

植物学报 ›› 2023, Vol. 58 ›› Issue (6): 966-981.DOI: 10.11983/CBB22248 cstr: 32102.14.CBB22248
董小云1,2, 魏家萍1, 崔俊美1, 武泽峰1, 郑国强1,2, 李辉1,2, 王莹1,2, 田海燕1,2, 刘自刚1,2,*( )
)
收稿日期:2022-10-20
接受日期:2023-03-08
出版日期:2023-11-01
发布日期:2023-11-27
通讯作者:
* E-mail: lzgworking@163.com
基金资助:
Xiaoyun Dong1,2, Jiaping Wei1, Junmei Cui1, Zefeng Wu1, Guoqiang Zheng1,2, Hui Li1,2, Ying Wang1,2, Haiyan Tian1,2, Zigang Liu1,2,*( )
)
Received:2022-10-20
Accepted:2023-03-08
Online:2023-11-01
Published:2023-11-27
Contact:
* E-mail: lzgworking@163.com
摘要: 温度是决定植物地理分布的主要环境因子之一, 分布于高纬度、高海拔地区的寒地植物, 在其生活史周期内通常会遭遇一段时期的零度以下低温。当环境温度降至冰点以下, 植物体内水分子趋向于形成冰晶体, 不断增大的冰晶对植物组织结构造成致命损伤。为适应冷冻环境, 寒地植物病程相关蛋白(PR)及相关的WRKY转录因子进化成为能与冰面特异吸附结合、高效抑制冰晶形成和生长的抗冻蛋白(AFPs)。目前, 已从冬黑麦(Secale cereale)等近百种植物中分离鉴定了AFPs。与昆虫AFPs相比, 植物AFPs具有极高的重结晶抑制活性, 可有效防止形成体内大冰晶。低温和病原菌均可诱导寒地植物合成AFP。有趣的是, 仅冷诱导合成的AFPs有水解酶/抗冻活性双重分子功能。然而, 越来越多的证据表明, PR-AFP仅具有水解酶/抗冻活性的其中一种, 其转化由翻译后肽差异折叠控制/调节。AFP因具有独特的分子功能与广阔的应用前景而逐渐成为植物学领域的研究热点。该文对近年来相关领域取得的研究进展进行系统综述。
董小云, 魏家萍, 崔俊美, 武泽峰, 郑国强, 李辉, 王莹, 田海燕, 刘自刚. 植物抗冻蛋白研究进展. 植物学报, 2023, 58(6): 966-981.
Xiaoyun Dong, Jiaping Wei, Junmei Cui, Zefeng Wu, Guoqiang Zheng, Hui Li, Ying Wang, Haiyan Tian, Zigang Liu. Research Progress in Plant Antifreeze Protein. Chinese Bulletin of Botany, 2023, 58(6): 966-981.
| 物种名称 | 材料 | AFPs的 亚细胞定位 | 分子量 (kDa) | 属性 | 同源蛋白 | 参考文献 |
|---|---|---|---|---|---|---|
| 欧白英 (Solanum dulcamara) | 茎 | 细胞膜和细 胞质 | 67 | 富含甘氨酸(23.7%), TH=-0.3°C, >30 mg?mL-1, 高RI活性 | WRKY转录因子 | Duman, |
| 冬黑麦 (Secale cereale) | 叶 | 质外体 | 15-38 | 0.1 mg?mL-1, TH=0.03°C | 几丁质酶、葡聚 糖酶和类甜蛋白 | Hon et al., |
| 南极发草 (Deschampsia antarctica) | 叶 | 质外体 | 29 | 富含亮氨酸 | 磺肽素受体激酶 | John et al., |
| 白菜型冬油菜 (Brassica rapa) | 叶 | 细胞质和细 胞核 | 38 | - | β-1,3-葡聚糖酶 | Liu et al., |
| 胡萝卜 (Daucus carota) | 根 | 质外体 | 36 | 1 mg?mL-1时, TH=-0.35°C, 高RI活性 | 多聚半乳糖醛酸 酶抑制剂蛋白 | Worrall et al., Meyer et al., |
| 沙冬青 (Ammopiptanthus mongolicus) | 叶 | 细胞质 | 40 | 热稳定蛋白, 糖基化, 20 mg?mL-1时, TH=0.9°C | 凝集素 | 费云标等, Yong et al., |
| 冬小麦 (Triticum aestivum) | 叶 | 质外体 | 21.3 | 热稳定蛋白, 富含β-折叠和无规则卷曲 | 类甜蛋白 | Chun et al., Kontogiorgo, |
| 黑麦草 (Lolium perenne) | 叶 | 质外体 | 29 | 有RI活性 | - | Sidebottom et al., Pudney et al., |
| 蓝叶云杉 (Picea pungens) | 叶 | 质外体 | 27 | 0.4 mg?mL-1时, TH=(2.02± 0.40)°C | 几丁质酶 | Jarzabek et al., |
| 无芒雀麦 (Bromus inermis) | 细胞培养 | 分泌物和质 外体 | 33 | 钙离子非依赖抗冻活性, 六角双锥体冰晶形态 | I型几丁质酶 | Nakamura et al., |
| 连翘 (Forsythia suspensa) | 树皮和叶 | 细胞质 | 20 | 热稳定蛋白, 高RI活性 (6 μg?mL-1) | 与脱水蛋白同源 | Simpson et al., |
| 蜡梅 (Chimonanthus praecox) | 花冠 | 质外体 | 33 | 1.5 mg?mL-1时, TH=0.52°C | I型几丁质酶 | Zhang et al., |
| 沙棘 (Hippophae rhamnoides) | 枝条 | 质外体 | 41 | 糖基化, 热不稳定蛋白, TH=0.19°C, 9倍RI | 多聚半乳糖醛酸 酶抑制剂蛋白 | Gupta et al., |
| 萝卜 (Raphanus sativus) | 块茎和叶 | 质外体 | 1.32 | 块茎: TH=(0.2±0.03)°C; 叶: TH=(0.18±0.02)°C | - | Kawahara et al., |
| 欧洲云杉 (Picea abies) | 叶 | 质外体 | 27和70 | 0.4 mg?mL-1时,TH=(2.19± 0.83)°C | 几丁质酶 | Sabala et al., Jarzabek et al., |
| 桃 (Prunus persica) | 树皮 | 细胞质、细胞 核和木质部薄壁细胞 | 60 | 热稳定蛋白, 28.3 μg?mL-1时, TH=0.06°C | 脱水蛋白 | Wisniewski et al., |
表1 不同植物中抗冻蛋白(AFPs)的性质
Table 1 Properties of different plant antifreeze proteins (AFPs)
| 物种名称 | 材料 | AFPs的 亚细胞定位 | 分子量 (kDa) | 属性 | 同源蛋白 | 参考文献 |
|---|---|---|---|---|---|---|
| 欧白英 (Solanum dulcamara) | 茎 | 细胞膜和细 胞质 | 67 | 富含甘氨酸(23.7%), TH=-0.3°C, >30 mg?mL-1, 高RI活性 | WRKY转录因子 | Duman, |
| 冬黑麦 (Secale cereale) | 叶 | 质外体 | 15-38 | 0.1 mg?mL-1, TH=0.03°C | 几丁质酶、葡聚 糖酶和类甜蛋白 | Hon et al., |
| 南极发草 (Deschampsia antarctica) | 叶 | 质外体 | 29 | 富含亮氨酸 | 磺肽素受体激酶 | John et al., |
| 白菜型冬油菜 (Brassica rapa) | 叶 | 细胞质和细 胞核 | 38 | - | β-1,3-葡聚糖酶 | Liu et al., |
| 胡萝卜 (Daucus carota) | 根 | 质外体 | 36 | 1 mg?mL-1时, TH=-0.35°C, 高RI活性 | 多聚半乳糖醛酸 酶抑制剂蛋白 | Worrall et al., Meyer et al., |
| 沙冬青 (Ammopiptanthus mongolicus) | 叶 | 细胞质 | 40 | 热稳定蛋白, 糖基化, 20 mg?mL-1时, TH=0.9°C | 凝集素 | 费云标等, Yong et al., |
| 冬小麦 (Triticum aestivum) | 叶 | 质外体 | 21.3 | 热稳定蛋白, 富含β-折叠和无规则卷曲 | 类甜蛋白 | Chun et al., Kontogiorgo, |
| 黑麦草 (Lolium perenne) | 叶 | 质外体 | 29 | 有RI活性 | - | Sidebottom et al., Pudney et al., |
| 蓝叶云杉 (Picea pungens) | 叶 | 质外体 | 27 | 0.4 mg?mL-1时, TH=(2.02± 0.40)°C | 几丁质酶 | Jarzabek et al., |
| 无芒雀麦 (Bromus inermis) | 细胞培养 | 分泌物和质 外体 | 33 | 钙离子非依赖抗冻活性, 六角双锥体冰晶形态 | I型几丁质酶 | Nakamura et al., |
| 连翘 (Forsythia suspensa) | 树皮和叶 | 细胞质 | 20 | 热稳定蛋白, 高RI活性 (6 μg?mL-1) | 与脱水蛋白同源 | Simpson et al., |
| 蜡梅 (Chimonanthus praecox) | 花冠 | 质外体 | 33 | 1.5 mg?mL-1时, TH=0.52°C | I型几丁质酶 | Zhang et al., |
| 沙棘 (Hippophae rhamnoides) | 枝条 | 质外体 | 41 | 糖基化, 热不稳定蛋白, TH=0.19°C, 9倍RI | 多聚半乳糖醛酸 酶抑制剂蛋白 | Gupta et al., |
| 萝卜 (Raphanus sativus) | 块茎和叶 | 质外体 | 1.32 | 块茎: TH=(0.2±0.03)°C; 叶: TH=(0.18±0.02)°C | - | Kawahara et al., |
| 欧洲云杉 (Picea abies) | 叶 | 质外体 | 27和70 | 0.4 mg?mL-1时,TH=(2.19± 0.83)°C | 几丁质酶 | Sabala et al., Jarzabek et al., |
| 桃 (Prunus persica) | 树皮 | 细胞质、细胞 核和木质部薄壁细胞 | 60 | 热稳定蛋白, 28.3 μg?mL-1时, TH=0.06°C | 脱水蛋白 | Wisniewski et al., |
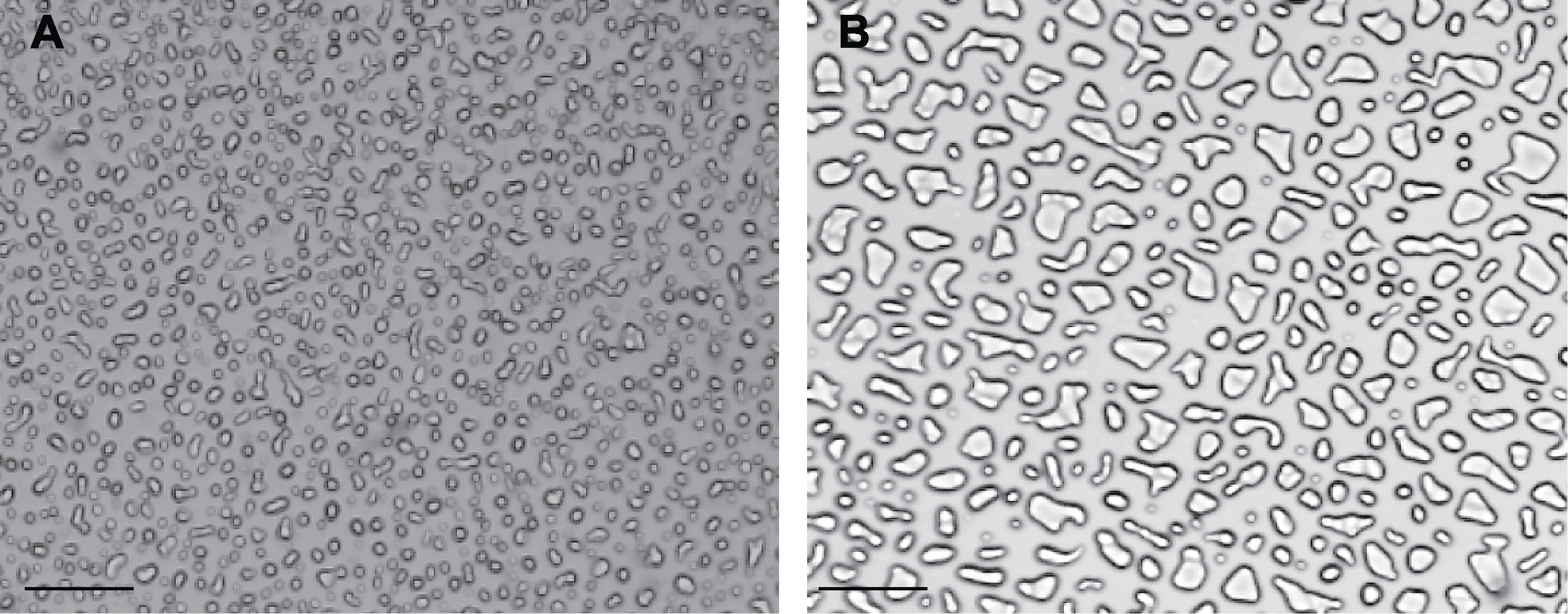
图1 白菜型冬油菜叶片质外体蛋白质粗提物冰晶形态修饰和重结晶抑制活性 (A) 低温处理幼苗叶片质外体粗提液在-7°C恒温50分钟后的冰晶生长情况(具有高重结晶抑制活性); (B) 常温处理幼苗叶片质外体粗提液在-7°C恒温50分钟后的冰晶生长情况; 图中有不规则冰晶体、尖长锥形冰晶体、近椭圆形冰晶体、梭形冰晶体和棒状冰晶体。Bars=10 μm
Figure 1 Ice crystal morphology modification and recrystallization inhibition activity of crude extracts of Brassica rapa leaf plastid exosome proteins (A) Growth of ice crystals after 50 min at -7°C in the crude extracts of plasmalemma exosomes from low temperature treated seedlings (with high recrystallization inhibition activity); (B) Growth of ice crystals after 50 min at -7°C in the crude extracts of plasmalemma exosomes from room temperature treated seedlings; The ice crystals in the figure are irregular ice crystals, pinted elongated conical ice crystals, subelliptical ice crystals, pike shaped ice crystals, and rod shaped ice crystals. Bars=10 μm
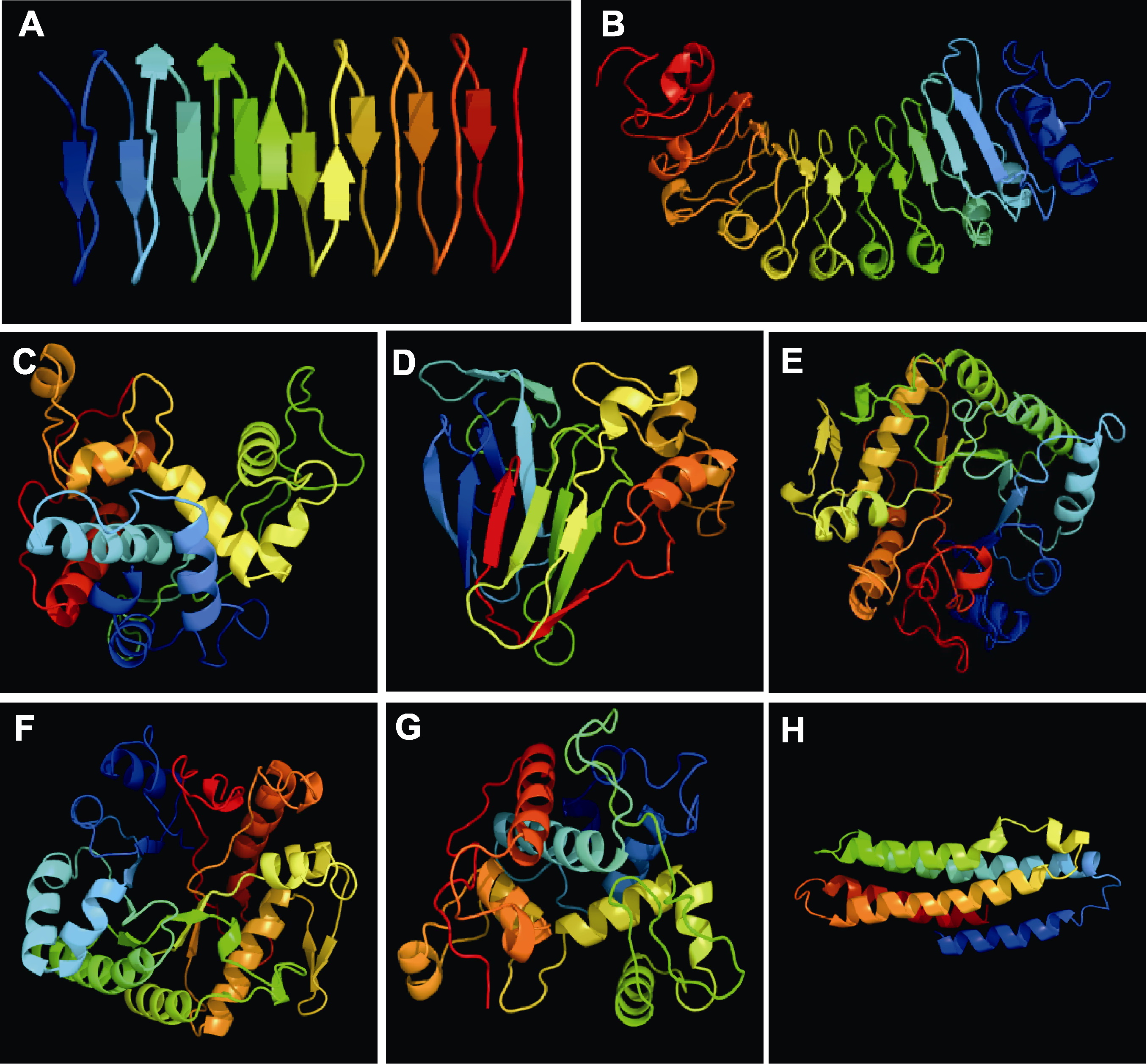
图2 几种植物抗冻蛋白(AFPs)的分子结构 (A) 黑麦草LpAFP (AJ277399.1); (B) 胡萝卜DcAFP (AF055480.1); (C) 欧白英I型内切几丁质-AFP (Q84LQ7); (D) 小麦类甜蛋白-AFP (AAM15877.1); (E) 白菜型冬油菜BrAFP1 (gi62361691); (F) 冬黑麦葡聚糖酶-AFP (CAJ58506.1); (G) 冬黑麦II型内切几丁质-AFP (AF280438.1); (H) 小球藻AFP (ABR01234.1)。通过在线软件Phyre 2.0 (http://www.sbg.bio.ic.ac.uk/phyre2/html/page.cgi?id =index)运行GenBank序列生成植物AFPs模型。
Figure 2 Molecular structure of antifreeze proteins (AFPs) in several plants (A) Lolium perenne LpAFP (AJ277399.1); (B) Daucus carota DcAFP (AF055480.1); (C) A type I endochitinase-AFP from Solanum dulcamara (Q84LQ7); (D) Triticum aestivum thaumatin like-AFP (AAM15877.1); (E) Brassica rapa BrAFP1 (gi62361691); (F) A glucanase-AFP from Secale cereale (CAJ58506.1); (G) A type II endochitinase-AFP from S. cereale (AF280438.1); (H) AFP from Chlorella vulgaris (ABR01234.1). Plant AFP models generated by running GenBank sequences through the Phyre 2.0 server (http://www.sbg.bio.ic.ac.uk/phyre2/html/page.cgi?id=index).
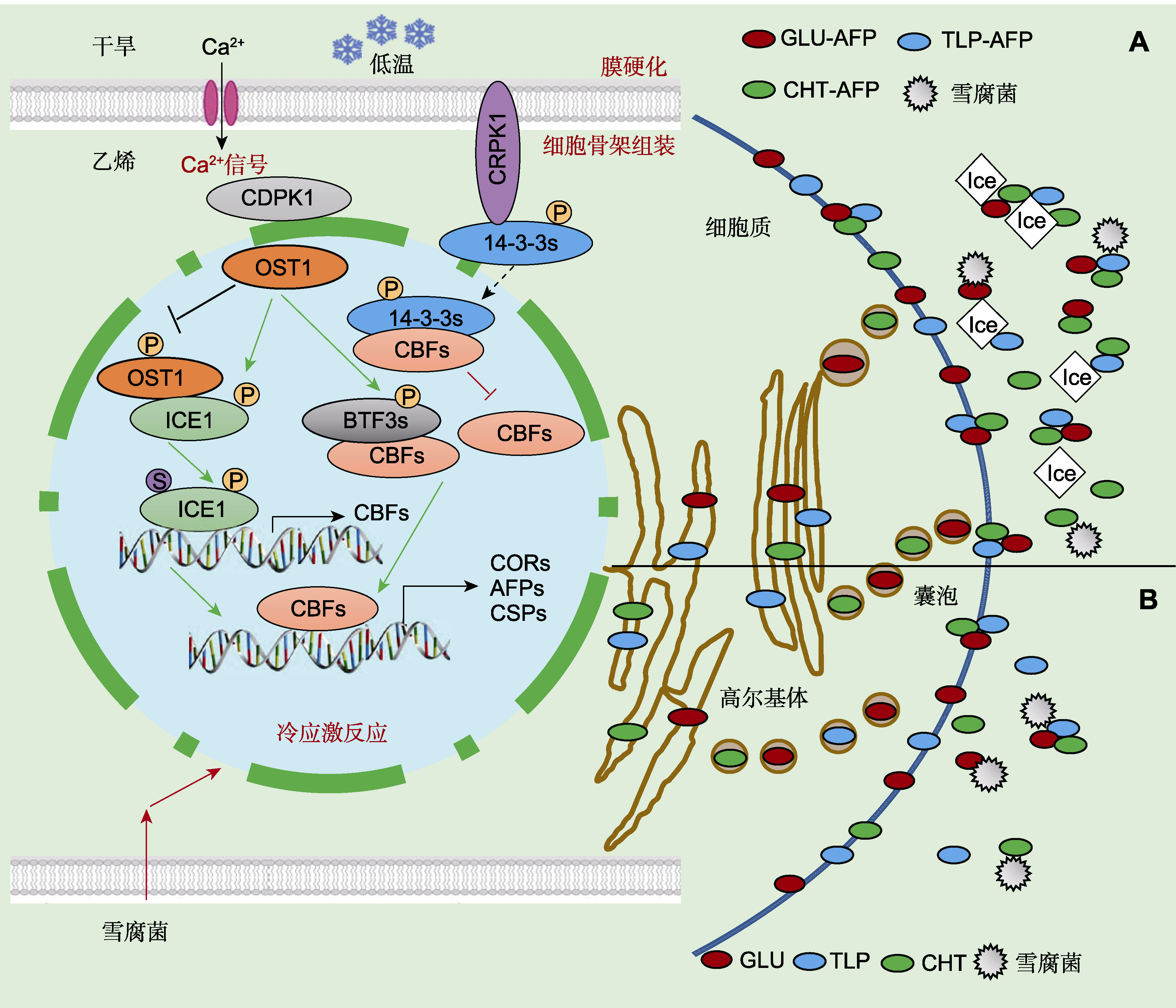
图3 低温下植物抗冻蛋白(AFPs)和致病相关蛋白(PRs)的分子调控模型(Griffith and Yaish, 2004; Guo et al., 2018) (A) 低温胁迫诱导COR、AFP和CSP等基因表达, 新合成的PR-AFP蛋白和PR蛋白通过内质网、高尔基体和囊泡分泌运输, 穿过质膜并聚集在细胞壁外空间, 形成寡聚体复合物, 抑制低温下病原菌生长; 这些蛋白质包括几丁质酶-AFPs (CHT-AFP)、葡聚糖酶-AFPs (GLU-AFP)和类甜蛋白-AFPs (TLP-AFP); 当环境温度降至冰点以下, PR-AFPs蛋白可特异吸附到冰晶表面, 抑制冰晶生长和重结晶; (B) PR蛋白(包括几丁质酶(CHT)、葡聚糖酶(GLU)和类甜蛋白(TLP))被合成并分泌到质外体中, 常温下用水杨酸(SA)、脱落酸(ABA)或雪腐病原菌处理植物, 仅诱导合成PR蛋白, 可抑制真菌病原体生长。
Figure 3 Molecular regulation models of antifreeze proteins (AFPs) and pathogenesis-related proteins (PRs) in plants under low temperature (Griffith and Yaish, 2004; Guo et al., 2018) (A) Cold stress induces COR, AFP and CSP gene expression; newly synthesized, dual-function PR-AFP proteins and PRs are secreted via the endoplasmic reticulum, Golgi bodies and vesicles that merge with the plasmalemma and accumulate on the outer cell wall surface; the AFPs, including chitinase-AFPs (CHT-AFP), glucanase-AFPs (GLU-AFP) and thaumatin-like AFPs (TLP-AFP), form oligomeric complexes that inhibit the growth of pathogens under low temperature; Below the freezing degree, the proteins are adsorbed onto the surfaces of ice and inhibit its growth and recrystallization; (B) PR proteins, including chitinases (CHT), glucanases (GLU), and thaumatin-like proteins (TLP), were synthesized and secreted into the apoplast, when plants are treated with salicylic acid (SA), abscisic acid (ABA) or snow mold at normal temperatures to inhibit the growth of fungal pathogens.
| 蛋白名称 | 蛋白来源 | 受体植物 | 转基因表型 | 参考文献 |
|---|---|---|---|---|
| afa3 | 冬季比目鱼 (Pseudopleuronectes americanus) | 烟草 | 重组蛋白在细胞内积累, 抑制冰晶重 结晶 | Hightower et al., |
| Type I afp | 冬季比目鱼 (P. americanus) | 烟草和马铃薯 | 重组proAFP蛋白在4°C积累, 但在25°C无积累; LT50降低1°C | Wallis et al., Kenward et al., |
| TaAFPI | 冬季比目鱼(P. americanus) | 冬小麦 | 蛋白质在质外体中积累; 表现出很强的抗冻活性, 即使在-7°C也表现出很强的耐冷性 | Khanna and Daggard, |
| DcAFP | 胡萝卜(Daucus carota) | 烟草和拟南芥 | 在-2°C表现出耐冷性, 在质外体积累 | Worrall et al., Meyer et al., |
| Type II afp | 美绒杜父鱼 (Hemitripterus americanus) | 烟草 | 积累的蛋白质无抗冻活性 | Kenward et al., |
| sbwAFP | 云杉蚜虫 (Choristoneura fumiferana) | 烟草 | 冰核蛋白在质外体中积累; 提取物显示TH/RI活性 | Holmberg et al., |
| THPI | 云杉蚜虫(C. fumiferana) | 拟南芥 | 转基因植株的耐寒性增强 | Zhu et al., |
| dAFP-1 | 火色甲虫 (Dendroides canadensis) | 拟南芥 | 在质外体中积累, TH=0.42°C | Huang et al., |
| MpAFP149 | 沙漠甲虫 (Microdera punctipennis) | 烟草 | 在质外体中积累; 在-1°C时, 耐冷性增强 | Wang et al., |
| LpIRIa和LpIRIb | 黑麦草(Lolium perenne) | 拟南芥 | 在-8-4°C范围内提高存活率 | Zhang et al., |
| LpAFP、LpIRI2和LpIRI3 | 多年生黑麦草(L. perenne) | 拟南芥 | 在-6°C时, 离子渗漏降低12%-39%; 在-8- -5°C范围内提高存活率 | Bredow et al., |
| BrAFP1 | 白菜型冬油菜(Brassica rapa) | 拟南芥 | 在-4°C提高存活率 | Dong et al., |
| IRIPs | 南极发草 (Deschampsia antarctica) | 拟南芥 | 过表达植株抗冻活性增强, 提取液中有RI活性 | John et al., |
表2 表达编码抗冻蛋白(AFP)基因的转基因植物
Table 2 Transgenic plants expressing a gene encoding an antifreeze protein (AFP)
| 蛋白名称 | 蛋白来源 | 受体植物 | 转基因表型 | 参考文献 |
|---|---|---|---|---|
| afa3 | 冬季比目鱼 (Pseudopleuronectes americanus) | 烟草 | 重组蛋白在细胞内积累, 抑制冰晶重 结晶 | Hightower et al., |
| Type I afp | 冬季比目鱼 (P. americanus) | 烟草和马铃薯 | 重组proAFP蛋白在4°C积累, 但在25°C无积累; LT50降低1°C | Wallis et al., Kenward et al., |
| TaAFPI | 冬季比目鱼(P. americanus) | 冬小麦 | 蛋白质在质外体中积累; 表现出很强的抗冻活性, 即使在-7°C也表现出很强的耐冷性 | Khanna and Daggard, |
| DcAFP | 胡萝卜(Daucus carota) | 烟草和拟南芥 | 在-2°C表现出耐冷性, 在质外体积累 | Worrall et al., Meyer et al., |
| Type II afp | 美绒杜父鱼 (Hemitripterus americanus) | 烟草 | 积累的蛋白质无抗冻活性 | Kenward et al., |
| sbwAFP | 云杉蚜虫 (Choristoneura fumiferana) | 烟草 | 冰核蛋白在质外体中积累; 提取物显示TH/RI活性 | Holmberg et al., |
| THPI | 云杉蚜虫(C. fumiferana) | 拟南芥 | 转基因植株的耐寒性增强 | Zhu et al., |
| dAFP-1 | 火色甲虫 (Dendroides canadensis) | 拟南芥 | 在质外体中积累, TH=0.42°C | Huang et al., |
| MpAFP149 | 沙漠甲虫 (Microdera punctipennis) | 烟草 | 在质外体中积累; 在-1°C时, 耐冷性增强 | Wang et al., |
| LpIRIa和LpIRIb | 黑麦草(Lolium perenne) | 拟南芥 | 在-8-4°C范围内提高存活率 | Zhang et al., |
| LpAFP、LpIRI2和LpIRI3 | 多年生黑麦草(L. perenne) | 拟南芥 | 在-6°C时, 离子渗漏降低12%-39%; 在-8- -5°C范围内提高存活率 | Bredow et al., |
| BrAFP1 | 白菜型冬油菜(Brassica rapa) | 拟南芥 | 在-4°C提高存活率 | Dong et al., |
| IRIPs | 南极发草 (Deschampsia antarctica) | 拟南芥 | 过表达植株抗冻活性增强, 提取液中有RI活性 | John et al., |
| [1] | 费云标, 孙龙华, 黄涛, 舒念红, 高素琴, 简令成 (1994). 沙冬青高活性抗冻蛋白的发现. 植物学报 36, 649-650. |
| [2] | 费云标, 魏令波, 高素琴, 陆漫春, 王保怀, 李芝芬, 张有民, 舒念红, 江勇, 王维香 (2000). 沙冬青抗冻蛋白的分离、纯化及其理化特性分析. 科学通报 45, 2185-2189. |
| [3] | 李文轲, 马春森 (2012). 抗冻蛋白特征、作用机理与预测新进展. 生命科学 24, 1089-1097. |
| [4] |
刘静妍, 施怡婷, 杨淑华 (2017). CBF: 平衡植物低温应答与生长发育的关键. 植物学报 52, 689-698.
DOI |
| [5] | 卢存福, 简令成, 匡廷云 (2000). 低温诱导唐古特红景天细胞分泌抗冻蛋白. 生物化学与生物物理进展 27, 555-559. |
| [6] | 王红, 简令成, 张举仁 (1994). 低温胁迫下水稻幼叶细胞内Ca2+水平的变化. 植物学报 36, 587-591. |
| [7] | 张振华, 陈介南, 卢孟柱, 章怀云, 刘伯斌 (2012). 胡萝卜与黄粉虫抗冻融合基因在拟南芥中的表达与抗冻性分析. 中国农学通报 28, 146-152. |
| [8] |
Aroca R, Amodeo G, Fernández-Illescas S, Herman E, Chaumont F, Chrispeels MJ (2005). The role of aquaporins and membrane damage in chilling and hydrogen peroxide induced changes in the hydraulic conductance of maize roots. Plant Physiol 137, 341-353.
DOI PMID |
| [9] | Bahari L, Yashunsky V, Braslavsky I (2015). Microscopic investigation of antifreeze proteins activity at cryogenic temperatures. Cryobiology 71, 570-573. |
| [10] |
Bredow M, Vanderbeld B, Walker VK (2017). Ice-binding proteins confer freezing tolerance in transgenic Arabidopsis thaliana. Plant Biotechnol J 15, 68-81.
DOI URL |
| [11] |
Cheung RCF, Ng TB, Wong JH (2017). Antifreeze proteins from diverse organisms and their applications: an overview. Curr Protein Pept Sci 18, 262-283.
DOI URL |
| [12] |
Chun JU, Yu XM, Griffith M (1998). Genetic studies of antifreeze proteins and their correlation with winter survival in wheat. Euphytica 102, 219-226.
DOI URL |
| [13] |
Collins T, Margesin R (2019). Psychrophilic lifestyles: mechanisms of adaptation and biotechnological tools. Appl Microbiol Biotechnol 103, 2857-2871.
DOI PMID |
| [14] |
Dave RS, Mitra RK (1998). A low temperature induced apoplastic protein isolated from Arachis hypogaea. Phytochemistry 49, 2207-2213.
PMID |
| [15] |
Davies PL (2014). Ice-binding proteins: a remarkable diversity of structures for stopping and starting ice growth. Trends Biochem Sci 39, 548-555.
DOI PMID |
| [16] |
DeVries AL (1983). Antifreeze peptides and glycopeptides in cold-water fishes. Annu Rev Physiol 45, 245-260.
PMID |
| [17] |
DeVries AL (1986). Antifreeze glycopeptides and peptides: interactions with ice and water. Methods Enzymol 127, 293-303.
PMID |
| [18] |
Dong XY, Liu ZG, Mi WB, Xu CM, Xu MX, Zhou Y, Zhen GQ, Cao XD, Fang XL, Mi C (2020). Overexpression of BrAFP1 gene from winter rapeseed (Brassica rapa) confers cold tolerance in Arabidopsis. Plant Physiol Biochem 155, 338-345.
DOI URL |
| [19] |
Duman JG (1994). Purification and characterization of a thermal hysteresis protein from a plant, the bittersweet nightshade Solanum dulcamara. Biochim Biophys Acta Protein Struct Mol Enzymol 1206, 129-135.
DOI URL |
| [20] |
Eickhoff L, Dreischmeier K, Zipori A, Sirotinskaya V, Adar C, Reicher N, Braslavsky I, Rudich Y, Koop T (2019). Contrasting behavior of antifreeze proteins: ice growth inhibitors and ice nucleation promoters. J Phys Chem Lett 10, 966-972.
DOI PMID |
| [21] |
Eskandari A, Leow TC, Rahman MBA, Oslan SN (2020). Antifreeze proteins and their practical utilization in industry, medicine, and agriculture. Biomolecules 10, 1649.
DOI URL |
| [22] |
Fan Y, Liu B, Wang H, Wang S, Wang J (2002). Cloning of an antifreeze protein gene from carrot and its influence on cold tolerance in transgenic tobacco plants. Plant Cell Rep 21, 296-301.
DOI URL |
| [23] |
Fletcher GL, Hew CL, Davies PL (2001). Antifreeze proteins of teleost fishes. Annu Rev Physiol 63, 359-390.
PMID |
| [24] |
Gao WJ, He M, Liu J, Ma X, Zhang Y, Dai SL, Zhou YW (2018). Overexpression of Chrysanthemum lavandulifolium ClCBF1 in Chrysanthemum morifolium ‘White Snow’ improves the level of salinity and drought tolerance. Plant Physiol Biochem 124, 50-58.
DOI URL |
| [25] |
Garnham CP, Gilbert JA, Hartman CP, Campbell RL, Laybourn-Parry J, Davies PL (2008). A Ca2+-dependent bacterial antifreeze protein domain has a novel β-helical ice-binding fold. Biochem J 411, 171-180.
DOI URL |
| [26] |
Gill SS, Tuteja N (2010). Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48, 909-930.
DOI URL |
| [27] |
Giubertoni G, Meister K, DeVries AL, Bakker HJ (2019). Determination of the solution structure of antifreeze glycoproteins using two-dimensional infrared spectroscopy. J Phys Chem Lett 10, 352-357.
DOI PMID |
| [28] |
Gong ZY, Ewart KV, Hu ZZ, Fletcher GL, Hew CL (1996). Skin antifreeze protein genes of the winter flounder, Pleuronectes americanus, encode distinct and active polypeptides without the secretory signal and prosequences. J Biol Chem 271, 4106-4112.
DOI PMID |
| [29] |
Griffith M, Ala P, Yang DSC, Hon WC, Moffatt BA (1992). Antifreeze protein produced endogenously in winter rye leaves. Plant Physiol 100, 593-596.
DOI PMID |
| [30] | Griffith M, Antikainen M, Hon WC, Pihakaski-Maunsbach K, Yu XM, Chun JU, Yang DSC (1997). Antifreeze proteins in winter rye. Physiol Plant 100, 327-332. |
| [31] |
Griffith M, Yaish MWF (2004). Antifreeze proteins in overwintering plants: a tale of two activities. Trends Plant Sci 9, 399-405.
PMID |
| [32] |
Gruneberg AK, Graham LA, Eves R, Agrawal P, Oleschuk RD, Davies PL (2021). Ice recrystallization inhibition activity varies with ice-binding protein type and does not correlate with thermal hysteresis. Cryobiology 99, 28-39.
DOI PMID |
| [33] |
Guo XY, Liu DF, Chong K (2018). Cold signaling in plants: insights into mechanisms and regulation. J Integr Plant Biol 60, 745-756.
DOI |
| [34] |
Gupta R, Deswal R (2012). Low temperature stress modulated secretome analysis and purification of antifreeze protein from Hippophae rhamnoides, a Himalayan wonder plant. J Proteome Res 11, 2684-2696.
DOI PMID |
| [35] |
Gupta R, Deswal R (2014a). Antifreeze proteins enable plants to survive in freezing conditions. J Biosci 39, 931-944.
DOI URL |
| [36] |
Gupta R, Deswal R (2014b). Refolding of β-stranded class I chitinases of Hippophae rhamnoides enhances the antifreeze activity during cold acclimation. PLoS One 9, e91723.
DOI URL |
| [37] |
Hightower R, Baden C, Penzes E, Lund P, Dunsmuir P (1991). Expression of antifreeze proteins in transgenic plants. Plant Mol Biol 17, 1013-1021.
PMID |
| [38] |
Hiilovaara-Teijo M, Hannukkala A, Griffith M, Yu XM, Pihakaski-Maunsbach K (1999). Snow-mold-induced apoplastic proteins in winter rye leaves lack antifreeze activity. Plant Physiol 121, 665-674.
PMID |
| [39] |
Holmberg N, Farrés J, Bailey JE, Kallio PT (2001). Targeted expression of a synthetic codon optimized gene, encoding the spruce budworm antifreeze protein, leads to accumulation of antifreeze activity in the apoplasts of transgenic tobacco. Gene 275, 115-124.
PMID |
| [40] |
Hon WC, Griffith M, Chong P, Yang DSC (1994). Extraction and isolation of antifreeze proteins from winter rye (Secale cereale L.) leaves. Plant Physiol 104, 971-980.
PMID |
| [41] |
Hon WC, Griffith M, Mlynarz A, Kwok YC, Yang DSC (1995). Antifreeze proteins in winter rye are similar to pathogenesis-related proteins. Plant Physiol 109, 879-889.
PMID |
| [42] |
Hoshino T, Odaira M, Yoshida M, Tsuda S (1999). Physiological and biochemical significance of antifreeze substances in plants. J Plant Res 112, 255-261.
DOI URL |
| [43] |
Huang T, Duman JG (2002). Cloning and characterization of a thermal hysteresis (antifreeze) protein with DNA- binding activity from winter bittersweet nightshade, Solanum dulcamara. Plant Mol Biol 48, 339-350.
PMID |
| [44] |
Huang T, Nicodemus J, Zarka DG, Thomashow MF, Wisniewski M, Duman JG (2002). Expression of an insect (Dendroides canadensis) antifreeze protein in Arabidopsis thaliana results in a decrease in plant freezing temperature. Plant Mol Biol 50, 333-344.
PMID |
| [45] |
Hussain HA, Men S, Hussain S, Zhang QW, Ashraf U, Anjum SA, Ali I, Wang LC (2020). Maize tolerance against drought and chilling stresses varied with root morphology and antioxidative defense system. Plants 9, 720.
DOI URL |
| [46] |
Jamalainen EA (1974). Resistance in winter cereals and grasses to low-temperature parasitic fungi. Annu Rev Phytopathol 12, 281-302.
DOI URL |
| [47] |
Jarząbek M, Pukacki PM, Nuc K (2009). Cold-regulated proteins with potent antifreeze and cryoprotective activities in spruces (Picea spp.). Cryobiology 58, 268-274.
DOI URL |
| [48] |
Jia ZC, Davies PL (2002). Antifreeze proteins: an unusual receptor-ligand interaction. Trends Biochem Sci 27, 101-106.
PMID |
| [49] |
John UP, Polotnianka RM, Sivakumaran KA, Chew O, Mackin L, Kuiper MJ, Talbot JP, Nugent GD, Mautord J, Schrauf GE, Spangenberg GC (2009). Ice recrystallization inhibition proteins (IRIPs) and freeze tolerance in the cryophilic Antarctic hair grass Deschampsia antarctica E. Desv. Plant Cell Environ 32, 336-348.
DOI URL |
| [50] | Kawahara H, Fujii A, Inoue M, Kitao S, Fukuoka J, Obata H (2009). Antifreeze activity of cold acclimated Japanese radish and purification of antifreeze peptide. Cryo Lett 30, 119-131. |
| [51] |
Kenward KD, Altschuler M, Hildebrand D, Davies PL (1993). Accumulation of type I fish antifreeze protein in transgenic tobacco is cold-specific. Plant Mol Biol 23, 377-385.
PMID |
| [52] |
Kenward KD, Brandle J, McPherson J, Davies PL (1999). Type II fish antifreeze protein accumulation in transgenic tobacco does not confer frost resistance. Transgenic Res 8, 105-117.
PMID |
| [53] |
Khanna HK, Daggard GE (2006). Targeted expression of redesigned and codon optimised synthetic gene leads to recrystallisation inhibition and reduced electrolyte leakage in spring wheat at sub-zero temperatures. Plant Cell Rep 25, 1336-1346.
PMID |
| [54] |
Knight CA, Duman JG (1986). Inhibition of recrystallization of ice by insect thermal hysteresis proteins: a possible cryoprotective role. Cryobiology 23, 256-262.
DOI URL |
| [55] |
Knight CA, Hallett J, DeVries AL (1988). Solute effects on ice recrystallization: an assessment technique. Cryobiology 25, 55-60.
PMID |
| [56] |
Kontogiorgos V, Regand A, Yada RY, Goff HD (2007). Isolation and characterization of ice structuring proteins from cold-acclimated winter wheat grass extract for recrystallization inhibition in frozen foods. J Food Biochem 31, 139-160.
DOI URL |
| [57] |
Kozuch DJ, Stillinger FH, Debenedetti PG (2020). Genetic algorithm approach for the optimization of protein antifreeze activity using molecular simulations. J Chem Theory Comput 16, 7866-7873.
DOI PMID |
| [58] |
Kuiper MJ, Davies PL, Walker VK (2001). A theoretical model of a plant antifreeze protein from Lolium perenne. Biophys J 81, 3560-3565.
PMID |
| [59] |
Liu K, Wang CL, Ma J, Shi GS, Yao X, Fang HP, Song YL, Wang JJ (2016). Janus effect of antifreeze proteins on ice nucleation. Proc Natl Acad Sci USA 113, 14739-14744.
DOI PMID |
| [60] |
Liu ZG, Dong XY, Ma L, Sun WC, Yang G, Fang Y, Wu JY, Li XC (2019). Separation and identification of Brassica rapa BrAFP and its gene cloning and expression under freezing stress. Plant Breeding 138, 193-201.
DOI URL |
| [61] |
Marentes E, Griffith M, Mlynarz A, Brush RA (1993). Proteins accumulate in the apoplast of winter rye leaves during cold acclimation. Physiol Plant 87, 499-507.
DOI URL |
| [62] |
Mazur P (1984). Freezing of living cells: mechanisms and implications. Am J Physiol-Cell Ph 247, C125-C142.
DOI URL |
| [63] |
Meyer K, Keil M, Naldrett MJ (1999). A leucine-rich repeat protein of carrot that exhibits antifreeze activity. FEBS Lett 447, 171-178.
DOI PMID |
| [64] |
Middleton AJ, Marshall CB, Faucher F, Bar-Dolev M, Braslavsky I, Campbell RL, Walker VK, Davies PL (2012). Antifreeze protein from freeze-tolerant grass has a beta-roll fold with an irregularly structured ice-binding site. J Mol Biol 416, 713-724.
DOI PMID |
| [65] | Muthukumaran J, Manivel P, Kannan M, Jeyakanthan J, Krishna R (2011). A framework for classification of antifreeze proteins in over wintering plants based on their sequence and structural features. J Bioinf Seq Anal 3, 70-88. |
| [66] |
Nakamura T, Ishikawa M, Nakatani H, Oda A (2008). Characterization of cold-responsive extracellular chitinase in bromegrass cell cultures and its relationship to antifreeze activity. Plant Physiol 147, 391-401.
DOI PMID |
| [67] | Naullage PM, Metya AK, Molinero V (2020). Computationally efficient approach for the identification of ice-binding surfaces and how they bind ice. J Chem Phys 153, 174-106. |
| [68] |
Payne SR, Sandford D, Harris A, Young OA (1994). The effects of antifreeze proteins on chilled and frozen meat. Meat Sci 37, 429-438.
DOI PMID |
| [69] |
Pihakaski-Maunsbach K, Griffith M, Antikainen M, Maunsbach AB (1996). Immunogold localization of glucanase-like antifreeze protein in cold acclimated winter rye. Protoplasma 191, 115-125.
DOI URL |
| [70] |
Pinhero RG, Rao MV, Paliyath G, Murr DP, Fletcher RA (1997). Changes in activities of antioxidant enzymes and their relationship to genetic and paclobutrazol-induced chilling tolerance of maize seedlings. Plant Physiol 114, 695-704.
PMID |
| [71] |
Pudney PDA, Buckley SL, Sidebottom CM, Twigg SN, Sevilla MP, Holt CB, Roper D, Telford JH, McArthur AJ, Lillford PJ (2003). The physico-chemical characterization of a boiling stable antifreeze protein from a perennial grass (Lolium perenne). Arch Biochem Biophys 410, 238-245.
PMID |
| [72] |
Rahman AT, Arai T, Yamauchi A, Miura A, Kondo H, Ohyama Y, Tsuda S (2019). Ice recrystallization is strongly inhibited when antifreeze proteins bind to multiple ice planes. Sci Rep 9, 2212.
DOI PMID |
| [73] |
Raymond JA, Janech MG, Fritsen CH (2009). Novel ice-binding proteins from a psychrophilic antarctic alga (Chlamydomonadaceae, Chlorophyceae). J Phycol 45, 130-136.
DOI PMID |
| [74] |
Sabala I, Egertsdotter U, von Fircks H, von Arnold S (1996). Abscisic acid-induced secretion of an antifreeze-like protein in embryogenic cell lines of Picea abies. J Plant Physiol 149, 163-170.
DOI URL |
| [75] |
Sabehat A, Weiss D, Lurie S (1998). Heat-shock proteins and cross-tolerance in plants. Physiol Plant 103, 437-441.
DOI URL |
| [76] |
Sandve SR, Rudi H, Asp T, Rognli OR (2008). Tracking the evolution of a cold stress associated gene family in cold tolerant grasses. BMC Evol Biol 8, 245.
DOI PMID |
| [77] |
Scotter AJ, Marshall CB, Graham LA, Gilbert JA, Garnham CP, Davies PL (2006). The basis for hyperactivity of antifreeze proteins. Cryobiology 53, 229-239.
PMID |
| [78] |
Shi YT, Ding YL, Yang SH (2015). Cold signal transduction and its interplay with phytohormones during cold acclimation. Plant Cell Physiol 56, 7-15.
DOI PMID |
| [79] |
Sidebottom C, Buckley S, Pudney P, Twigg S, Jarman C, Holt C, Telford J, McArthur A, Worrall D, Hubbard R, Lillford P (2000). Heat-stable antifreeze protein from grass. Nature 406, 256.
DOI |
| [80] |
Simpson DJ, Smallwood M, Twigg S, Doucet CJ, Ross J, Bowles DJ (2005). Purification and characterisation of an antifreeze protein from Forsythia suspensa (L.). Cryobiology 51, 230-234.
DOI URL |
| [81] |
Smallwood M, Worrall D, Byass L, Elias L, Ashford D, Doucet CJ, Holt C, Telford J, Lillford P, Bowles DJ (1999). Isolation and characterization of a novel antifreeze protein from carrot (Daucus carota). Biochem J 340, 385-391.
DOI URL |
| [82] |
Snider CS, Hsiang T, Zhao GY, Griffith M (2000). Role of ice nucleation and antifreeze activities in pathogenesis and growth of snow molds. Phytopathology 90, 354-361.
DOI PMID |
| [83] |
Thomashow MF (1999). Plant cold acclimation: freezing tolerance genes and regulatory mechanisms. Annu Rev Plant Physiol Plant Mol Biol 50, 571-599.
DOI URL |
| [84] |
Tronsmo AM (1984). Predisposing effects of low temperature on resistance to winter stress factors in grasses. Acta Agric Scand 34, 210-220.
DOI URL |
| [85] | Tronsmo AM (1993). Resistance to winter stress factors in Half-Sib families of Dactylis glomerata, tested in a controlled environment. Acta Agric Scand Sect B-Soil Plant Sci 43, 89-96. |
| [86] |
Tyshenko MG, Doucet D, Davies PL, Walker VK (1997). The antifreeze potential of the spruce budworm thermal hysteresis protein. Nat Biotechnol 15, 887-890.
PMID |
| [87] |
Urbańczyk M, Góra J, Latajka R, Sewald N (2017). Antifreeze glycopeptides: from structure and activity studies to current approaches in chemical synthesis. Amino Acids 49, 209-222.
DOI PMID |
| [88] |
Urrutia ME, Duman JG, Knight CA (1992). Plant thermal hysteresis proteins. Biochim Biophys Acta Protein Struct Mol Enzymol 1121, 199-206.
DOI URL |
| [89] |
Wallis JG, Wang HY, Guerra DJ (1997). Expression of a synthetic antifreeze protein in potato reduces electrolyte release at freezing temperatures. Plant Mol Biol 35, 323-330.
DOI PMID |
| [90] |
Wang Y, Qiu LM, Dai CY, Wang J, Luo JM, Zhang FC, Ma J (2008). Expression of insect (Microdera puntipennis dzungarica) antifreeze protein MpAFP149 confers the cold tolerance to transgenic tobacco. Plant Cell Rep 27, 1349-1358.
DOI PMID |
| [91] |
Wisniewski M, Webb R, Balsamo R, Close TJ, Yu XM, Griffith M (1999). Purification, immunolocalization, cryoprotective, and antifreeze activity of PCA60: a dehydrin from peach (Prunus persica). Physiol Plant 105, 600-608.
DOI URL |
| [92] |
Worrall D, Elias L, Ashford D, Smallwood M, Sidebottom C, Lillford P, Telford J, Holt C, Bowles D (1998). A carrot leucine-rich-repeat protein that inhibits ice recrystallization. Science 282, 115-117.
PMID |
| [93] |
Yaish MWF, Doxey AC, McConkey BJ, Moffatt BA, Griffith M (2006). Cold-active winter rye glucanases with ice-binding capacity. Plant Physiol 141, 1459-1472.
PMID |
| [94] |
Yan SP, Zhang QY, Tang ZC, Su WA, Sun WN (2006). Comparative proteomic analysis provides new insights into chilling stress responses in rice. Mol Cell Proteomics 5, 484-496.
DOI URL |
| [95] |
Ye QL, Eves R, Campbell RL, Davies PL (2020). Crystal structure of an insect antifreeze protein reveals ordered waters on the ice-binding surface. Biochem J 477, 3271-3286.
DOI PMID |
| [96] |
Yeh S, Moffatt BA, Griffith M, Xiong F, Yang DSC, Wiseman SB, Sarhan F, Danyluk J, Xue YQ, Hew CL, Doherty-Kirby A, Lajoie G (2000). Chitinase genes responsive to cold encode antifreeze proteins in winter cereals. Plant Physiol 124, 1251-1264.
PMID |
| [97] |
Yeh Y, Feeney RE (1996). Antifreeze proteins: structures and mechanisms of function. Chem Rev 96, 601-618.
DOI PMID |
| [98] | Yong J, Fei Y, Wei L (2000). Purification and identification of an antifreeze protein from the leaves of Ammopiptanthus mongolicus. Acta Bot Sin 6, 67-73. |
| [99] |
Yu XM, Griffith M (1999). Antifreeze proteins in winter rye leaves form oligomeric complexes. Plant Physiol 119, 1361-1369.
PMID |
| [100] |
Yu XM, Griffith M (2001). Winter rye antifreeze activity increases in response to cold and drought, but not abscisic acid. Physiol Plant 112, 78-86.
DOI URL |
| [101] |
Yu XM, Griffith M, Wiseman SB (2001). Ethylene induces antifreeze activity in winter rye leaves. Plant Physiol 126, 1232-1240.
PMID |
| [102] | Zandiyeh S, Ebrahimi F, Sabbaghian M (2018). Application of antifreeze proteins for sperm cryopreservation. Crimson Publ 1, 61-62. |
| [103] |
Zhang CZ, Fei SZ, Arora R, Hannapel DJ (2010). Ice recrystallization inhibition proteins of perennial ryegrass enhance freezing tolerance. Planta 232, 155-164.
DOI PMID |
| [104] |
Zhang DQ, Liu B, Feng DR, He YM, Wang JF (2004). Expression, purification, and antifreeze activity of carrot antifreeze protein and its mutants. Protein Expr Purif 35, 257-263.
DOI URL |
| [105] |
Zhang DQ, Wang HB, Liu B, Feng DR, He YM, Wang JF (2006). Carrot antifreeze protein does not exhibit the polygalacturonase-inhibiting activity of PGIP family. Acta Genet Sin 33, 1027-1036.
DOI URL |
| [106] | Zhang SH, Wei Y, Liu JL, Yu HM, Yin JH, Pan HY, Baldwin TC (2011). An apoplastic chitinase CpCHT1 isolated from the corolla of wintersweet exhibits both antifreeze and antifungal activities. Biol Plant 55, 141-148. |
| [107] |
Zhu B, Xiong AS, Peng RH, Xu J, Jin XF, Meng XR, Yao QH (2010). Over-expression of ThpI from Choristoneura fumiferana enhances tolerance to cold in Arabidopsis. Mol Biol Rep 37, 961-966.
DOI PMID |
| [1] | 谢露露, 崔青青, 董春娟, 尚庆茂. 植物嫁接愈合分子机制研究进展[J]. 植物学报, 2020, 55(5): 634-643. |
| [2] | 王劲东,周豫,余佳雯,范晓磊,张昌泉,李钱峰,刘巧泉. miR172-AP2模块调控植物生长发育及逆境响应的研究进展[J]. 植物学报, 2020, 55(2): 205-215. |
| [3] | 陈颖, 王婷, 华学军. 脯氨酸转运相关基因的研究进展[J]. 植物学报, 2018, 53(6): 754-763. |
| [4] | 李明, 李长生, 赵传志, 李爱芹, 王兴军. 植物SPL转录因子研究进展[J]. 植物学报, 2013, 48(1): 107-116. |
| [5] | 李莎, 姜凌, 王崇英, 张春义. 叶酸在植物体内功能的研究进展[J]. 植物学报, 2012, 47(5): 525-533. |
| [6] | 张辉 汤文开 谭新 龚路路 李学宝. 棉纤维发育及其相关基因表达调控研究进展[J]. 植物学报, 2007, 24(02): 127-133. |
| [7] | 戴晓峰;肖玲;武玉花;吴刚;卢长明. 植物脂肪酸去饱和酶及其编码基因研究进展[J]. 植物学报, 2007, 24(01): 105-113. |
| [8] | 许莉 郑文竹 左正宏 周涵韬 许玉德. 玉米a-微管蛋白分子生物学研究进展[J]. 植物学报, 1999, 16(05): 488-494. |
| [9] | 向旭 傅家瑞. 脱落酸应答基因的表达调控及其与逆境胁迫的关系[J]. 植物学报, 1998, 15(03): 11-16. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||