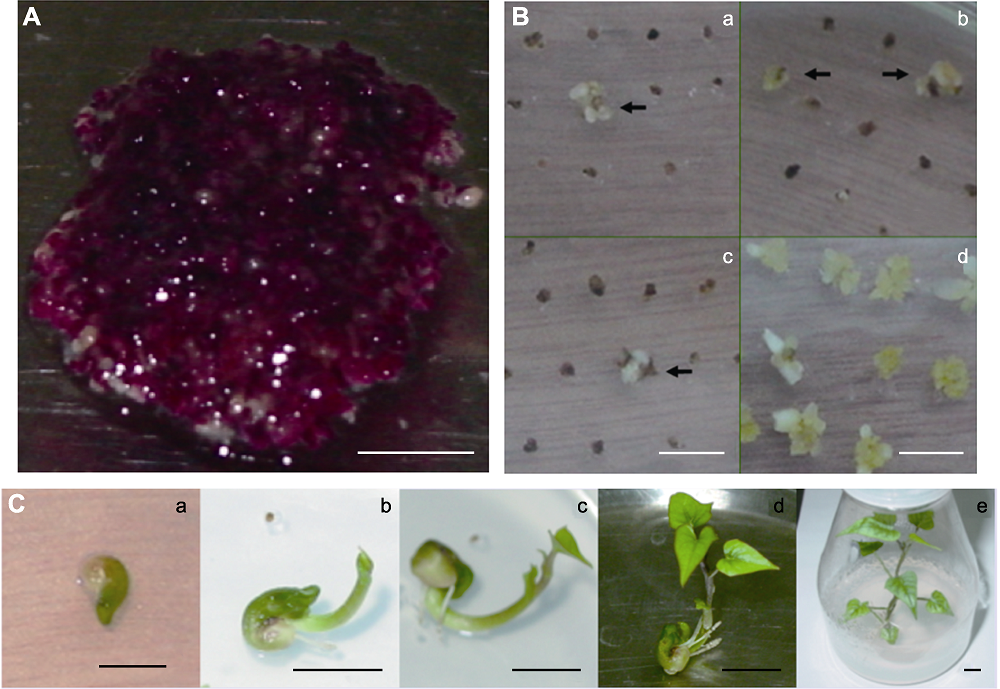
图3 甘薯抗性体胚及其转基因植株再生过程 (A) 用于转化的甘薯胚性悬浮细胞在MS+0.2 mg·L-1 2,4-D液体培养基中28°C黑暗振荡培养32周; (B) 选择与非选择培养4周的胚性愈伤组织, a-c为选择性培养基(MS+0.2 mg·L-1 2,4-D+100 mg·L-1 Carb+0.8 mg·L-1 GAP), 箭头所指为抗性愈伤组织; d为未加除草剂的对照; (C) 转基因植株再生过程, a: 再生组织; b: 生芽; c: 生叶; d: 成株; e: 成苗。Bars=1 cm
Figure 3 Sweet potato resistant somatic embryo and the regeneration of transgenic plants (A) Sweet potato embryogenic suspension cells cultivated in MS+0.2 mg·L-1 2,4-D liquid medium at 28°C for 32 weeks; (B) Embryogenic callus cultivated in selective and non-selective medium for 4 weeks, a-c: Embryogenic callus cultivated in selective medium (MS+0.2 mg·L-1 2,4-D+100 mg·L-1 Carb+0.8 mg·L-1 GAP), resistant callus was marked by arrows; d: Embryogenic callus cultivated in control medium without herbicide; (C) The processes of transgenic plant regeneration, a: Reproductive tissue; b: Bud; c: Leaf; d: Seedling; e: Reproductive plant. Bars=1 cm
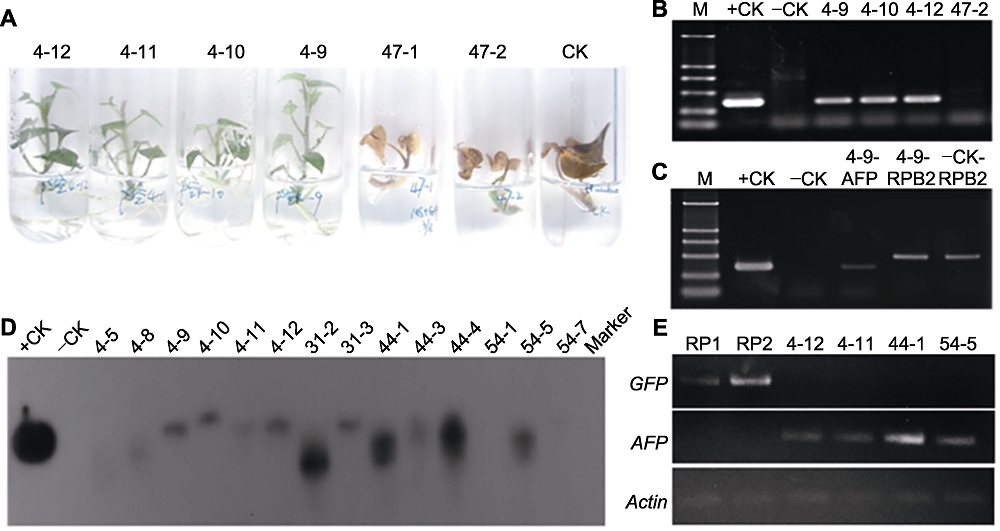
图4 TmAFP转基因甘薯植株的鉴定 (A) 采用除草剂(1.0 mg·L-1 GAP)对再生植株进行筛选(4-12、4-11、4-10、4-9: 抗性植株; 47-1、47-2: 非抗性植株; CK: 非转基因对照); (B) PCR快速鉴定转基因植株(AFP片段长度353 bp) (M: D2000 DNA marker (下同); +CK: pSUIBEV3-AFP质粒正对照; -CK: 未转化对照植株; 4-9、4-10、4-12: GAP抗性植株; 47-2: 非抗性植株); (C) 转基因植株的PCR检测(+CK: pSUIBEV3-AFP质粒作正对照扩增AFP基因; -CK: 未转化对照植株扩增AFP基因; 4-9-AFP: 转基因植株扩增AFP基因; 4-9-RPB2: 转基因植株扩增RPB2基因; -CK-RPB2: 未转化对照植株扩增RPB2基因); (D) Southern杂交(+CK: pCAMBIA-AFP质粒正对照; -CK: 未转化对照植株; 其余为不同转基因株系); (E) 转基因植株RT-PCR检测(RP1、RP2: 转pCAMBIA空载植株; 4-12、4-11、44-1、54-5: 转pCAMBIA-AFP株系)。
Figure 4 Detection of TmAFP in the transgenic sweet potato plants (A) Screening with 1.0 mg·L-1 GAP (Line 4-12, 4-11, 4-10, 4-9: Resistant seedlings; Line 47-1, 47-2: Non-resistant seedlings; CK: Non-transgenic control); (B) Amplified 353-bp fragment of AFP gene (M: D2000 molecular weight marker; +CK: pSUIBEV3-AFP vector as positive control; -CK: Non-transgenic seedlings as negative control; Line 4-9, 4-10, 4-12: GAP resistant seedlings; Line 47-2: Non-resistant seedlings); (C) PCR detection of transgenic seedlings (+CK: Amplifying AFP gene using pSUIBEV3-AFP as template; -CK: Amplifying AFP gene using non-transgenic seedling; 4-9-AFP: Amplifying AFP gene in transgenic seedling; 4-9-RPB2: Amplifying RPB2 gene in transgenic seedling; -CK-RPB2: Amplifying RPB2 gene in non-transgenic seedling); (D) Southern blotting analysis (+CK: pCAMBIA-AFP vector as positive control; -CK: Non-transgenic seedlings as negative control; The others represent different transgenic seedling lines); (E) RT-PCR detection of transgenic seedlings (RP1, RP2: Transgenic seedlings with empty pCAMBIA vector; Line 4-12, 4-11, 44-1, 54-5: Transgenic seedlings with pCAMBIA-AFP vector).
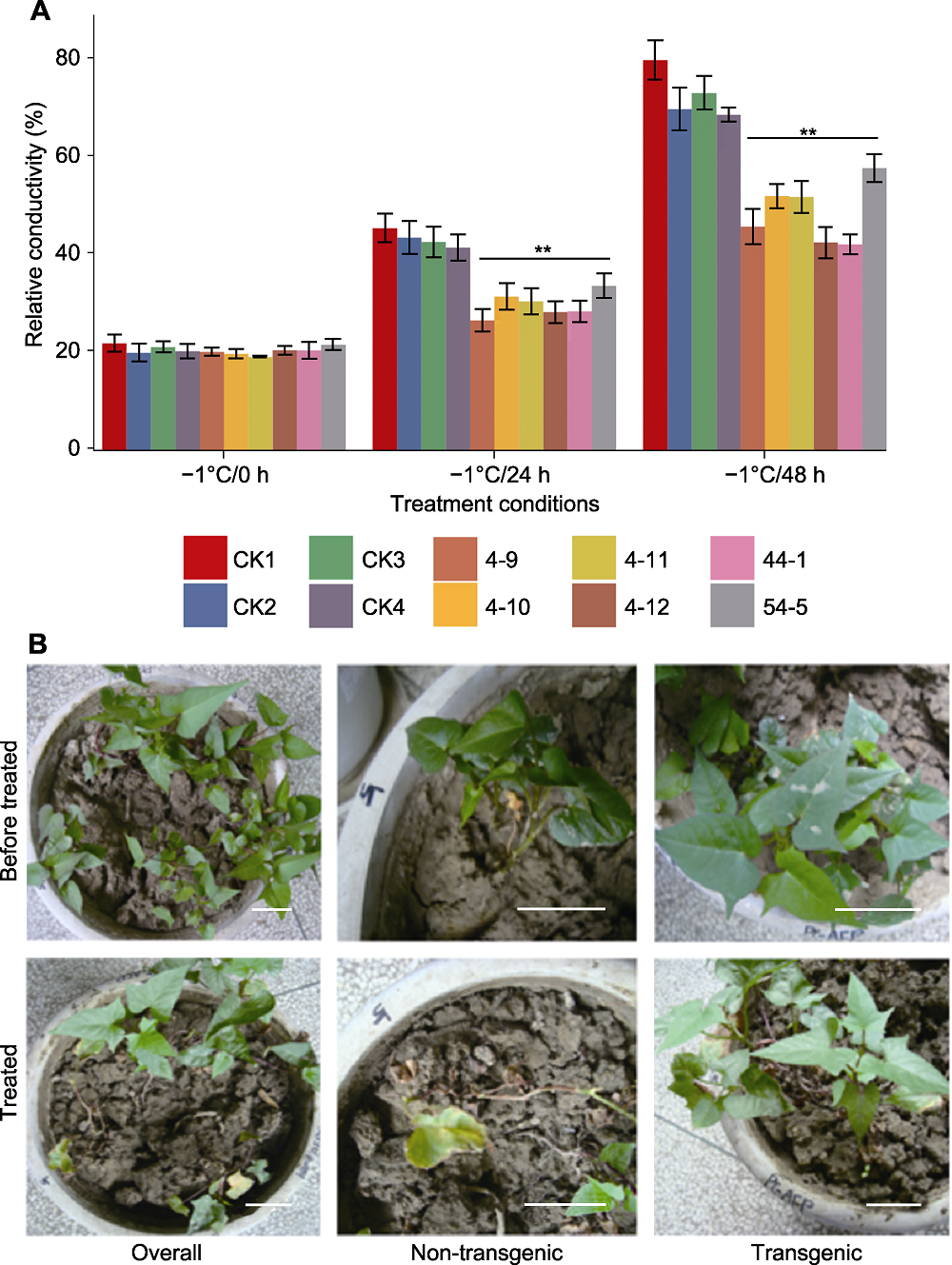
图5 TmAFP转基因甘薯植株抗冻能力的检测 (A) 不同冷处理条件下植株电导率变化(CK1: 非转基因对照; CK2-4: 转空载的非抗冻植株; 4-9、4-10、4-11、4-12、44-1和54-5: TmAFP转基因株系; ** 表示差异极显著(P<0.01)); (B) -1°C条件下处理15小时后转移至室温条件, 转基因甘薯植株及对照的表型变化(Overall: 盆中同时栽种CK1、4-9、4-10、4-11、4-12; Non-transgenic: CK1放大图; Transgenic: 转基因株系4-9放大图)。Bars=5 cm
Figure 5 Detection of freezing-tolerance ability of TmAFP transgenic sweet potato plants (A) Conductivity assay under different freeze-treatments (CK1: Non-transgenic control; CK2-4: Transgenic plants with empty vector; 4-9, 4-10, 4-11, 4-12, 44-1, 54-5: TmAFP transgenic lines; ** indicate extremely significant differences (P<0.01)); (B) Phenotypic changes of transgenic sweet potato plants and controls after 15 h treatment at -1°C (Overall: CK1, 4-9, 4-10, 4-11, 4-12 planted in the same pot; Non-transgenic: Zoomed in CK1; Transgenic: Zoomed in transgenic line 4-9). Bars=5 cm
| [1] | 蔺忠龙, 李维薇, 白现广, 吕广磊, 程在全 (2009). 植物抗冻基因最新研究进展. 北方园艺 ( 1), 119-123. |
| [2] | 刘忠渊, 王芸, 吕国栋, 王贤磊, 张富春, 马纪 (2006). Tenebriomolitor抗冻蛋白基因家族cDNA片段的克隆、序列分析及原核表达. 遗传 28, 1532-1540. |
| [3] | 马代夫, 李洪民, 李秀英, 谢逸平, 李强 (2005). 甘薯育种与甘薯产业发展. 见: 全国甘薯育种与产业化学术研讨会. 成都: 中国作物学会. pp. 3-10. |
| [4] | 阮龙, 高正良, 陈义红, 张玮, 张云华, 吴跃进, 邵希文 (2010). 干旱耐逆基因(HS1)转化甘薯获得转基因植株. 激光生物学报 19, 552-556. |
| [5] | 王欣, 过晓明, 李强, 唐忠厚, 郭尚洙, 马代夫 (2011). 转逆境诱导型启动子SWPA2驱动Cu/ZnSOD和APX基因甘薯(Ipomoea batatas (L.) Lam.)耐盐性. 分子植物育种 9, 754-759. |
| [6] | 王艳, 马纪, 黄薇, 邱立明, 叶锋, 张富春 (2009). 叶绿体型转昆虫抗冻蛋白基因烟草的耐寒性. 作物学报 35, 1253-1260. |
| [7] | 臧宁, 翟红, 王玉萍, 于波, 何绍贞, 刘庆昌 (2007). 表达bar基因的抗除草剂转基因甘薯的获得. 分子植物育种 5, 475-479. |
| [8] | 翟红, 何绍贞, 赵宁, 刘庆昌 (2017). 甘薯生物技术育种研究进展. 江苏师范大学学报(自然科学版) 35, 25-29. |
| [9] | 张振华, 陈介南, 卢孟柱, 章怀云, 刘伯斌 (2012). 胡萝卜与黄粉虫抗冻融合基因在拟南芥中的表达与抗冻性分析. 中国农学通报 28(31), 146-152. |
| [10] | 瓜谷郁三( 谢国生, 李合生译 ) (2004). 植物逆境生物化学及分子生物学: 着重热带薯类. 北京: 中国农业出版社. pp. 202-204. |
| [11] | Cutler AJ, Saleem M, Kendall E, Gusta LV, Georges F, Fletcher GL (1989). Winter flounder antifreeze protein improves the cold hardiness of plant tissues. J Plant Physiol 135, 351-354. |
| [12] | Fan WJ, Zhang M, Zhang HX, Zhang P (2012). Improved tolerance to various abiotic stresses in transgenic sweet potato ( Ipomoea batatas) expressing spinach betaine aldehyde dehydrogenase. PLoS One 7, e37344. |
| [13] | Liu DG, He SZ, Song XJ, Zhai H, Liu N, Zhang DD, Ren ZT, Liu QC (2015). IbSIMT1, a novel salt-induced methyltransferase gene from Ipomoea batatas, is involved in salt tolerance. Plant Cell Tissue Organ Cult 120, 701-715. |
| [14] | Liu DG, He SZ, Zhai H, Wang LJ, Zhao Y, Wang B, Li RJ, Liu QC (2014a). Overexpression of IbP5CR enhances salt tolerance in transgenic sweetpotato. Plant Cell Tissue Organ Cult 117, 1-16. |
| [15] | Liu DG, Wang LJ, Zhai H, Song XJ, He SZ, Liu QC (2014b). A novel α/β-hydrolase gene IbMas enhances salt tolerance in transgenic sweetpotato. PLoS One 9, e115128. |
| [16] | Liu QC (2011). Sweet potato omics and biotechnology in China. Plant Omics 4, 295-301. |
| [17] | Mwanga ROM, Andrade MI, Carey EE, Low JW, Yencho GC, Grüneberg WJ (2017). Sweetpotato (Ipomoea batatas L.). In: Campos H, Caligari PDS, eds. Genetic Improvement of Tropical Crops. Cham: Springer. pp. 181-218. |
| [18] | Nada H, Furukawa Y (2011). Growth inhibition at the ice prismatic plane induced by a spruce budworm antifreeze protein: a molecular dynamics simulation study. Phys Chem Chem Phys 13, 19936-19942. |
| [19] | Pearce RS (1999). Molecular analysis of acclimation to cold. Plant Growth Regul 29, 47-76. |
| [20] | Perl A, Perl-Treves R, Galili S, Aviv D, Shalgi E, Malkin S, Galun E (1993). Enhanced oxidative-stress defense in transgenic potato expressing tomato Cu, Zn superoxide dismutases. Theor Appl Genet 85, 568-576. |
| [21] | Ramya L, Ramakrishnan V (2016). Interaction of tenebrio molitor antifreeze protein with ice crystal: insights from molecular dynamics simulations. Mol Inform 35, 268-277. |
| [22] | Wang B, Zhai H, He SZ, Zhang H, Ren ZT, Zhang DD, Liu QC (2016). A vacuolar Na+/H+ antiporter gene, IbNHX2, enhances salt and drought tolerance in transgenic sweetpotato. Sci Hortic 201, 153-166. |
| [23] | Wang C, Pakhomova S, Newcomer ME, Christner BC, Luo BH (2017). Structural basis of antifreeze activity of a bacterial multi-domain antifreeze protein. PLoS One 12, e0187169. |
| [24] | Wang LJ, He SZ, Zhai H, Liu DG, Wang YN, Liu QC (2013). Molecular cloning and functional characterization of a salt tolerance-associated gene IbNFU1 from sweetpotato. J Integr Agric 12, 27-35. |
| [25] | Wang WX, Vinocur B, Altman A (2003). Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. Planta 218, 1-14. |
| [26] | Yang Y, Guan S, Zhai H, He S, Liu Q (2009). Development and evaluation of a storage root-bearing sweetpotato somatic hybrid between Ipomoea batatas(L.) Lam. and I. triloba L. Plant Cell Tissue Organ Cult 99, 83-89. |
| [27] | Yue CW, Zhang YZ (2009). Cloning and expression of Tenebrio molitor antifreeze protein in Escherichia coli. Mol Biol Rep 36, 529-536. |
| [28] | Zhai H, Wang FB, Si ZZ, Huo JX, Xing L, An YY, He SZ, Liu QC (2016). A myo-inositol-1-phosphate synthase gene, IbMIPS1, enhances salt and drought tolerance and stem nematode resistance in transgenic sweet potato. Plant Biotechnol J 14, 592-602. |
| [29] | Zhang JH, Davies WJ (1987). Increased synthesis of ABA in partially dehydrated root tips and ABA transport from roots to leaves. J Exp Bot 38, 2015-2023. |
| [1] | 苏晨, 牛钰凡, 徐航, 王希岭, 于英俊, 何雨晴, 王雷. 生物钟与光温环境信号互作网络研究进展[J]. 植物学报, 2025, 60(3): 315-341. |
| [2] | 董小云, 魏家萍, 崔俊美, 武泽峰, 郑国强, 李辉, 王莹, 田海燕, 刘自刚. 植物抗冻蛋白研究进展[J]. 植物学报, 2023, 58(6): 966-981. |
| [3] | 张波, 任长忠. 燕麦基因组学与分子育种研究进展[J]. 植物学报, 2022, 57(6): 785-791. |
| [4] | 李三和, 刘凯, 闸雯俊, 徐华山, 李培德, 周雷, 游艾青. 转BPH9和Bar基因抗褐飞虱耐除草剂水稻‘H23’对非靶标生物的影响[J]. 生物多样性, 2021, 29(4): 488-494. |
| [5] | 刘丽燕, 冯锦霞, 刘文鑫, 万贤崇. 干旱胁迫对转PtPIP2;8基因84K杨苗木光合、生长和根系结构的影响[J]. 植物生态学报, 2020, 44(6): 677-686. |
| [6] | 李格,孟小庆,李宗芸,朱明库. 甘薯盐胁迫响应基因IbMYB3的表达特征及生物信息学分析[J]. 植物学报, 2020, 55(1): 38-48. |
| [7] | 马燕婕, 何浩鹏, 沈文静, 刘标, 薛堃. 转基因玉米对田间节肢动物群落多样性的影响[J]. 生物多样性, 2019, 27(4): 419-432. |
| [8] | 杨德卫,王莫,韩利波,唐定中,李生平. 水稻稻瘟病抗性基因的克隆、育种利用及稻瘟菌无毒基因研究进展[J]. 植物学报, 2019, 54(2): 265-276. |
| [9] | 刘华, 常晓蕾, 蒋玮, 白蓝, 郑曙峰, 王金斌, 王维, 潘爱虎, 王荣谈, 唐雪明. 华东三省转Bt基因棉花种植对边际水体中Cry1Ab/c蛋白残留的影响[J]. 生物多样性, 2016, 24(12): 1373-1380. |
| [10] | 陈世璇, 张振南, 王波, 朱燕, 龚月桦, 孙冬梅, 邓馨. 复苏植物旋蒴苣苔J结构域蛋白编码基因BhDNAJC2的 克隆、表达与功能[J]. 植物学报, 2015, 50(2): 180-190. |
| [11] | 王烨, 顾兴芳, 张圣平, 苗晗, 陈国华, 谢丙炎. RNAi载体导入黄瓜的遗传转化体系[J]. 植物学报, 2014, 49(2): 183-189. |
| [12] | 戴思兰, 黄河, 付建新, 洪艳. 观赏植物分子育种研究进展[J]. 植物学报, 2013, 48(6): 589-607. |
| [13] | 巴超杰, 薛静, 陈绪清, 杨凤萍, 张立全, 李向龙, 张晓东. 利用花青素可视化跟踪表达系统快速筛选表达Cry1Ab/c基因的转基因玉米[J]. 植物学报, 2013, 48(1): 59-64. |
| [14] | 储成才. 转基因生物技术育种: 机遇还是挑战?[J]. 植物学报, 2013, 48(1): 10-22. |
| [15] | 杨维才. 植物转基因技术——回顾与前瞻[J]. 植物学报, 2013, 48(1): 6-9. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||