

Chinese Bulletin of Botany ›› 2017, Vol. 52 ›› Issue (4): 453-464.DOI: 10.11983/CBB17044 cstr: 32102.14.CBB17044
Previous Articles Next Articles
Shengchun Zhang, Qingming Li, Chengwei Yang*
Received:2017-03-09
Accepted:2017-05-06
Online:2017-07-01
Published:2017-05-05
Contact:
Yang Chengwei
About author:# Co-first authors
Shengchun Zhang, Qingming Li, Chengwei Yang. Arabidopsis Metalloprotease FtSH4 Regulates Leaf Senescence Through Auxin and Reactive Oxygen Species[J]. Chinese Bulletin of Botany, 2017, 52(4): 453-464.
| Primer name | Primer sequence (5ʹ-3ʹ) |
|---|---|
| PER33F | TCTTCTCCATCACTTCTTCTTA |
| PER33R | ATCCTCCAACACATATTCTCTA |
| PER37F | CGCCAACACTCTTTGACAACAAG |
| PER37R | ACTCATCCTTATCATTGCCTTCGC |
| ARF2F | AATATAGCACCTTCATCTCCT |
| ARF2R | ATCACACTCTACACTCTCAG |
| ARF7F | GCTAATGCTAATAACAGTCCTT |
| ARF7R | TCCACATTCTTCAGTCTCAA |
| SAG12F | ATGATGAGCAAGCACTGATGAAGG |
| SAG12R | TCCGTTAGTAGATTCGCCGTATCC |
| SAG13F | GCGACAACATAAGGACGAACTCTG |
| SAG13R | GAAGACAAAGAAATGCCACAAGCG |
| SAG101F | GGGATGAGAGACGATGTGAGAGAG |
| SAG101R | CGGGTGTTCATAAACTCGGTCAAG |
| SEN1F | GGACATCCGACTAGAGCCATCAAC |
| SEN1R | ATCGCCGTGAAGCCAGCAG |
| SEN4F | AACCGCCAATTTCCACACTTACTC |
| SEN4R | CTCTTGTTGCCCAATCGTCTGC |
| UBQ10F | CCGACTACAACATTCAGAAG |
| UBQ10R | TATCAATGGTGTCAGAACTCT |
Table 1 Primers used in this study
| Primer name | Primer sequence (5ʹ-3ʹ) |
|---|---|
| PER33F | TCTTCTCCATCACTTCTTCTTA |
| PER33R | ATCCTCCAACACATATTCTCTA |
| PER37F | CGCCAACACTCTTTGACAACAAG |
| PER37R | ACTCATCCTTATCATTGCCTTCGC |
| ARF2F | AATATAGCACCTTCATCTCCT |
| ARF2R | ATCACACTCTACACTCTCAG |
| ARF7F | GCTAATGCTAATAACAGTCCTT |
| ARF7R | TCCACATTCTTCAGTCTCAA |
| SAG12F | ATGATGAGCAAGCACTGATGAAGG |
| SAG12R | TCCGTTAGTAGATTCGCCGTATCC |
| SAG13F | GCGACAACATAAGGACGAACTCTG |
| SAG13R | GAAGACAAAGAAATGCCACAAGCG |
| SAG101F | GGGATGAGAGACGATGTGAGAGAG |
| SAG101R | CGGGTGTTCATAAACTCGGTCAAG |
| SEN1F | GGACATCCGACTAGAGCCATCAAC |
| SEN1R | ATCGCCGTGAAGCCAGCAG |
| SEN4F | AACCGCCAATTTCCACACTTACTC |
| SEN4R | CTCTTGTTGCCCAATCGTCTGC |
| UBQ10F | CCGACTACAACATTCAGAAG |
| UBQ10R | TATCAATGGTGTCAGAACTCT |
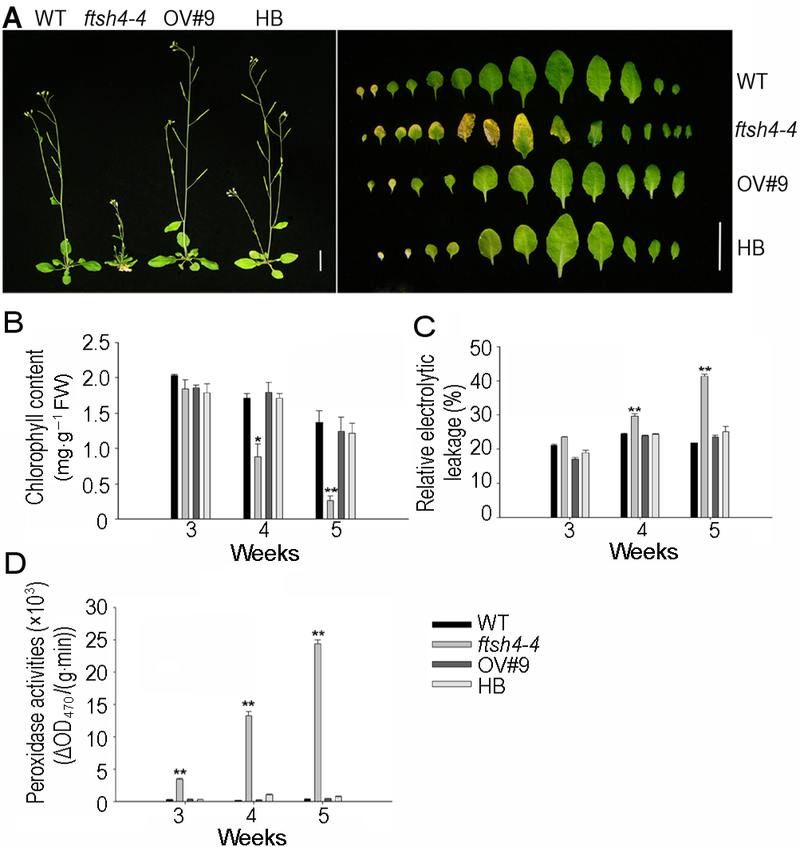
Figure 1 FtSH4 mutation causes leaf senescence of Arabi- dopsis^(A) The phenotype of premature senescence observed in the ftsh4-4 mutant (Bar=1 cm); (B) The chlorophyll content decreased in ftsh4-4 mutant; (C) The relative electrolytic leakage increased in ftsh4-4 mutant; (D) The peroxidase activities increased in ftsh4-4 mutant. * indicates significant difference at P<0.05; ** indicates significant difference at P<0.01 (Student’s t-test).
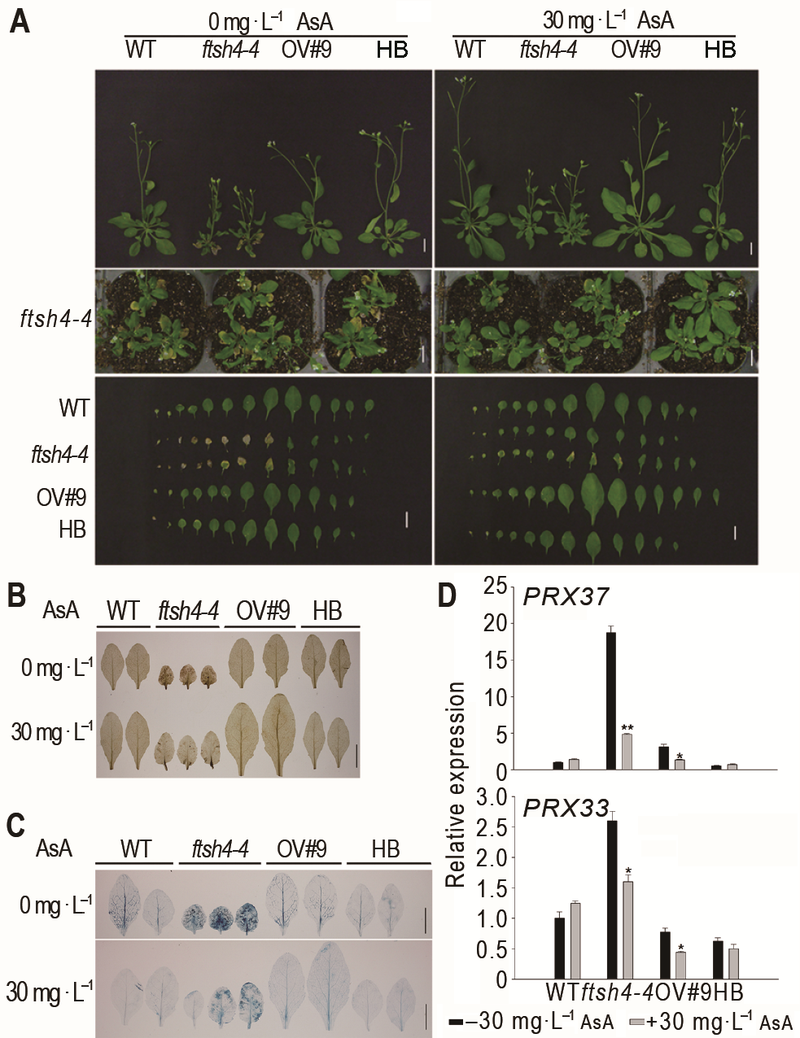
Figure 2 Exogenous AsA rescued the leaf senescence phenotype of Arabidopsis ftsh4-4 mutant^(A) Exogenous AsA rescued the leaf senescence phenotype of ftsh4-4 mutant (Bar=1 cm); (B) Exogenous AsA reduced the H2O2 level of ftsh4-4 mutant (Bar=1 cm); (C) Exogenous AsA reduced the cell death of ftsh4-4 mutant (Bar=1 cm); (D) Exogenous AsA reduced the expression of peroxidase genes in ftsh4-4 mutant. * indicates significant difference at P<0.05; ** indicates significant difference at P<0.01 (Student’s t-test).
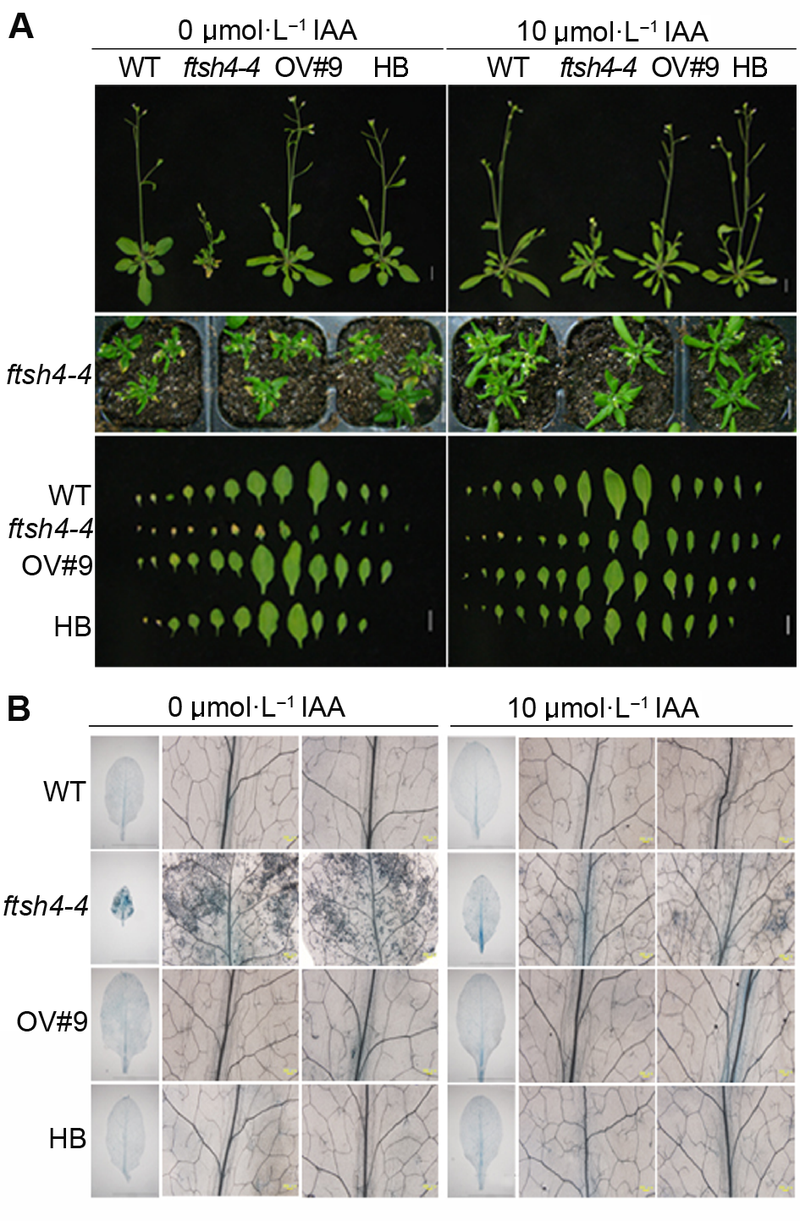
Figure 3 Exogenous IAA restored the leaf senescence phenotype of Arabidopsis ftsh4-4 mutant^(A) Exogenous IAA restored the leaf senescence phenotype of ftsh4-4 mutant (Bar=1 cm); (B) Exogenous IAA reduced the cell death of ftsh4-4 mutant
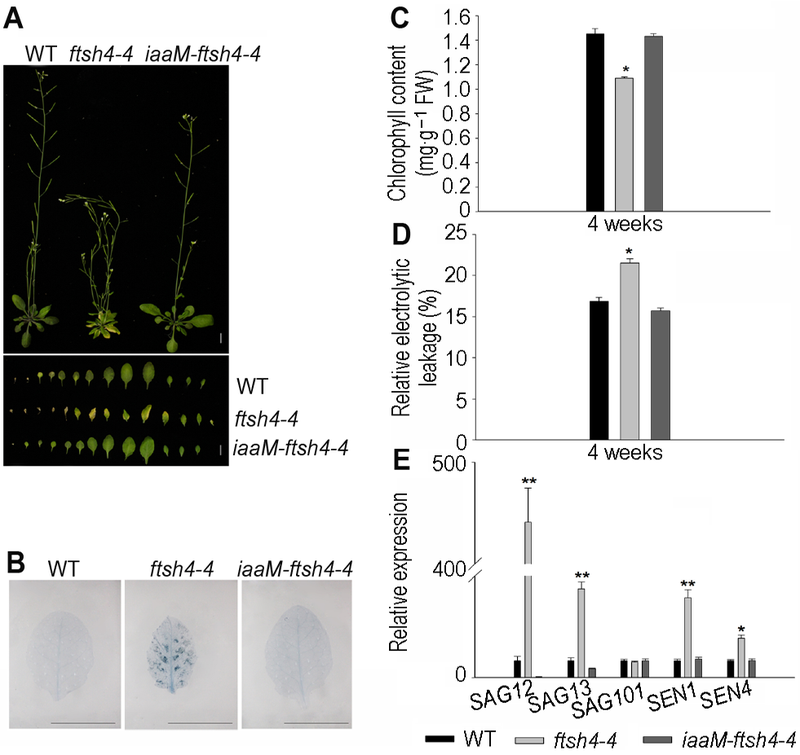
Figure 4 Increasing endogenous IAA restored the leaf senescence phenotype of Arabidopsis ftsh4-4 mutant^(A) Increasing endogenous IAA restored the leaf senescence phenotype of ftsh4-4 mutant (Bar=1 cm); (B) Cell death decreased in iaaM-ftsh4-4 (Bar=1 cm); (C) The chlorophyll content of iaaM-ftsh4-4 transgeneic line restored to the wild type level; (D) The relative electrolytic leakage of iaaM- ftsh4-4 transgeneic line restored to the wild type level; (E) Endogenous IAA decreased the expression of senescence-associated genes SAG12, SAG13, SAG101, SEN1 and SEN4 in ftsh4-4 mutant. * indicates significant difference at P<0.05; ** indicates significant difference at P<0.01 (Student’s t-test).
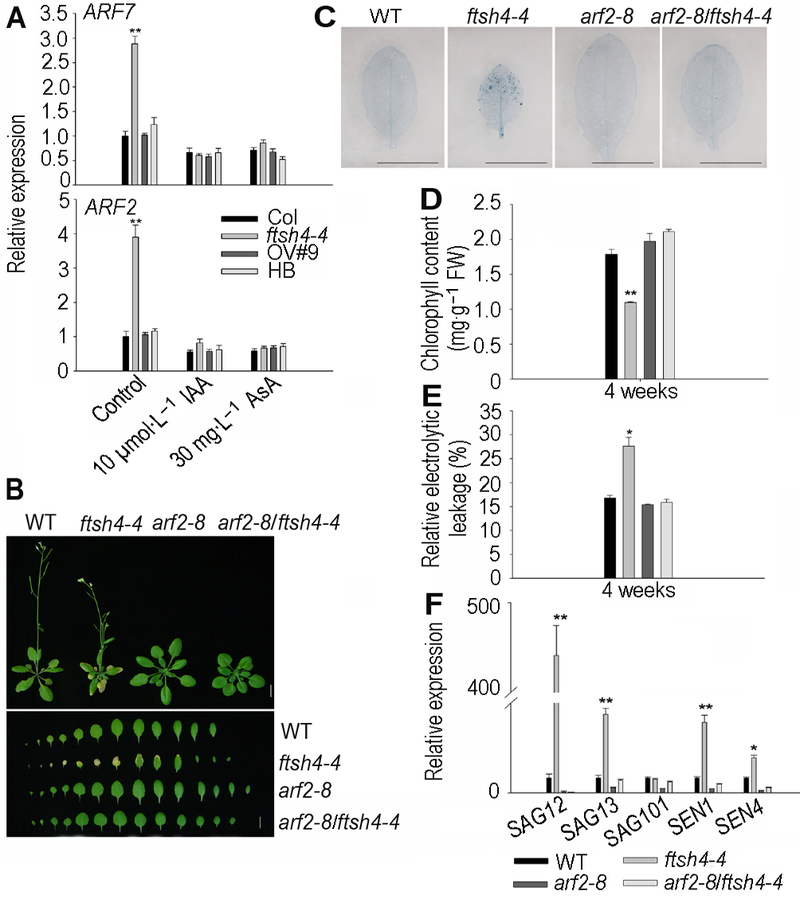
Figure 5 ARF2 is involved in FtSH4-mediated leaf senescence of Arabidopsis^(A) The expression levels of ARF2 and ARF7 increased in the ftsh4-4 and were inhibited by the exogenous IAA and AsA; (B) ARF2 mutation rescued the leaf senescence of ftsh4-4 mutant (Bar=1 cm); (C) ARF2 mutation reduced the cell death of ftsh4-4 mutant (Bar=1 cm); (D) ARF2 mutation increased the chlorophyll content of ftsh4-4 mutant; (E) ARF2 mutation reduced the relative electrolytic leakage of ftsh4-4 mutant; (F) ARF2 mutation reduced the expression of senescence-associated genes SAG12, SAG13, SAG101, SEN1 and SEN4. * indicates significant difference at P<0.05; ** indicates significant difference at P<0.01 (Student’s t-test).
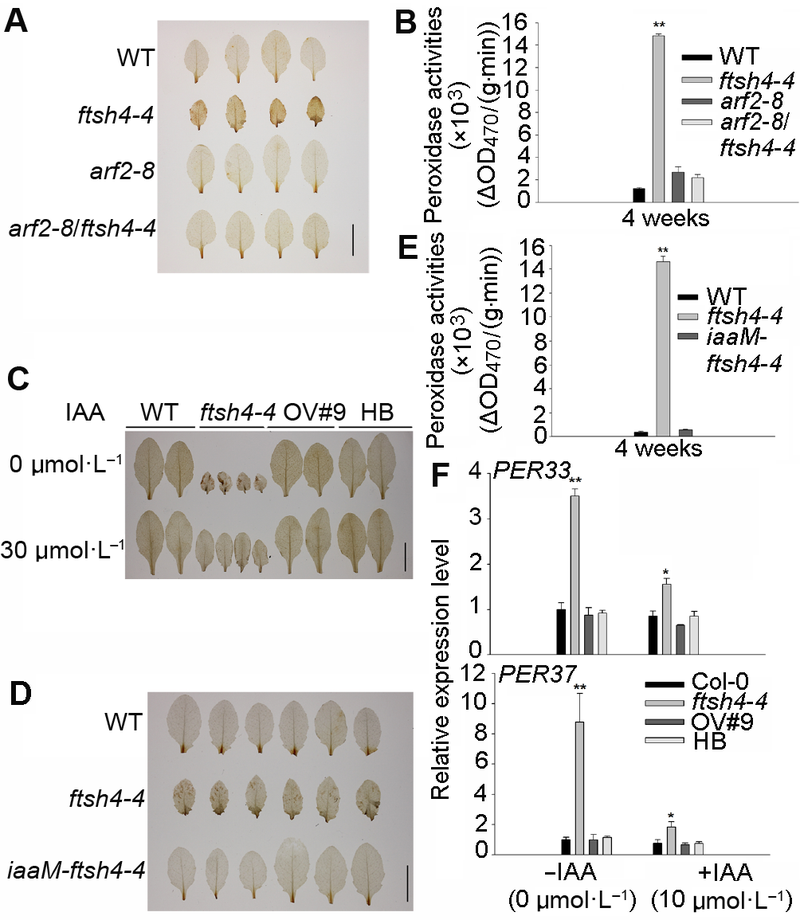
Figure 6 IAA treatment reduces the H2O2 level and peroxidase activities of Arabidopsis ftsh4-4 mutant^(A) ARF2 mutation reduced the H2O2 level of ftsh4-4 mutant (Bar=1 cm); (B) ARF2 mutation reduced the peroxidase activities of ftsh4-4 mutant; (C) Exogenous IAA rescued the H2O2 level of ftsh4-4 mutant (Bar=1 cm); (D) Increasing endogenous IAA reduced the H2O2 level of ftsh4-4 mutant (Bar=1 cm); (E) Increasing endogenous IAA reduced the peroxidase activities of ftsh4-4 mutant; (F) Exogenous IAA reduced the peroxidase genes expression of ftsh4-4 mutant. * indicates significant difference at P<0.05; ** indicates significant difference at P<0.01 (Student’s t-test).
| [1] | Apel K, Hirt H (2004). Reactive oxygen species: metabolism, oxidative stress, and signal transduction.Annu Rev Plant Biol 55, 373-399. |
| [2] | Arnon DI (1949). Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol 24, 1-15. |
| [3] | Bashandy T, Guilleminot J, Vernoux T, Caparros-Ruiz D, Ljung K, Meyer Y, Reichheld JP (2010). Interplay bet- ween the NADP-linked thioredoxin and glutathione systems in Arabidopsis auxin signaling.Plant Cell 22 376-391. |
| [4] | Blomster T, Salojarvi J, Sipari N, Brosche M, Ahlfors R, Keinanen M, Overmyer K, Kangasjarvi J (2011). Apo- plastic reactive oxygen species transiently decrease auxin signaling and cause stress-induced morphogenic res- ponse in Arabidopsis.Plant Physiol 157, 1866-1883. |
| [5] | Camilleri C, Jouanin L (1991). The TR-DNA region carrying the auxin synthesis genes of the Agrobacterium rhizo- genes agropine-type plasmid pRiA4: nucleotide sequence analysis and introduction into tobacco plants. Mol Plant Microbe Interact 4, 155-162. |
| [6] | Chen GH, Liu CP, Chen SC, Wang LC (2012). Role of ARABIDOPSIS A-FIFTEEN in regulating leaf senescence involves response to reactive oxygen species and is dependent on ETHYLENE INSENSITIVE2. J Exp Bot 63, 275-292. |
| [7] | Chen JP, Burke JJ, Velten J, Xin ZU (2006). FtsH11 protease plays a critical role in Arabidopsis thermotolerance.Plant J 48, 73-84. |
| [8] | Ellis CM, Nagpal P, Young JC, Hagen G, Guilfoyle TJ, Reed JW (2005). AUXIN RESPONSE FACTOR1 and AUXIN RESPONSE FACTOR2 regulate senescence and floral organ abscission in Arabidopsis thaliana. Development 132, 4563-4574. |
| [9] | Garcia-Lorenzo M, Sjodin A, Jansson S, Funk C (2006). Protease gene families in Populus and Arabidopsis. BMC Plant Biol 6, 30. |
| [10] | Gazarian IG, Lagrimini LM, Mellon FA, Naldrett MJ, Ashby GA, Thorneley RN (1998). Identification of skatolyl hydroperoxide and its role in the peroxidase-catalysed oxidation of indol-3-yl acetic acid.Biochem J 333, 223-232. |
| [11] | Gibala M, Kicia M, Sakamoto W, Gola EM, Kubrakiewicz J, Smakowska E, Janska H (2009). The lack of mitochondrial AtFtsH4 protease alters Arabidopsis leaf morphology at the late stage of rosette development under short-day photoperiod.Plant J 59, 685-699. |
| [12] | Guo Y, Gan SS (2012). Convergence and divergence in gene expression profiles induced by leaf senescence and 27 senescence-promoting hormonal, pathological and environmental stress treatments.Plant Cell Environ 35, 644-655. |
| [13] | He J, Duan Y, Hua D, Fan G, Wang L, Liu Y, Chen Z, Han L, Qu LJ, Gong Z (2012). DEXH box RNA helicase- mediated mitochondrial reactive oxygen species production in Arabidopsis mediates crosstalk between abscisic acid and auxin signaling.Plant Cell 24, 1815-1833. |
| [14] | Hou K, Wu W, Gan SS (2013). SAUR36, a small auxin up RNA gene, is involved in the promotion of leaf senescence in Arabidopsis.Plant Physiol 161, 1002-1009. |
| [15] | Huang YC, Chang YL, Hsu JJ, Chuang HW (2008). Trans- criptome analysis of auxin-regulated genes of Arabidopsis thaliana. Gene 420, 118-124. |
| [16] | Jibran R, Hunter DA, Dijkwel PP (2013). Hormonal regulation of leaf senescence through integration of developmental and stress signals.Plant Mol Biol 82, 547-561. |
| [17] | Joo JH, Bae YS, Lee JS (2001). Role of auxin-induced reactive oxygen species in root gravitropism.Plant Physiol 126, 1055-1060. |
| [18] | Kant S, Bi YM, Zhu T, Rothstein SJ (2009). SAUR39, a small auxin-up RNA gene, acts as a negative regulator of auxin synthesis and transport in rice.Plant Physiol 151, 691-701. |
| [19] | Kato Y, Miura E, Ido K, Ifuku K, Sakamoto W (2009). The variegated mutants lacking chloroplastic FtsHs are defective in D1 degradation and accumulate reactive oxygen sp- ecies.Plant Physiol 151, 1790-1801. |
| [20] | Kim JI, Murphy AS, Baek D, Lee SW, Yun DJ, Bressan RA, Narasimhan ML (2011). YUCCA6 over-expression demonstrates auxin function in delaying leaf senescence inArabidopsis thaliana. J Exp Bot 62, 3981-3992. |
| [21] | Kolodziejczak M, Kolaczkowska A, Szczesny B, Urantowka A, Knorpp C, Kieleczawa J, Janska H (2002). A higher plant mitochondrial homologue of the yeast m-AAA protease-molecular cloning, localization, and putative func- tion.J Biol Chem 277, 43792-43798. |
| [22] | Kovtun Y, Chiu WL, Tena G, Sheen J (2000). Functional analysis of oxidative stress-activated mitogen-activated protein kinase cascade in plants.Proc Natl Acad Sci USA 97, 2940-2945. |
| [23] | Li Z, Peng J, Wen X, Guo H (2012). Gene network analysis and functional studies of senescence-associated genes reveal novel regulators of Arabidopsis leaf senescence.J Integr Plant Biol 54, 526-539. |
| [24] | Lim PO, Kim HJ, Nam HG (2007). Leaf senescence.Annu Rev Plant Biol 58, 115-136. |
| [25] | Lim PO, Lee IC, Kim J, Kim HJ, Ryu JS, Woo HR, Nam HG (2010). Auxin response factor 2 (ARF2) plays a major role in regulating auxin-mediated leaf longevity.J Exp Bot 61, 1419-1430. |
| [26] | Ljung K, Hull AK, Kowalczyk M, Marchant A, Celenza J, Cohen JD, Sandberg G (2002). Biosynthesis, conjugation, catabolism and homeostasis of indole-3-acetic acid in Arabidopsis thaliana. Plant Mol Biol 49, 249-272. |
| [27] | Malnoe A, Wang F, Girard-Bascou J, Wollman FA, de Vitry C (2014). Thylakoid FtsH protease contributes to photosystem II and cytochrome b6f remodeling in Chlamydomonas reinhardtii under stress conditions.Plant Cell 26, 373-390. |
| [28] | Meudt WJ, Gaines TP (1967). Studies on the oxidation of indole-3-acetic acid by peroxidase enzymes. I. Colorimetric determination of indole-3-acetic acid oxidation produ- cts.Plant Physiol 144, 118-128. |
| [29] | Mittler R, Vanderauwera S, Suzuki N, Miller G, Tognetti VB, Vandepoele K, Gollery M, Shulaev V, Van Breu- segem F (2011). ROS signaling: the new wave?Trends Plant Sci 16, 300-309. |
| [30] | Nakagami H, Soukupova H, Schikora A, Zarsky V, Hirt H (2006). A mitogen-activated protein kinase kinase kinase mediates reactive oxygen species homeostasis in Arabidopsis.J Biol Chem 281, 38697-38704. |
| [31] | Nolden M, Ehses S, Koppen M, Bernacchia A, Rugarli EI, Langer T (2005). The m-AAA protease defective in hereditary spastic paraplegia controls ribosome assembly in mitochondria.Cell 123, 277-289. |
| [32] | Okushima Y, Fukaki H, Onoda M, Theologis A, Tasaka M (2007). ARF7 and ARF19 regulate lateral root formation via direct activation ofLBD/ASL genes in Arabidopsis. Pla- nt Cell 19, 118-130. |
| [33] | Piechota J, Kolodziejczak M, Juszczak I, Sakamoto W, Janska H (2010). Identification and characterization of high molecular weight complexes formed by matrix AAA proteases and prohibitins in mitochondria ofArabidopsis thaliana. J Biol Chem 285, 12512-12521. |
| [34] | Potters G, Pasternak TP, Guisez Y, Palme KJ, Jansen MA (2007). Stress-induced morphogenic responses: growing out of trouble?Trends Plant Sci 12, 98-105. |
| [35] | Queval G, Issakidis-Bourguet E, Hoeberichts FA, Vandorpe M, Gakiere B, Vanacker H, Miginiac-Maslow M, Van Breusegem F, Noctor G (2007). Conditional oxidative stress responses in the Arabidopsis photorespiratory mutant cat2 demonstrate that redox state is a key modulator of daylength-dependent gene expression, and define photoperiod as a crucial factor in the regulation of H2O2- induced cell death. Plant J 52, 640-657. |
| [36] | Ren G, Zhou Q, Wu S, Zhang Y, Zhang L, Huang J, Sun Z, Kuai B (2010). Reverse genetic identification of CRN1 and its distinctive role in chlorophyll degradation in Arabidopsis.J Integr Plant Biol 52, 496-504. |
| [37] | Romano CP, Robson PR, Smith H, Estelle M, Klee H (1995). Transgene-mediated auxin overproduction in Ara- bidopsis: hypocotyl elongation phenotype and interactions with the hy6-1 hypocotyl elongation and axr1 auxin-resis- tant mutants.Plant Mol Biol 27, 1071-1083. |
| [38] | Sakamoto W, Tamura T, Hanba-Tomita Y, Murata M (2002). The VAR1 locus of Arabidopsis encodes a chloroplastic FtsH and is responsible for leaf variegation in the mutant alleles.Genes Cells 7, 769-780. |
| [39] | Salleh FM, Evans K, Goodall B, Machin H, Mowla SB, Mur LA, Runions J, Theodoulou FL, Foyer CH, Rogers HJ (2012). A novel function for a redox-related LEA protein (SAG21/AtLEA5) in root development and biotic stress re- sponses.Plant Cell Environ 35, 418-429. |
| [40] | Savitsky PA, Gazaryan IG, Tishkov VI, Lagrimini LM, Ruzgas T, Gorton L (1999). Oxidation of indole-3-acetic acid by dioxygen catalysed by plant peroxidases: specifi- city for the enzyme structure.Biochem J 340, 579-583. |
| [41] | Sierla M, Rahikainen M, Salojarvi J, Kangasjarvi J, Kangasjarvi S (2013). Apoplastic and chloroplastic redox signaling networks in plant stress responses.Antioxid Re- dox Signal 18, 2220-2239. |
| [42] | Suzuki N, Miller G, Morales J, Shulaev V, Torres MA, Mittler R (2011). Respiratory burst oxidases: the engines of ROS signaling.Curr Opin Plant Biol 14, 691-699. |
| [43] | Wagner R, Aigner H, Pruzinska A, Jankanpaa HJ, Jansson S, Funk C (2011). Fitness analyses of Arabidopsis thaliana mutants depleted of FtsH metalloproteases and characterization of three FtsH6 deletion mutants exposed to high light stress, senescence and chilling. New Phytol 191, 449-458. |
| [44] | Wang P, Song CP (2008). Guard-cell signaling for hydrogen peroxide and abscisic acid.New Phytol 178, 703-718. |
| [45] | Watanabe M, Balazadeh S, Tohge T, Erban A, Giavalisco P, Kopka J, Mueller-Roeber B, Fernie AR, Hoefgen R (2013). Comprehensive dissection of spatiotemporal meta- bolic shifts in primary, secondary, and lipid metabolism during developmental senescence in Arabidopsis.Plant Physiol 162, 1290-1310. |
| [46] | Woodward AW, Bartel B (2005). Auxin: regulation, action, and interaction.Ann Bot 95, 707-735. |
| [47] | Xu F, Meng T, Li P, Yu Y, Cui Y, Wang Y, Gong Q, Wang NN (2011). A soybean dual-specificity kinase, GmSARK, and its Arabidopsis homolog, AtSARK, regulate leaf senescence through synergistic actions of auxin and ethyle- ne.Plant Physiol 157, 2131-2153. |
| [48] | Yuan HM, Liu WC, Jin Y, Lu YT (2013). Role of ROS and auxin in plant response to metal-mediated stress.Plant Signal Behav 8, e24671. |
| [49] | Zentgraf U, Laun T, Miao Y (2010). The complex regulation of WRKY53 during leaf senescence of Arabidopsis thali- ana.Eur J Cell Biol 89, 133-137. |
| [50] | Zhang S, Wu J, Yuan D, Zhang D, Huang Z, Xiao L, Yang C (2014). Perturbation of auxin homeostasis caused by mitochondrial FtSH4 gene-mediated peroxidase accumulation regulates Arabidopsis architecture.Mol Plant 7, 856-873. |
| [51] | Zhang S, Li C, Wang R, Chen Y, Shu S, Huang R, Zhang D, Xiao S, Yao N, Li J, Yang CW (2017). The mitochondrial protease FtSH4 regulates leaf senescence via WRKY- dependent salicylic acid signal.Plant Physiol 173, 2294-2307. |
| [52] | Zimmermann P, Heinlein C, Orendi G, Zentgraf U (2006). Senescence-specific regulation of catalases in Arabidopsis thaliana(L.) Heynh. Plant Cell Environ 29, 1049-1060. |
| [1] | Yuhan Liu, Qijiang Cao, Shihan Zhang, Yihui Li, Jing Wang, Xiaomeng Tan, Xiaoru Liu, Xianling Wang. Mechanism of AtFTCD-L in Root Response to Soil Compaction [J]. Chinese Bulletin of Botany, 2025, 60(4): 1-0. |
| [2] | Wenli Yang, Zhao Li, Zhiming Liu, Zhihua Zhang, Jinsheng Yang, Yanjie Lü, Yongjun Wang. Senescence Characteristics of Maize Leaves at Different Maturity Stages and Their Effect on Phyllosphere Bacteria [J]. Chinese Bulletin of Botany, 2024, 59(6): 1024-1040. |
| [3] | Yanjun Jing, Rongcheng Lin. Blue Light Receptor CRY2 Transforms into a ‘dark dancer’ [J]. Chinese Bulletin of Botany, 2024, 59(6): 878-882. |
| [4] | Yan Luo, Qiyuan Liu, Yuanbing Lü, Yue Wu, Yaoyu Tian, Tian An, Zhenhua Li. Photothermal Sensitivity of Phytochrome Mutants During Seed Germination in Arabidopsis thaliana [J]. Chinese Bulletin of Botany, 2024, 59(5): 752-762. |
| [5] | Yuying Zhou, Hui Chen, Simu Liu. Research Progress on Auxin Responsive Non-canonical Aux/IAA Proteins in Plants [J]. Chinese Bulletin of Botany, 2024, 59(4): 651-658. |
| [6] | Yanxiao Chen, Yaping Li, Jinjun Zhou, Lixia Xie, Yongbin Peng, Wei Sun, Yanan He, onghui Jiang, Zenglan Wang, Chongke Zheng, Xianzhi Xie. Effect of Amino Acid Point Mutations on the Structure and Function of Phytochrome B in Arabidopsis thaliana [J]. Chinese Bulletin of Botany, 2024, 59(3): 481-494. |
| [7] | Jixuan Yang, Xuefei Wang, Hongya Gu. Genetic Basis of Flowering Time Variations in Tibetan Arabidopsis thaliana [J]. Chinese Bulletin of Botany, 2024, 59(3): 373-382. |
| [8] | Yuejing Zhang, Hetian Sang, Hanqi Wang, Zhenzhen Shi, Li Li, Xin Wang, Kun Sun, Ji Zhang, Hanqing Feng. Research Progress of Plant Signaling in Systemic Responses to Abiotic Stresses [J]. Chinese Bulletin of Botany, 2024, 59(1): 122-133. |
| [9] | Xiangpei Kong, Mengyue Zhang, Zhaojun Ding. There Is a Way Out-new Breakthroughs in Extracellular Auxin Sensing [J]. Chinese Bulletin of Botany, 2023, 58(6): 861-865. |
| [10] | Yuan Yuan, Enhebayaer, Qi Yanhua. Research Advances in Biological Functions of GH3 Gene Family in Plants [J]. Chinese Bulletin of Botany, 2023, 58(5): 770-782. |
| [11] | Gang Wang, Ertao Wang. The Broad-spectrum Innate Resistance Against Clubroot Disease Conferred by WeiTsing is Mechanistically Revealed [J]. Chinese Bulletin of Botany, 2023, 58(3): 356-358. |
| [12] | Shuyao Zhou, Jianming Li, Juan Mao. AtGH3.17-mediated Regulation of Auxin and Brassinosteroid Response in Arabidopsis thaliana [J]. Chinese Bulletin of Botany, 2023, 58(3): 373-384. |
| [13] | Yongguang Li, Hui Ren, Yingjie Zhang, Ruining Li, Hao Ai, Xianzhong Huang. Analysis of the molecular evolution of the PEBP gene family in cruciferous plants [J]. Biodiv Sci, 2022, 30(6): 21545-. |
| [14] | Ye Qing, Yan Xiaoyan, Chen Huize, Feng Jinlin, Han Rong. Effect of Nitrogen-doped Graphene Quantum Dots on Growth Direction of Primary Root in Arabidopsis thaliana [J]. Chinese Bulletin of Botany, 2022, 57(5): 623-634. |
| [15] | Liu Xiaolong, Ji Ping, Yang Hongtao, Ding Yongdian, Fu Jialing, Liang Jiangxia, Yu Congcong. Priming Effect of Abscisic Acid on High Temperature Stress During Rice Heading-flowering Stage [J]. Chinese Bulletin of Botany, 2022, 57(5): 596-610. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||