

植物学报 ›› 2018, Vol. 53 ›› Issue (5): 661-670.DOI: 10.11983/CBB17141 cstr: 32102.14.CBB17141
曾引伟, 曹玉曼, 沙煦旸, 李淑霞, 杨培志, 呼天明, 刘金隆*( )
)
收稿日期:2017-07-30
接受日期:2017-12-05
出版日期:2018-09-01
发布日期:2018-11-29
通讯作者:
刘金隆
作者简介:† 共同第一作者。
基金资助:
Zeng Yinwei, Cao Yuman, Sha Xuyang, Li Shuxia, Yang Peizhi, Hu Tianming, Liu Jinlong*( )
)
Received:2017-07-30
Accepted:2017-12-05
Online:2018-09-01
Published:2018-11-29
Contact:
Liu Jinlong
About author:† These authors contributed equally to this paper
摘要: 实时观测根瘤及根系形态对于豆科植物研究具有重要意义, 但目前还缺乏一个便于观测根系、高效结瘤、适宜生长且经济实用的豆科植物培养体系。以蒺藜苜蓿(Medicago truncatula)为植物材料, 建立了一种可实时观测根瘤及根系形态的纸袋水培法, 并与其它常用方法进行对比。结果表明, 依赖于石英砂等固体介质栽培蒺藜苜蓿对根瘤和根系形态的实时观测造成障碍, 而水培和喷雾培养等方法的根瘤菌接种效率不高, 且不便观测侧根发育情况。采用纸袋水培法探讨了褪黑素对蒺藜苜蓿根系发育的影响, 发现褪黑素具有降低根瘤形成效率、抑制侧根伸长、增加侧根数目以及增大侧根与主根之间夹角等作用。因此, 纸袋水培法能够高效接种根瘤菌且为实时无损伤观测根瘤及根系形态提供了可能, 是一种适用于豆科植物简单有效的培养方法。
曾引伟, 曹玉曼, 沙煦旸, 李淑霞, 杨培志, 呼天明, 刘金隆. 一种简单有效的非损伤观测根瘤和根系形态的方法. 植物学报, 2018, 53(5): 661-670.
Zeng Yinwei, Cao Yuman, Sha Xuyang, Li Shuxia, Yang Peizhi, Hu Tianming, Liu Jinlong. An Observation Method of Nodule and Root Morphology without Damage in Real-time. Chinese Bulletin of Botany, 2018, 53(5): 661-670.
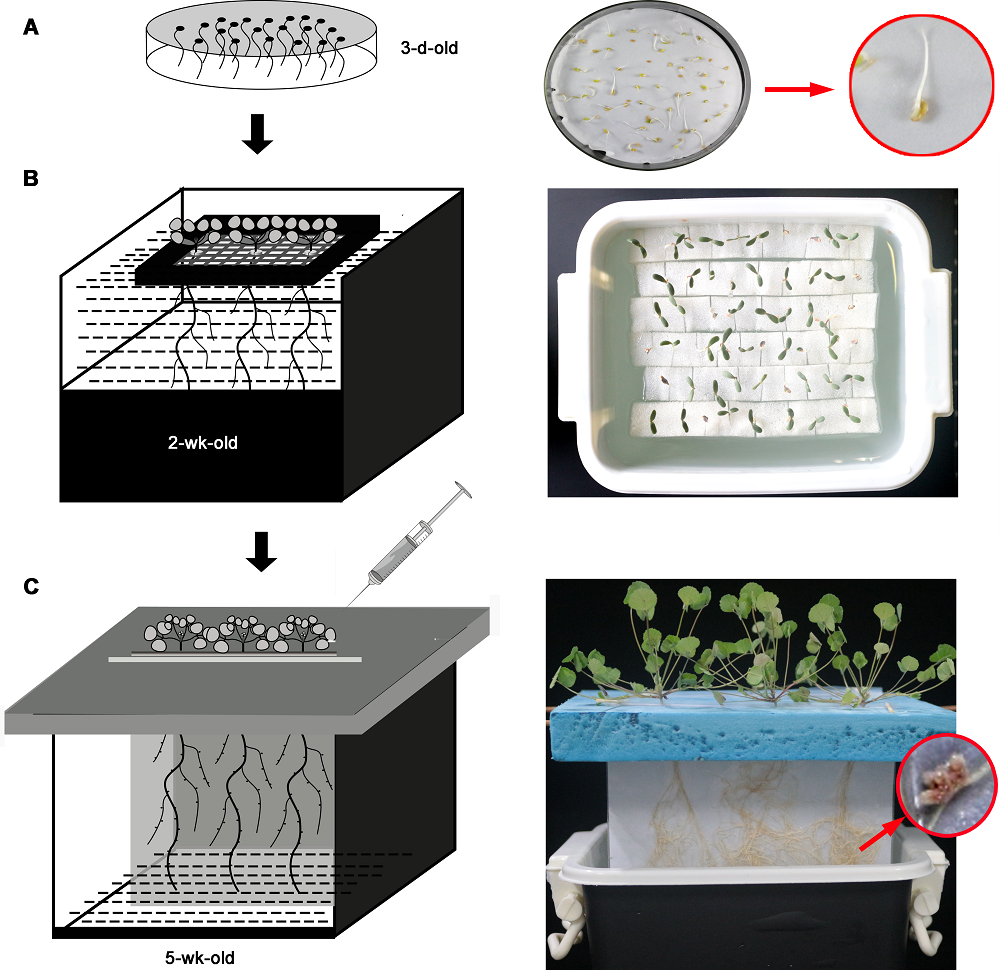
图1 纸袋水培法示意图(右侧为对应实物图)(A) 种子发芽; (B) 水培育苗; (C) 纸袋水培装置
Figure 1 Diagram of paper-based Ziplock bag hydroponics system (right side is the picture of the actual object)(A) Seed germination; (B) Water culture seedlings; (C) Paper-based Ziplock bag hydroponics device
| Treatment | Number of nodule per plant | Lateral root length (cm) | Number of lateral root per plant | Angle of lateral root with primary root (°) | Secondary lateral root |
|---|---|---|---|---|---|
| Control | 70.7±2.5 | 4.1±0.2 | 86.3±4.2 | 52.3±2.1 | YES |
| Melatonin (50 μmol·mL-1) | 21.3±1.5*** | 1.7±0.2*** | 143.3±7.1*** | 85.7±3.1*** | NO |
表1 褪黑素(50 μmol∙mL-1)处理对蒺藜苜蓿侧根发育及根瘤接种效果的影响(平均值±标准差, n=12)
Table 1 Effects of melatonin (50 μmol·mL-1) treatment on lateral root development and the inoculation of nodules in Medicago truncatula (means±SD, n=12)
| Treatment | Number of nodule per plant | Lateral root length (cm) | Number of lateral root per plant | Angle of lateral root with primary root (°) | Secondary lateral root |
|---|---|---|---|---|---|
| Control | 70.7±2.5 | 4.1±0.2 | 86.3±4.2 | 52.3±2.1 | YES |
| Melatonin (50 μmol·mL-1) | 21.3±1.5*** | 1.7±0.2*** | 143.3±7.1*** | 85.7±3.1*** | NO |

图2 不同培养方式对蒺藜苜蓿接种根瘤及根系形态的影响(A)-(C) 沙培法, (A) 沙培装置; (B) 根系及接种根瘤情况; (C) 掉落的根系; (D)-(F) 水培法, (D) 水培整体; (E) 根系形态; (F) E图中的局部根系及根瘤放大; (G)-(I) 喷雾培养法, (G) 喷雾培养装置; (H) 根系形态; (I) H图中的局部根系及根瘤放大; (J)-(M) 纸袋水培法, (J) 地上部分(左侧为R108, 右侧是A17); (K) 根系形态; (L) 地上部分及根系, (M) L图中的局部根系及根瘤放大。红色箭头为根瘤, 绿色箭头为石英砂; 除J和K图中蒺藜苜蓿为7周龄, 其余均为6周龄。
Figure 2 Effects of different culture methods on inoculation of nodules and root morphology of Medicago truncatula(A)-(C) Sand culture method, (A) Sand culture device; (B) Root morphology and nodules; (C) The lost roots; (D)-(F) Hydroponics, (D) Hydroponics; (E) Root morphology; (F) The enlarged image of local roots and nodules of Figure E; (G)-(I) Aeroponics, (G) Aeroponics device; (H) Root morphology; (I) The enlarged image of local roots and nodules of Figure H; (J)-(M) Paper-based Ziplock bag hydroponics method, (J) Above-ground parts of M. truncatula (the left side is R108, the right is A17); (K) Root morphology; (L) The whole plants of M. truncatula; (M) The enlarged image of local roots and nodules of Figure L. Red arrows for the nodules, green arrows for the quartz sand; The M. truncatula is 6 weeks old except Figure J and K which is 7 weeks old.
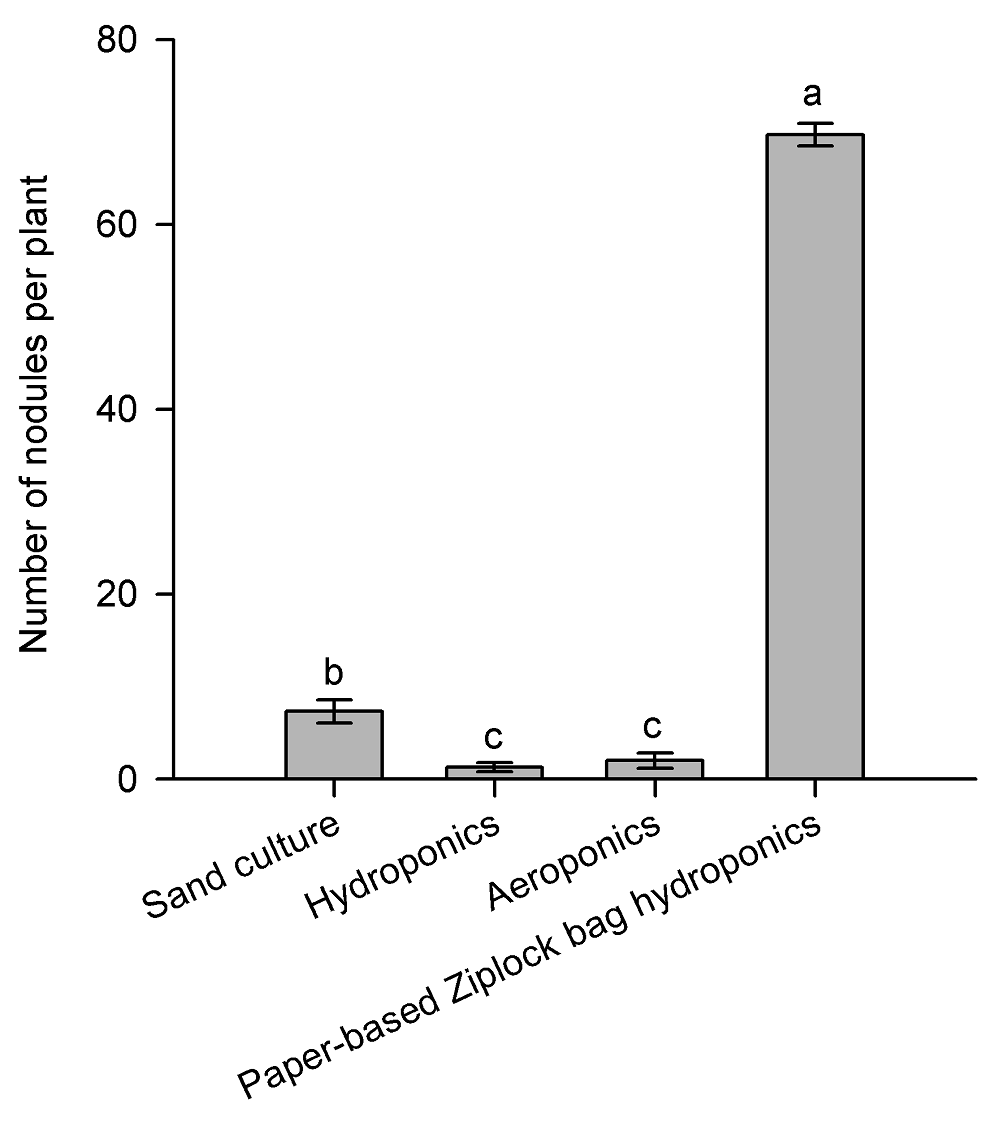
图3 不同培养方式对蒺藜苜蓿根瘤数目的影响(平均值±标准差, n=12)不同小写字母表示不同方法之间差异显著(P<0.05)。
Figure 3 Effects of different culture methods on the number of inoculated nodules in Medicago truncatula (means±SD, n=12)Different lowercase letters represent significant difference among different methods (P<0.05).
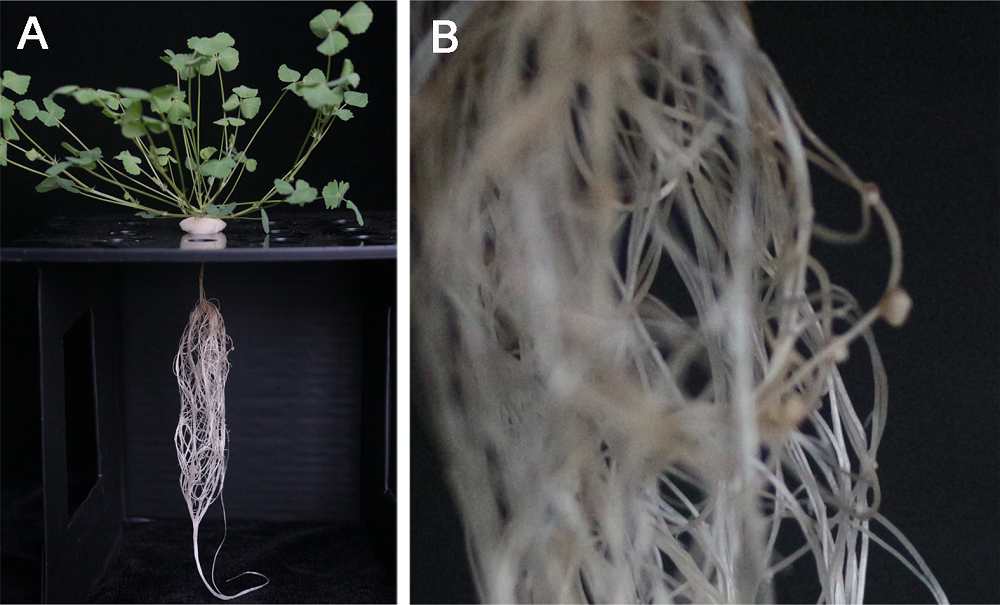
图4 蒺藜苜蓿水培(单株)根瘤数目(A) 整株情况; (B) 接瘤局部根系
Figure 4 The number of inoculated nodules of Medicago truncatula by hydroponics (single plant)(A) The whole plant; (B) The local roots with nodules
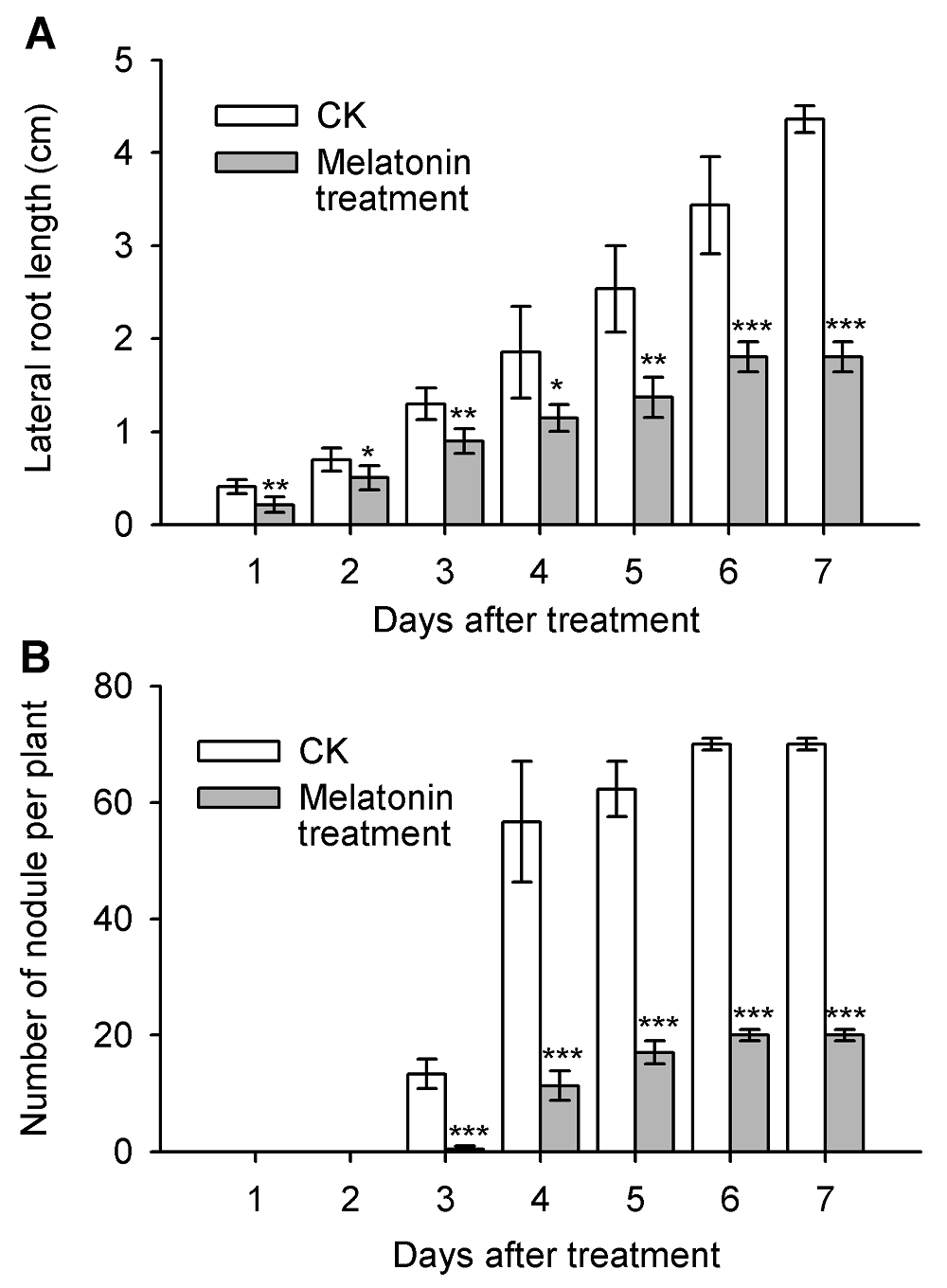
图5 褪黑素(50 μmol·mL-1)不同处理时间对蒺藜苜蓿侧根生长及根瘤形成的影响(n=12)(A) 侧根生长的动态变化; (B) 根瘤的形成。* P<0.05水平差异显著; ** P<0.01水平差异非常显著; *** P<0.001水平差异极显著。
Figure 5 Effects of melatonin (50 μmol·mL-1) on nodule formation and lateral root development of Medicago truncatula(A) Dynamic changes of lateral root growth; (B) The formation of nodules. * Represent significant differences at P<0.05; ** Represent highly significant differences at P<0.01; *** Represent extremely significant differences at P<0.001.
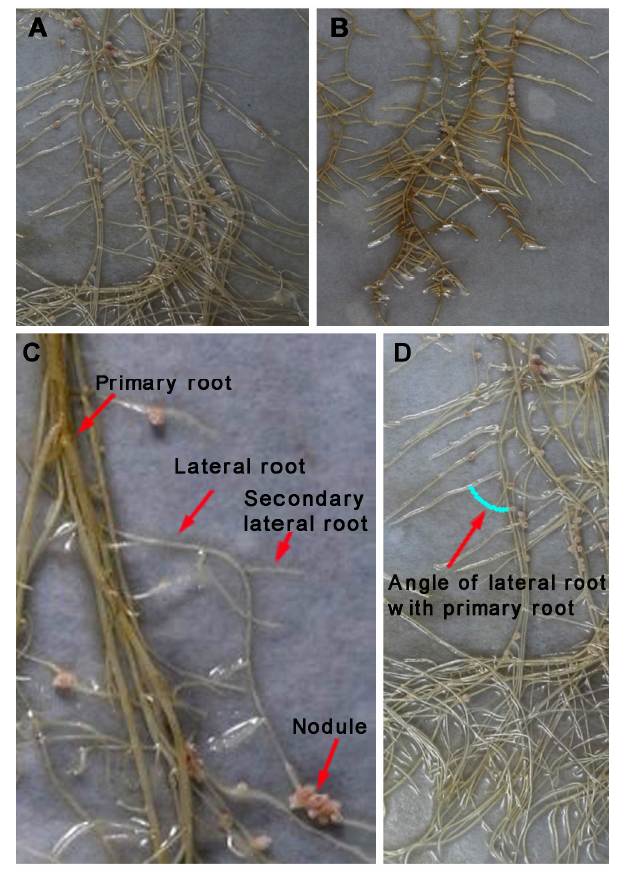
图6 褪黑素(50 μmol?mL–1)对蒺藜苜蓿(6周龄)根系的影响(A) 对照; (B) 褪黑素处理; (C) 主根、侧根、二级侧根和根瘤; (D) 主侧根夹角
Figure 6 Effect of melatonin (50 μmol?mL–1) on the root system of Medicago truncatula (6 weeks old)(A) Control; (B) Melatonin treatment; (C) Primary root, lateral root, secondary lateral root and nodules; (D) Angle of lateral root with primary root
| 1 | 陈文新, 陈文峰 (2004). 发挥生物固氮作用减少化学氮肥用量. 中国农业科技导报 6(6), 3-6. |
| 2 | 褚贵新, 沈其荣, 曹金留, 茆泽圣, 钟增涛, 赵龙 (2003). 旱作水稻与花生间作系统中的氮素固定与转移及其对土壤肥力的影响. 土壤学报 40, 717-723. |
| 3 | 梁如玉, 李登煜 (1984). 用试管水培法鉴定豆科作物的结瘤性能. 土壤肥料 (3), 37-38. |
| 4 | 章家恩, 高爱霞, 徐华勤, 罗明珠 (2009). 玉米/花生间作对土壤微生物和土壤养分状况的影响. 应用生态学报 20, 1597-1602. |
| 5 | Arnao MB, Hernández-Ruiz J (2007). Melatonin promotes adventitious-and lateral root regeneration in etiolated hypocotyls of Lupinus albus L. J Pineal Res 42, 147-152. |
| 6 | Arnao MB, Hernández-Ruiz J (2015). Functions of melatonin in plants: a review.J Pineal Res 59, 133-150. |
| 7 | Bergersen FJ (1965). Ammonia? An early stable product of nitrogen fixation by soybean root nodules F.J. Bergersen.Aust J Biol Sci 18, 1-9. |
| 8 | Clark RT, Famoso AN, Zhao KY, Shaff JE, Craft EJ, Bustamante CD, McCouch SR, Aneshansley DJ, Kochian LV (2013). High-throughput two-dimensional root system phenotyping platform facilitates genetic analysis of root growth and development.Plant Cell Environ 36, 454-466. |
| 9 | Danso SKA, Alexander M (1974). Survival of two strains of Rhizobium in soil. Soil Sci Soc Am J 38, 86-89. |
| 10 | De M Rangel W, De Oliveira Longatti SM, Ferreira PAA, Bonaldi DS, Guimarães AA, Thijs S, Weyens N, Vangronsveld J, Moreira FMS (2017). Leguminosae native nodulating bacteria from a gold mine As-contaminated soil: multi-resistance to trace elements, and possible role in plant growth and mineral nutrition.Int J Phytoremediation 19, 925-936. |
| 11 | Di Giacomo E, Laffont C, Sciarra F, Iannelli MA, Frugier F, Frugis G (2017). KNAT3/4/5-like class 2 KNOX transcription factors are involved in Medicago truncatula symbiotic nodule organ development. New Phytol 213, 822-837. |
| 12 | Dickstein R, Hu XJ, Yang J, Ba L, Coque L, Kim DJ, Cook DR, Yeung AT (2002). Differential expression of tandemly duplicated Enod8 genes in Medicago. Plant Sci 163, 333-343. |
| 13 | Famoso AN, Clark RT, Shaff JE, Craft E, McCouch SR, Kochian LV (2010). Development of a novel aluminum tolerance phenotyping platform used for comparisons of cereal aluminum tolerance and investigations into rice aluminum tolerance mechanisms.Plant Physiol 153, 1678-1691. |
| 14 | Farías-Rodríguez R, Mellor RB, Arias C, Peña-Cabriales JJ (1998). The accumulation of trehalose in nodules of several cultivars of common bean (Phaseolus vulgaris) and its correlation with resistance to drought stress. Phy- siol Plantarum 102, 353-359. |
| 15 | Hund A, Trachsel S, Stamp P (2009). Growth of axile and lateral roots of maize: I development of a phenotying platform.Plant Soil 325, 335-349. |
| 16 | Jahnke S, Menzel MI, Van Dusschoten D, Roeb GW, Bühler J, Minwuyelet S, Blümler P, Temperton VM, Hombach T, Streun M, Beer S, Khodaverdi M, Ziemons K, Coenen HH, Schurr U (2009). Combined MRI-PET dissects dynamic changes in plant structures and functions.Plant J 59, 634-644. |
| 17 | Jeudy C, Adrian M, Baussard C, Bernard C, Bernaud E, Bourion V, Busset H, Cabrera-Bosquet L, Cointault F, Han SM, Lamboeuf M, Moreau D, Pivato B, Prudent M, Trouvelot S, Truong HN, Vernoud V, Voisin AS, Wipf D, Salon C (2016). RhizoTubes as a new tool for high throughput imaging of plant root development and architecture: test, comparison with pot grown plants and validation.Plant Methods 12, 31. |
| 18 | Jeudy C, Ruffel S, Freixes S, Tillard P, Santoni AL, Morel S, Journet EP, Duc G, Gojon A, Lepetit M, Salon C (2010). Adaptation of Medicago truncatula to nitrogen limi- tation is modulated via local and systemic nodule developmental responses. New Phytol 185, 817-828. |
| 19 | Johnson HW, Means UM, Weber CR (1965). Competition for nodule sites between strains of Rhizobium japonicum applied as inoculum and strains in the soil. Agron J 57, 179-185. |
| 20 | Keyser HH, Bohlool BB, Hu TS, Weber DF (1982). Fast- growing rhizobia isolated from root nodules of soybean.Science 215, 1631-1632. |
| 21 | Kiers ET, Rousseau RA, West SA, Denison RF (2003). Host sanctions and the legume-rhizobium mutualism.Nature 425, 78-81. |
| 22 | Kistner C, Parniske M (2002). Evolution of signal transduction in intracellular symbiosis.Trends Plant Sci 7, 511-518. |
| 23 | Kuchenbuch RO, Ingram KT, Buczko U (2006). Effects of decreasing soil water content on seminal lateral roots of young maize plants.J Plant Nutr Soil Sci 169, 841-848. |
| 24 | Labidi N, Mahmoudi H, Dorsaf M, Slama I, Abdelly C (2009). Assessment of intervarietal differences in drought tolerance in chickpea using both nodule and plant traits as indicators.J Plant Breed Crop Sci 1(4), 80-86. |
| 25 | Le Marié C, Kirchgessner N, Marschall D, Walter A, Hund A (2014). Rhizoslides: paper-based growth system for non-destructive, high throughput phenotyping of root development by means of image analysis.Plant Methods 10, 13. |
| 26 | Liang CZ, Li AF, Yu H, Li WZ, Liang CZ, Guo SD, Zhang R, Chu CC (2017). Melatonin regulates root architecture by modulating auxin response in rice.Front Plant Sci 8, 134. |
| 27 | Mooney SJ, Pridmore TP, Helliwell J, Bennett MJ (2012). Developing X-ray computed tomography to non-invasively image 3-D root systems architecture in soil.Plant Soil 352, 1-22. |
| 28 | Nagel KA, Putz A, Gilmer F, Heinz K, Fischbach A, Pfeifer J, Faget M, Blossfeld S, Ernst M, Dimaki C, Kastenholz B, Kleinert AK, Galinski A, Scharr H, Fiorani F, Schurr U (2012). GROWSCREEN-Rhizo is a novel phenotyping robot enabling simultaneous measurements of root and shoot growth for plants grown in soil-filled rhizotrons.Funct Plant Biol 39, 891-904. |
| 29 | Nap JP, Bisseling T (1990). Developmental biology of a plant-prokaryote symbiosis: the legume root nodule.Science 250, 948-954. |
| 30 | O'Gara F, Shanmugam KT (1976). Regulation of nitrogen fixation by rhizobia export of fixed N2 as NH4+.Biochim Biophys Acta 437, 313-321. |
| 31 | Patterson III WA, Olson JJ (1983). Effects of heavy metals on radicle growth of selected woody species germinated on filter paper, mineral and organic soil substrates.Can J Forest Res 13, 233-238. |
| 32 | Pelagio-Flores R, Muñoz-Parra E, Ortiz-Castro R, López- Bucio J (2012). Melatonin regulates Arabidopsis root system architecture likely acting independently of auxin signaling.J Pineal Res 53, 279-288. |
| 33 | Planchamp C, Balmer D, Hund A, Mauch-Mani B (2013). A soil-free root observation system for the study of root-micro- organism interactions in maize.Plant Soil 367, 605-614. |
| 34 | Popp C, Ott T (2011). Regulation of signal transduction and bacterial infection during root nodule symbiosis.Curr Opin Plant Biol 14, 458-467. |
| 35 | Reddy CS, Pattanaik C, Mohapatra A, Biswal AK (2007). Phytosociological observations on tree diversity of tropical forest of Similipal Biosphere Reserve, Orissa, India.TAIWANIA 52, 352-359. |
| 36 | Reddy PM, Rendón-Anaya M, de los Dolores del Río M, Khandual S (2007). Flavonoids as signaling molecules and regulators of root nodule development.Dyn Soil Dyn Plant 1, 83-94. |
| 37 | Reimer R, Stich B, Melchinger AE, Schrag TA, Sørensen AP, Stamp P, Hund A (2013). Root response to temperature extremes: association mapping of temperate maize (Zea mays L). Maydica 58, 156-168. |
| 38 | Rispail N, Dita MA, González-Verdejo C, Pérez-de-Luque A, Castillejo MA, Prats E, Román B, Jorrín J, Rubiales D (2007). Plant resistance to parasitic plants: molecular approaches to an old foe.New Phytol 173, 703-712. |
| 39 | Robinson D, Hodge A, Griffiths BS, Fitter AH (1999). Plant root proliferation in nitrogen-rich patches confers competitive advantage.Proc Roy Soc B-Biol Sci 266, 431-435. |
| 40 | Ruta N, Stamp P, Liedgens M, Fracheboud Y, Hund A (2010). Collocations of QTLs for seedling traits and yield components of tropical maize under water stress conditions.Crop Sci 50, 1385-1392. |
| 41 | Ryle GJA, Powell CE, Gordon AJ (1978). Effect of source of nitrogen on the growth of Fiskeby soya bean: the carbon economy of whole plants.Ann Bot 42, 637-648. |
| 42 | Smit AL, Groenwold J (2005). Root characteristics of selected field crops: data from the Wageningen Rhizolab (1990-2002).Plant Soil 272, 365-384. |
| 43 | Smith S, De Smet I (2012). Root system architecture: insights from Arabidopsis and cereal crops.Philos Trans Roy Soc B Biol Sci 367,1441-1452. |
| 44 | Sparks DL (2003). Environmental Soil Chemistry, 2nd edn. London: Academic Press. pp. 75-133. |
| 45 | Stracke S, Kistner C, Yoshida S, Mulder L, Sato S, Kaneko T, Tabata S, Sandal N, Stougaard J, Szczyglowski K, Parniske M (2002). A plant receptor-like kinase required for both bacterial and fungal symbiosis.Nature 417, 959-962. |
| 46 | Streeter JG, Salminen SO (1992). Evidence supporting a non-phloem source of water for export of solutes in the xylem of soybean root nodules.Plant Cell Environ 15, 735-741. |
| 47 | Tilman D, Cassman KG, Matson PA, Naylor R, Polasky S (2002). Agricultural sustainability and intensive production practices.Nature 418, 671-677. |
| 48 | Truchet G, Barker DG, Camut S, De Billy F, Vasse J, Huguet T (1989). Alfalfa nodulation in the absence of Rhizobium. Mol Gen Genet 219, 65-68. |
| 49 | Udvardi MK, Day DA (1997). Metabolite transport across symbiotic membranes of legume nodules.Annu Rev Plant Biol 48, 493-523. |
| 50 | Zahran HH (1999). Rhizobium-legume symbiosis and nitrogen fixation under severe conditions and in an arid climate. Microbiol Mol Biol Rev 63, 968-989. |
| 51 | Zhu JM, Kaeppler SM, Lynch JP (2005). Mapping of QTLs for lateral root branching and length in maize (Zea mays L.) under differential phosphorus supply. Theor Appl Genet 111, 688-695. |
| 52 | Zipfel C, Oldroyd GED (2017). Plant signaling in symbiosis and immunity.Nature 543, 328-336. |
| [1] | 杜锦瑜, 孙震, 苏彦龙, 王贺萍, 刘亚玲, 吴振映, 何峰, 赵彦, 付春祥. 蒙古冰草咖啡酸氧甲基转移酶基因AmCOMT1的鉴定及功能分析[J]. 植物学报, 2024, 59(3): 383-396. |
| [2] | 刘位会, 宋小艳, 才仁多杰, 丁路明, 王长庭. 退化程度对高寒草甸不同优势植物根系形态性状和生物量的影响[J]. 植物生态学报, 2024, 48(12): 1666-1682. |
| [3] | 李林, 孙毅, 杨晓琼, 方海东, 闫帮国. 七彩花生根瘤内生菌对氮添加的响应及其与植物化学计量特征的关系[J]. 植物生态学报, 2024, 48(10): 1374-1384. |
| [4] | 张敏, 桑英, 宋金凤. 水培富贵竹的根压及其影响因素[J]. 植物生态学报, 2023, 47(7): 1010-1019. |
| [5] | 蔡淑钰, 刘建新, 王国夫, 吴丽元, 宋江平. 褪黑素促进镉胁迫下番茄种子萌发的调控机理[J]. 植物学报, 2023, 58(5): 720-732. |
| [6] | 冯晓敏, 高翔, 臧华栋, 胡跃高, 任长忠, 郝志萍, 吕慧卿, 曾昭海. 燕麦-绿豆间作效应及氮素转移特性[J]. 植物学报, 2023, 58(1): 122-131. |
| [7] | 张琦, 张文静, 袁宪凯, 李明, 赵强, 杜艳丽, 杜吉到. 褪黑素对盐胁迫下普通菜豆芽期核酸修复的调控机制[J]. 植物学报, 2023, 58(1): 108-121. |
| [8] | 艾金祥, 宋嘉怡, 严浙楠, 王志超, 陈文倩, 吴玉环, 王燕燕, 潘蕾蕾, 许俞韬, 刘鹏. 褪黑素对铅胁迫下虎舌红和朱砂根生理响应及DNA损伤的调控效应[J]. 植物学报, 2022, 57(2): 171-181. |
| [9] | 刘德帅, 姚磊, 徐伟荣, 冯美, 姚文孔. 褪黑素参与植物抗逆功能研究进展[J]. 植物学报, 2022, 57(1): 111-126. |
| [10] | 李强, 黄迎新, 周道玮, 丛山. 土壤氮磷添加下豆科草本植物生物固氮与磷获取策略的权衡机制[J]. 植物生态学报, 2021, 45(3): 286-297. |
| [11] | 王银柳, 耿倩倩, 黄建辉, 王常慧, 李磊, 哈斯木其尔, 牛国祥. 氮肥和种植密度对达乌里胡枝子的生长与生物固氮的影响[J]. 植物生态学报, 2021, 45(1): 13-22. |
| [12] | 刘承武, 赵忠. 豆科植物SHR-SCR模块——根瘤“奠基细胞”的命运推手[J]. 植物学报, 2020, 55(6): 661-665. |
| [13] | 邹显花, 胡亚楠, 韦丹, 陈思同, 吴鹏飞, 马祥庆. 磷高效利用杉木对低磷胁迫的适应性与内源激素的相关性[J]. 植物生态学报, 2019, 43(2): 139-151. |
| [14] | 艾文琴, 姜瀚原, 李欣欣, 廖红. 一种高效研究大豆根瘤共生固氮的营养液栽培体系[J]. 植物学报, 2018, 53(4): 519-527. |
| [15] | 魏解冰, 狄少康, 文江祁, 庞永珍. 植物Tnt1反转录转座子突变体库的特征及应用[J]. 植物学报, 2016, 51(6): 817-826. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||