

Chinese Bulletin of Botany ›› 2024, Vol. 59 ›› Issue (6): 1024-1040.DOI: 10.11983/CBB24037 cstr: 32102.14.CBB24037
Special Issue: 玉米生物学与分子设计(2024年59卷6期)
• RESEARCH PAPERS • Previous Articles Next Articles
Wenli Yang1,2,3,†, Zhao Li3,†, Zhiming Liu2, Zhihua Zhang3, Jinsheng Yang2, Yanjie Lü1,2,*( ), Yongjun Wang1,2,*(
), Yongjun Wang1,2,*( )
)
Received:2024-03-09
Accepted:2024-05-27
Online:2024-11-10
Published:2024-06-11
Contact:
*E-mail: lvyanjie_1977@163.com;yjwang2004@126.com
About author:†These authors contributed equally to this paper
Wenli Yang, Zhao Li, Zhiming Liu, Zhihua Zhang, Jinsheng Yang, Yanjie Lü, Yongjun Wang. Senescence Characteristics of Maize Leaves at Different Maturity Stages and Their Effect on Phyllosphere Bacteria[J]. Chinese Bulletin of Botany, 2024, 59(6): 1024-1040.
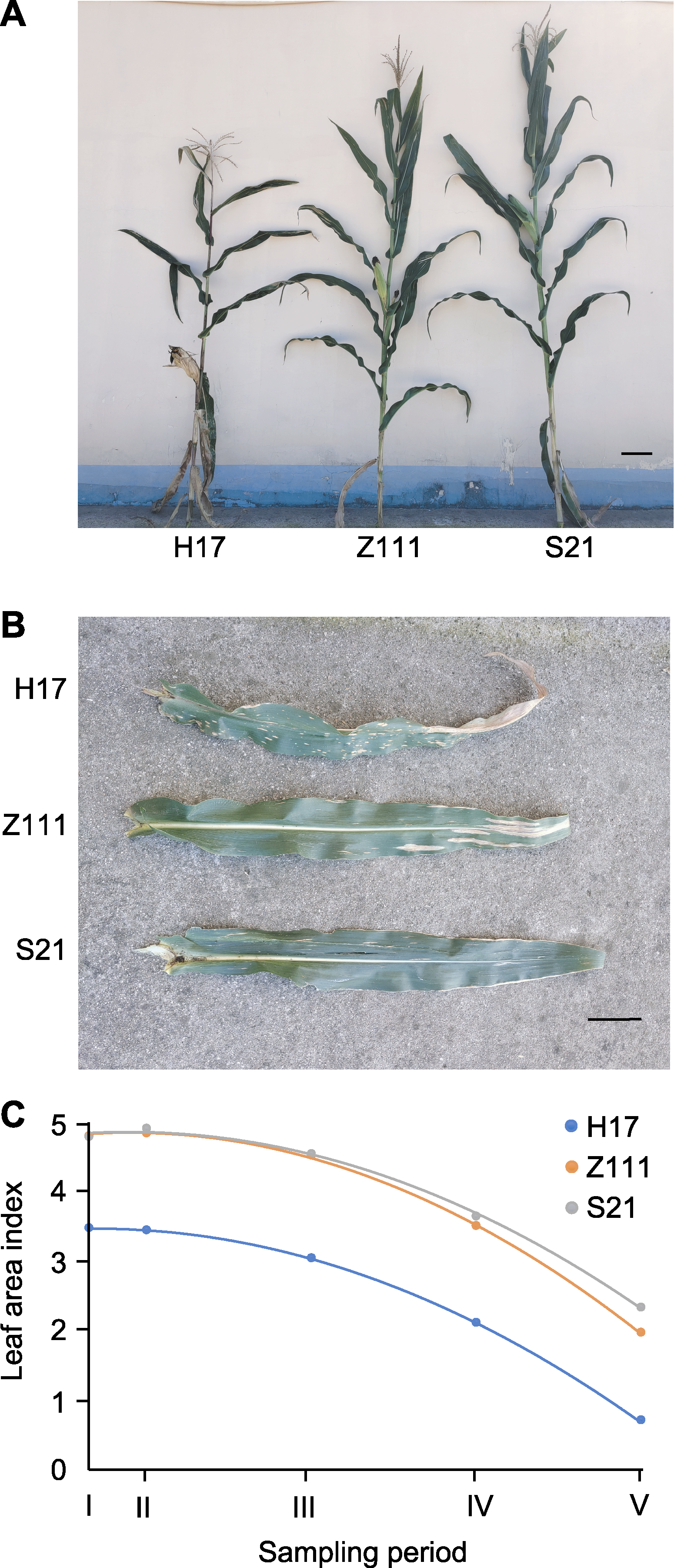
Figure 1 Leaf phenotype of three maize varieties at different sampling times (A) Whole maize plant at the sampling time point IV (bar=20 cm); (B) Ear leaf at the sampling time point IV (bar=10 cm); (C) Changes in leaf area index at different sampling period
| Hybrids | Senescence equation | Fit coefficient (R2) | Senescence traits parameter | ||||
|---|---|---|---|---|---|---|---|
| RGLAM (%) | Vm (%) | Vmax (%) | Ts (days) | Tmax (days) | |||
| H17 | y=e4.2169-0.0823x/(1+e4.2169-0.0823x) | 0.9918 | 20.60 | 1.19 | 2.06 | 10.88 | 51.2 |
| Z111 | y=e4.0155-0.0744x/(1+e4.0155-0.0744x) | 0.9935 | 40.69 | 0.89 | 1.86 | 11.20 | 54.0 |
| S21 | y=e3.7835-0.0655x/(1+e3.7835-0.0655x) | 0.9954 | 47.61 | 0.78 | 1.64 | 12.30 | 57.8 |
Table 1 Leaf senescence characteristics of three maize varieties
| Hybrids | Senescence equation | Fit coefficient (R2) | Senescence traits parameter | ||||
|---|---|---|---|---|---|---|---|
| RGLAM (%) | Vm (%) | Vmax (%) | Ts (days) | Tmax (days) | |||
| H17 | y=e4.2169-0.0823x/(1+e4.2169-0.0823x) | 0.9918 | 20.60 | 1.19 | 2.06 | 10.88 | 51.2 |
| Z111 | y=e4.0155-0.0744x/(1+e4.0155-0.0744x) | 0.9935 | 40.69 | 0.89 | 1.86 | 11.20 | 54.0 |
| S21 | y=e3.7835-0.0655x/(1+e3.7835-0.0655x) | 0.9954 | 47.61 | 0.78 | 1.64 | 12.30 | 57.8 |
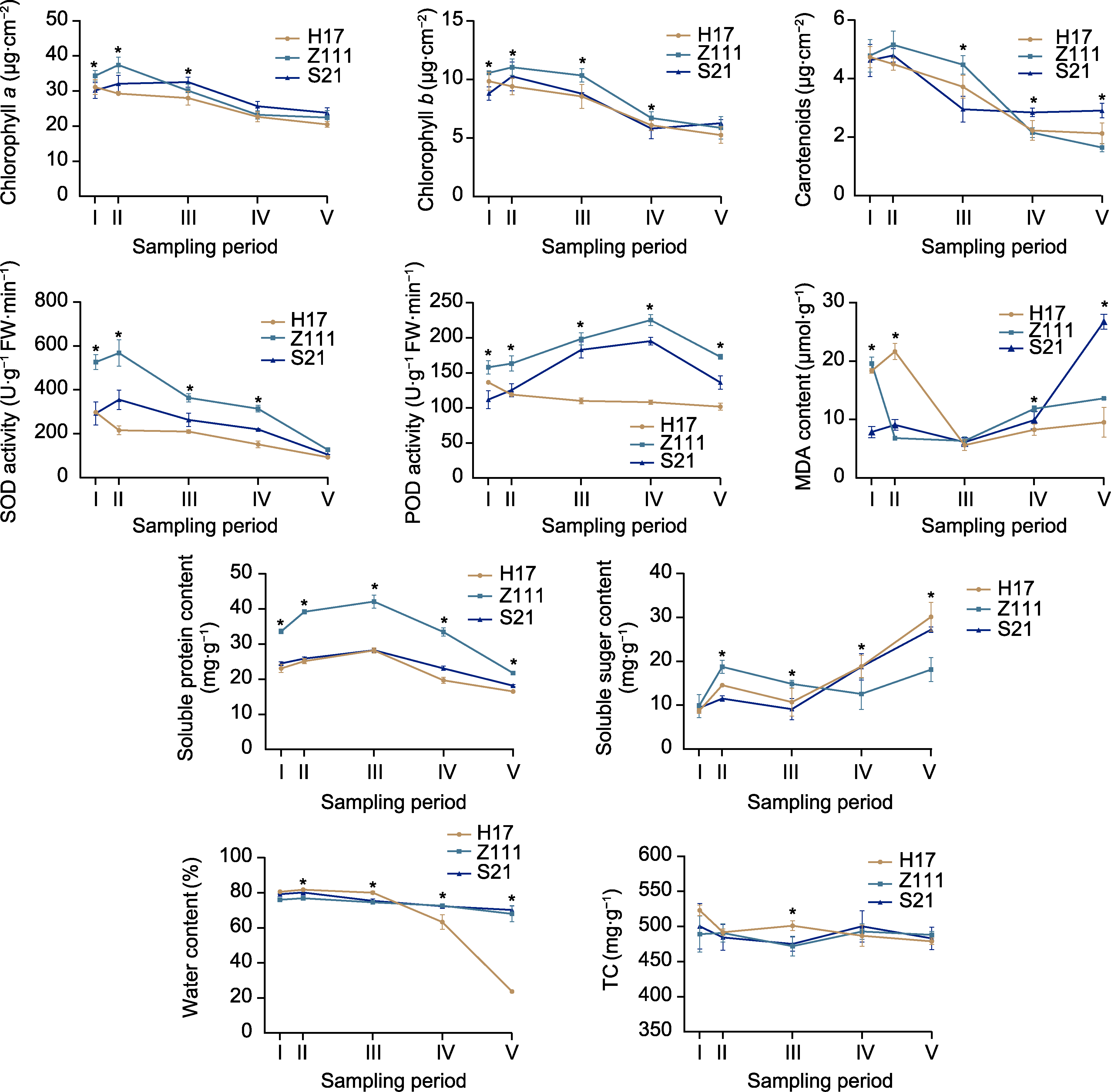
Figure 2 Analysis of leaf physiological and biochemical indexes of three maize varieties SOD: Superoxide dismutase; POD: Peroxidase; MDA: Malondialdehyde; TC: Total carbon. * indicate significant differences among the three varieties at the same sampling period (P<0.05).
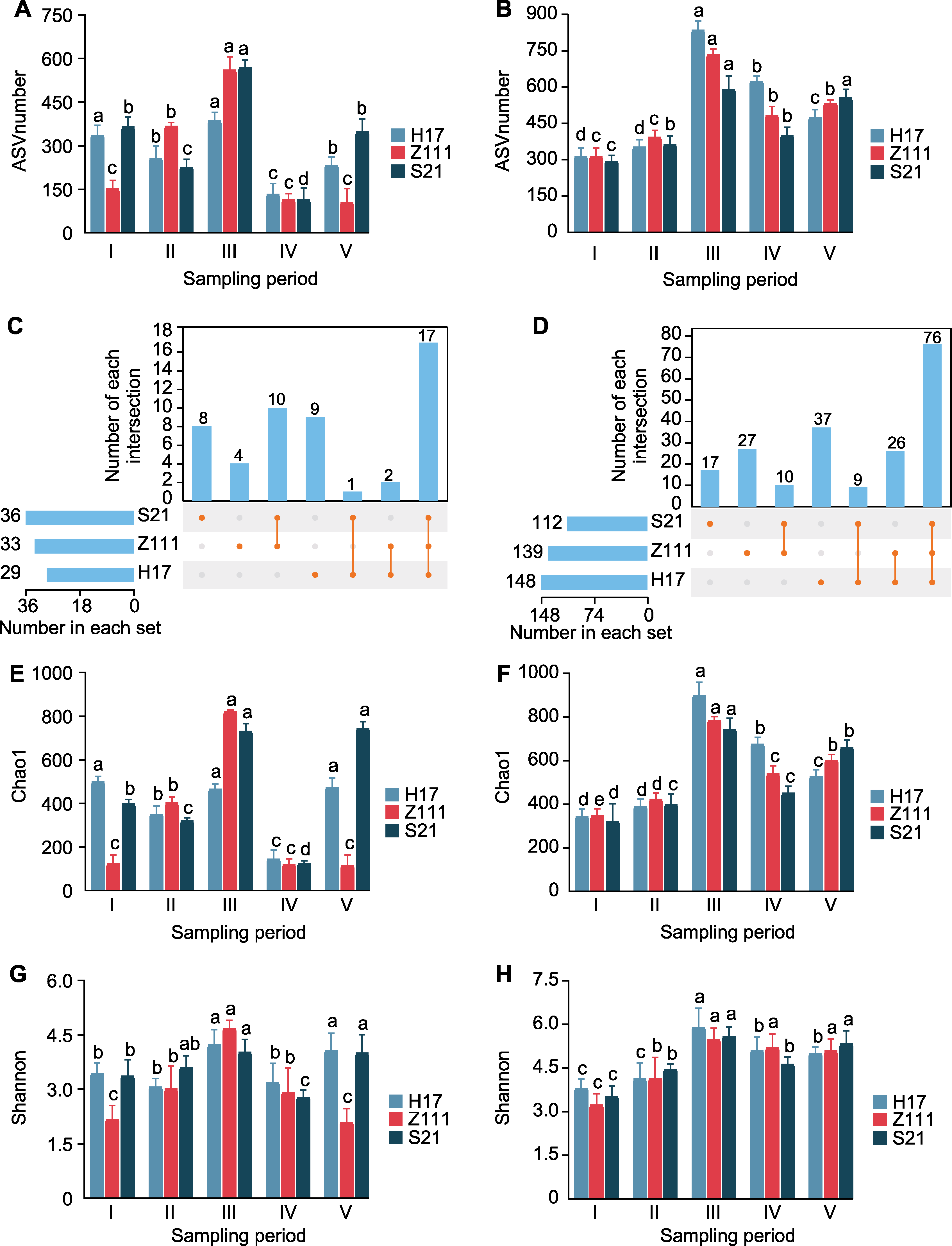
Figure 3 Changes of leaf microorganisms of three maize varieties in different development periods (A) Total number of endogenous bacterial amplicon sequence variants (ASVs) in different samples; (B) Total number of exogenous bacterial ASVs in different samples; (C) Endogenous bacterial shared ASVs in different samples; (D) Exogenous bacterial shared ASVs in different samples; (E) Chao1 diversity index of endogenous bacterial communities in different samples; (F) Chao1 diversity index of exogenous bacterial communities in different samples; (G) Shannon’s diversity index of endogenous bacterial communities in different samples; (H) Shannon’s diversity index of exogenous bacterial communities in different samples. Different lowercase letters indicate significant differences among different development periods of the same species (P<0.05).
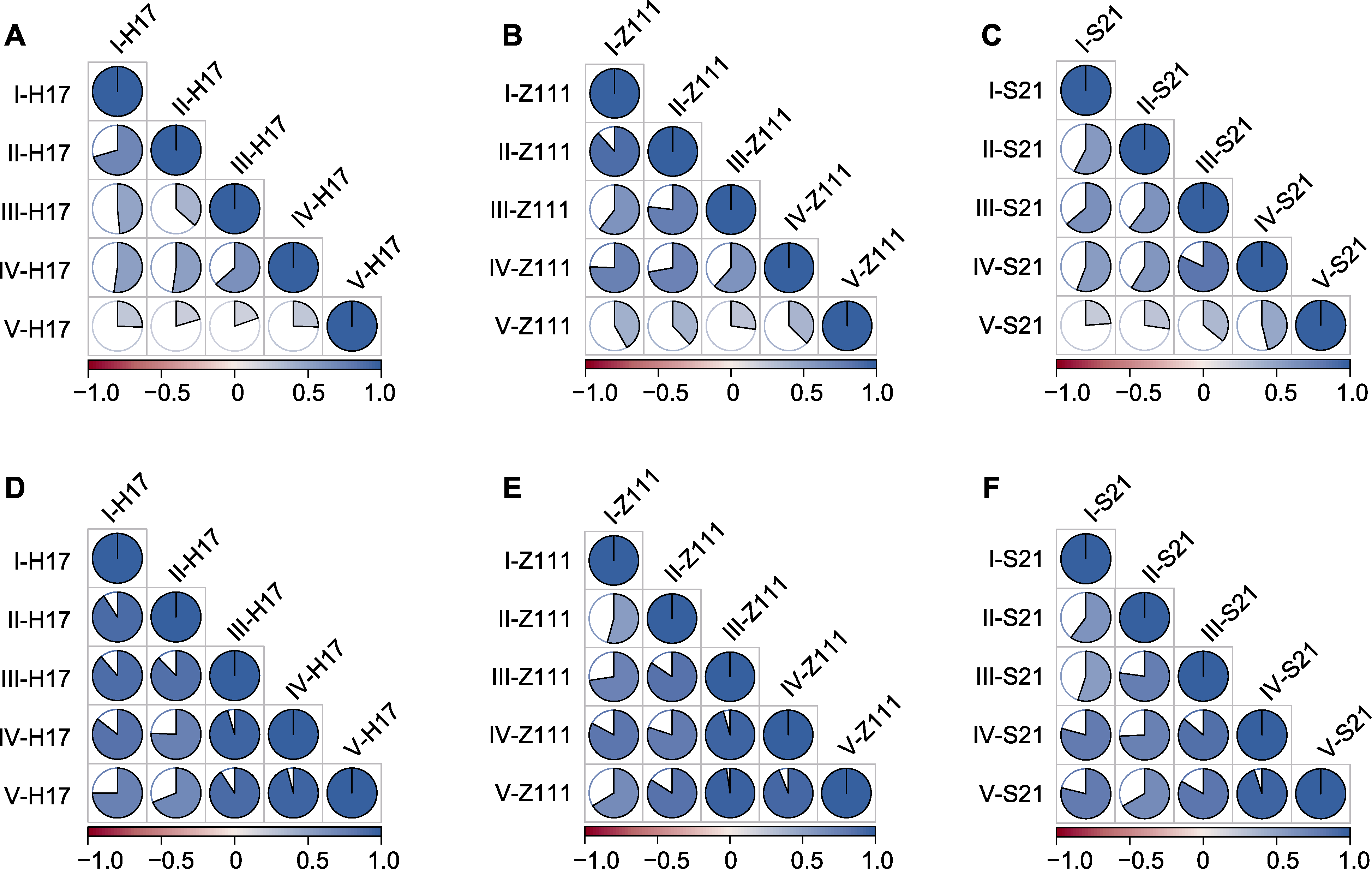
Figure 4 Correlation among leaf microbial communities of three maize varieties at different development time points (A)-(C) Correlation of endogenous bacterial communities in leaves of three maize varieties; (D)-(F) Correlation of exogenous bacterial communities in leaves of three maize varieties. Pie charts and colors represent Pearson correlation coefficient values.
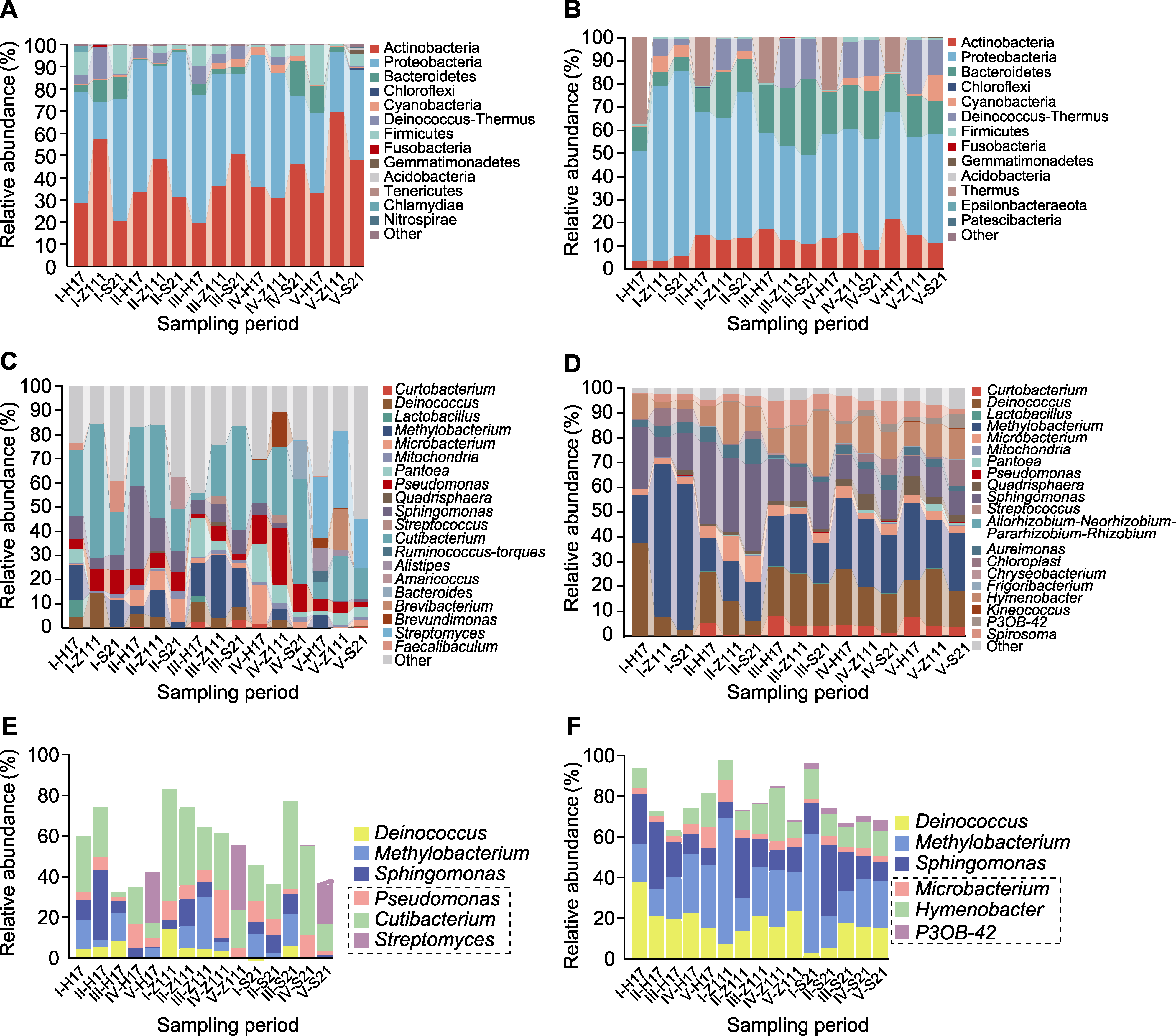
Figure 5 Leaf microbial community composition of 3 maize varieties at different maturity stages (A) The community composition of endogenous bacteria at the phylum level; (B) The community composition of exogenous bacteria at the phylum level; (C) The community composition of endogenous bacteria at the genus level; (D) The community composition of exogenous bacteria at the genus level; (E) Composition of horizontally dominant communities of endogenous bacterial genera (the dashed box indicate specific dominant genera, and outside the dashed box indicate dominant genera shared with exogenous bacteria); (F) Composition of horizontally dominant communities of exogenous bacterial genera (the dashed box indicate specific dominant genera, and outside the dashed box indicate dominant genera shared with endogenous bacteria).
| Samples | Nodes | Edges | Density | Average path length |
|---|---|---|---|---|
| I-H17 | 14 | 20 | 0.235 | 1.812 |
| I-Z111 | 19 | 27 | 0.171 | 2.417 |
| I-S21 | 19 | 32 | 0.222 | 2.625 |
| II-H17 | 22 | 40 | 0.253 | 2.633 |
| II-Z111 | 18 | 31 | 0.298 | 2.252 |
| II-S21 | 21 | 34 | 0.258 | 2.717 |
| III-H17 | 25 | 46 | 0.275 | 2.771 |
| III-Z111 | 29 | 41 | 0.329 | 2.256 |
| III-S21 | 27 | 37 | 0.278 | 2.820 |
| IV-H17 | 21 | 38 | 0.202 | 2.157 |
| IV-Z111 | 22 | 30 | 0.125 | 2.160 |
| IV-S21 | 22 | 34 | 0.184 | 2.638 |
| V-H17 | 15 | 22 | 0.195 | 1.447 |
| V-Z111 | 18 | 28 | 0.185 | 1.707 |
| V-S21 | 19 | 33 | 0.122 | 1.612 |
Table 2 Properties of endogenous bacterial co-occurrence network attributes in three maize varieties at different periods (I-V)
| Samples | Nodes | Edges | Density | Average path length |
|---|---|---|---|---|
| I-H17 | 14 | 20 | 0.235 | 1.812 |
| I-Z111 | 19 | 27 | 0.171 | 2.417 |
| I-S21 | 19 | 32 | 0.222 | 2.625 |
| II-H17 | 22 | 40 | 0.253 | 2.633 |
| II-Z111 | 18 | 31 | 0.298 | 2.252 |
| II-S21 | 21 | 34 | 0.258 | 2.717 |
| III-H17 | 25 | 46 | 0.275 | 2.771 |
| III-Z111 | 29 | 41 | 0.329 | 2.256 |
| III-S21 | 27 | 37 | 0.278 | 2.820 |
| IV-H17 | 21 | 38 | 0.202 | 2.157 |
| IV-Z111 | 22 | 30 | 0.125 | 2.160 |
| IV-S21 | 22 | 34 | 0.184 | 2.638 |
| V-H17 | 15 | 22 | 0.195 | 1.447 |
| V-Z111 | 18 | 28 | 0.185 | 1.707 |
| V-S21 | 19 | 33 | 0.122 | 1.612 |
| Samples | Nodes | Edges | Density | Average path length |
|---|---|---|---|---|
| I-H17 | 63 | 184 | 0.039 | 2.466 |
| I-Z111 | 65 | 160 | 0.040 | 2.937 |
| I-S21 | 53 | 130 | 0.037 | 2.445 |
| II-H17 | 77 | 212 | 0.045 | 2.101 |
| II-Z111 | 84 | 289 | 0.048 | 1.993 |
| II-S21 | 89 | 371 | 0.056 | 2.161 |
| III-H17 | 150 | 632 | 0.056 | 3.481 |
| III-Z111 | 138 | 599 | 0.065 | 3.483 |
| III-S21 | 136 | 605 | 0.067 | 3.300 |
| IV-H17 | 120 | 321 | 0.051 | 3.139 |
| IV-Z111 | 95 | 144 | 0.037 | 2.676 |
| IV-S21 | 81 | 125 | 0.049 | 2.743 |
| V-H17 | 85 | 127 | 0.033 | 2.559 |
| V-Z111 | 92 | 127 | 0.031 | 2.595 |
| V-S21 | 74 | 117 | 0.040 | 2.632 |
Table 3 Properties of exogenous bacterial co-occurrence network attributes in three maize varieties at different periods (I-V)
| Samples | Nodes | Edges | Density | Average path length |
|---|---|---|---|---|
| I-H17 | 63 | 184 | 0.039 | 2.466 |
| I-Z111 | 65 | 160 | 0.040 | 2.937 |
| I-S21 | 53 | 130 | 0.037 | 2.445 |
| II-H17 | 77 | 212 | 0.045 | 2.101 |
| II-Z111 | 84 | 289 | 0.048 | 1.993 |
| II-S21 | 89 | 371 | 0.056 | 2.161 |
| III-H17 | 150 | 632 | 0.056 | 3.481 |
| III-Z111 | 138 | 599 | 0.065 | 3.483 |
| III-S21 | 136 | 605 | 0.067 | 3.300 |
| IV-H17 | 120 | 321 | 0.051 | 3.139 |
| IV-Z111 | 95 | 144 | 0.037 | 2.676 |
| IV-S21 | 81 | 125 | 0.049 | 2.743 |
| V-H17 | 85 | 127 | 0.033 | 2.559 |
| V-Z111 | 92 | 127 | 0.031 | 2.595 |
| V-S21 | 74 | 117 | 0.040 | 2.632 |
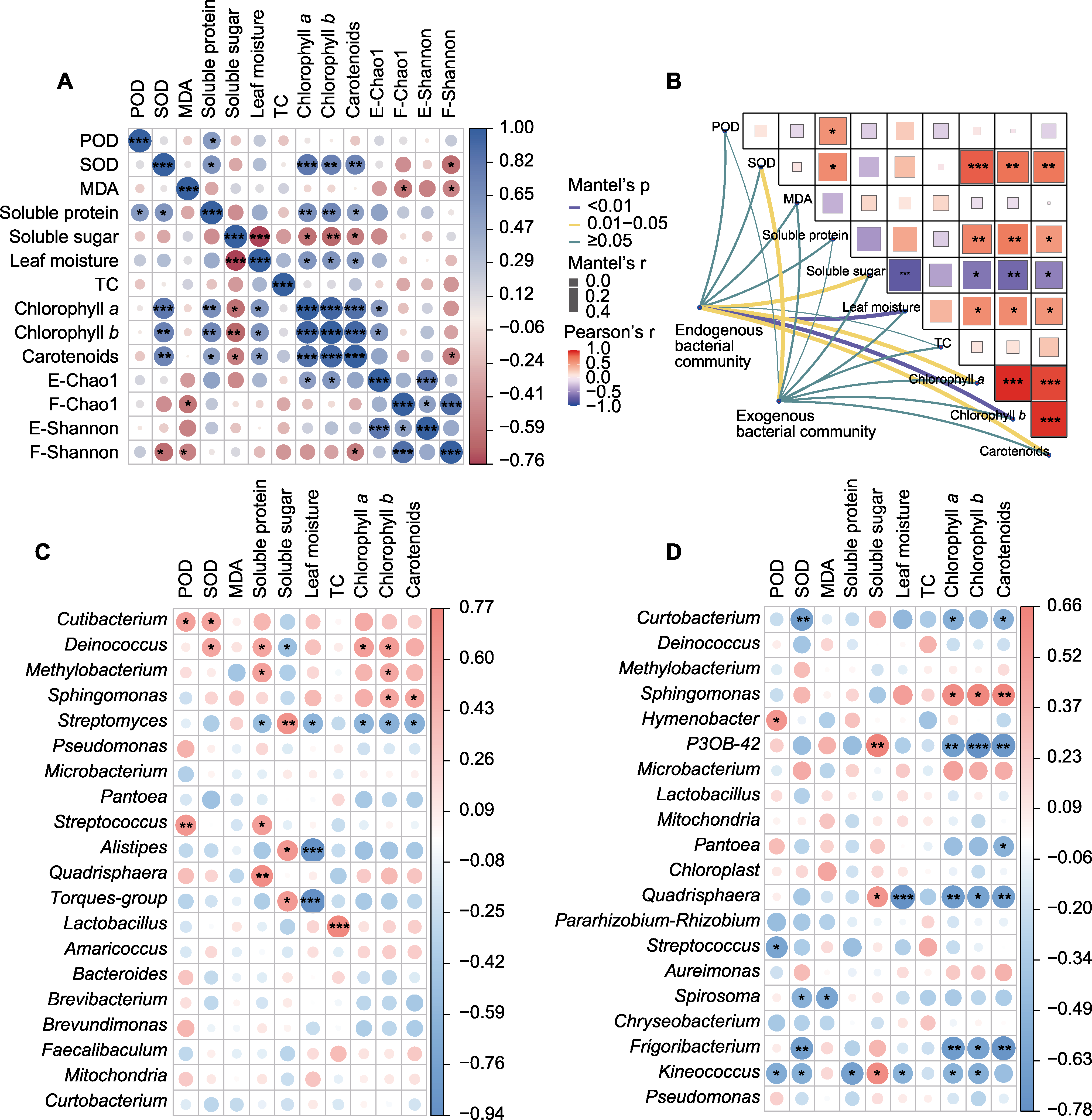
Figure 6 Relationships between leaf physicochemical properties and phyllosphere microorganisms of three maize varieties at different reproductive stages (A) Correlation between leaf physicochemical properties and bacterial diversity (E: Endogenous; F: Exogenous); (B) Mantel correlation between leaf physicochemical properties and leaf endogenous and exogenous bacterial community structure; (C) Correlation analysis between endogenous bacterial dominant species and leaf physicochemical properties; (D) Correlation analysis between exogenous bacterial dominant species and leaf physicochemical properties. POD, SOD, MDA and TC are the same as shown in Figure 2. *, **, and *** indicate significant differences at 0.05, 0.01, and 0.001 levels, respectively.
| [1] | Arnault G, Mony C, Vandenkoornhuyse P (2023). Plant microbiota dysbiosis and the Anna Karenina Principle. Trends Plant Sci 28, 18-30. |
| [2] | Borrell AK, Hammer GL, Henzell RG (2000). Does maintaining green leaf area in sorghum improve yield under drought? II. Dry matter production and yield. Crop Sci 40, 1037-1048. |
| [3] |
Bulgarelli D, Schlaeppi K, Spaepen S, Schulze-Lefert P (2013). Structure and functions of the bacterial microbiota of plants. Annu Rev Plant Biol 64, 807-838.
DOI PMID |
| [4] |
Canfield DE, Glazer AN, Falkowski PG (2010). The evolution and future of Earth’s nitrogen cycle. Science 330, 192-196.
DOI PMID |
| [5] | Chen JX, Wang XF (2015). Experimental Guide to Plant Physiology. Guangzhou: South China University of Technology Press. pp. 72-73. (in Chinese) |
| 陈建勋, 王晓峰 (2015). 植物生理学实验指导. 广州: 华南理工大学出版社. pp. 72-73. | |
| [6] | Chen YF, Zhou DB, Qi DF, Gao ZF, Xie JH, Luo YP (2018). Growth promotion and disease suppression ability of a Streptomyces sp. CB-75 from banana rhizosphere soil. Front Microbiol 8, 2704. |
| [7] | Cheng JE, Su P, Zhang ZH, Zheng LM, Wang ZY, Hamid MR, Dia JP, Du XH, Chen LJ, Zhai ZY, Kong XT, Liu Y, Zhang DY (2022). Metagenomic analysis of the dynamical conversion of photosynthetic bacterial communities in different crop fields over different growth periods. PLoS One 17, e0262517. |
| [8] | Duan J, Liang CY, Huang YW (1997). Studies on leaf senescence of hybrid rice at flowering and grain formation stage. Acta Phytophysiol Sin 23, 139-144. (in Chinese) |
| 段俊, 梁承邺, 黄毓文 (1997). 杂交水稻开花结实期间叶片衰老. 植物生理学报 23, 139-144. | |
| [9] |
Eiler A, Heinrich F, Bertilsson S (2012). Coherent dynamics and association networks among lake bacterioplankton taxa. ISME J 6, 330-342.
DOI PMID |
| [10] |
Fürnkranz M, Wanek W, Richter A, Abell G, Rasche F, Sessitsch A (2008). Nitrogen fixation by phyllosphere bacteria associated with higher plants and their colonizing epiphytes of a tropical lowland rainforest of Costa Rica. ISME J 2, 561-570.
DOI PMID |
| [11] | Goudjal Y, Toumatia O, Sabaou N, Barakate M, Mathieu F, Zitouni A (2013). Endophytic actinomycetes from spontaneous plants of Algerian Sahara: indole-3-acetic acid production and tomato plants growth promoting activity. World J Microbiol Biotechnol 29, 1821-1829. |
| [12] | Hernández JA, Jiménez A, Mullineaux P, Sevilia F (2000). Tolerance of pea (Pisum sativum L.) to long-term salt stress is associated with induction of antioxidant defences. Plant Cell Environ 23, 853-862. |
| [13] | Hou Q (2020). The Rhizosphere Microbial Diversity During the Growth Period of Continuous Cropping Potato and the Effect of Fertilization. Master’s thesis. Beijing: Chinese Academy of Agricultural Sciences. pp. 13-17. (in Chinese) |
| 侯乾 (2020). 连作马铃薯全生育期根际微生物多样性研究及施肥对其的影响. 硕士论文. 北京: 中国农业科学院. pp. 13-17. | |
| [14] | Hu TT, Yuan LN, Wang JF, Kang SZ, Li FS (2010). Antioxidation responses of maize roots and leaves to partial root-zone irrigation. Agric Water Manage 98, 164-171. |
| [15] | Hunter PJ, Hand P, Pink D, Whipps JM, Bending GD (2010). Both leaf properties and microbe-microbe interactions influence within-species variation in bacterial population diversity and structure in the lettuce (Lactuca species) phyllosphere. Appl Environ Microbiol 76, 8117-8125. |
| [16] |
Kolvenbach BA, Corvini PFX (2012). The degradation of alkylphenols by Sphingomonas sp. strain TTNP3—a review on seven years of research. New Biotechnol 30, 88-95.
DOI PMID |
| [17] | Li ZW (2014). The Expression Alteration of Various Genes Related to Sugar Metabolism in Senescing Leaves and Its Antioxidation Modulation for esl Mutant. PhD dissertation. Hangzhou: Zhejiang University. pp. 17-25. (in Chinese) |
| 李兆伟 (2014). 水稻叶片早衰突变体的糖代谢基因表达与抗氧化生理调控. 博士论文. 杭州: 浙江大学. pp. 17-25. | |
| [18] | Lindow SE, Brandl MT (2003). Microbiology of the phyllosphere. Appl Environ Microbiol 69, 1875-1883. |
| [19] | Liu H, Wei LL, Zhu LF, Wei H, Bai YX, Liu XL, Li SB (2023). Research progress of Sphingomonas. Microbiol China 50, 2738-2752. (in Chinese) |
| 刘辉, 韦璐璐, 朱龙发, 韦豪, 白云霞, 刘小玲, 李树波 (2023). 鞘氨醇单胞菌的研究进展. 微生物学通报 50, 2738-2752. | |
| [20] | Liu KC, Wang QC, Zhang HS, Feng K (2003). Advance in research of physiological mechanism and genetic traits of stay-green of maize. Shandong Agric Sci 35(2), 48-51. (in Chinese) |
| 刘开昌, 王庆成, 张海松, 冯凯 (2003). 玉米叶片保绿性生理机理及遗传特性研究进展. 山东农业科学 35(2), 48-51. | |
| [21] | Liu YY, Zhang C, Jiang SJ, Zhou ZF, Chen M, Zhang W, Wang J (2015). Functional analysis of drB0118 gene in response to abiotic stress in Deinococcus radiodurans. Microbiol China 42, 1474-1481. (in Chinese) |
| 刘盈盈, 张陈, 江世杰, 周正富, 陈明, 张维, 王劲 (2015). 非生物胁迫下耐辐射异常球菌drB0118基因功能分析. 微生物学通报 42, 1474-1481. | |
| [22] | Luo LY, Wang P, Zhai ZY, Su P, Tan XQ, Zhang DY, Zhang Z, Liu Y (2019). The effects of Rhodopseudomonas palustris PSB06 and CGA009 with different agricultural applications on rice growth and rhizosphere bacterial communities. AMB Express 9, 173. |
| [23] | Ma YY, Chu HY (2021). Field sampling and sample storage of wheat-associated microbiomes. Bio-101 e2003668. doi: 10.21769/BioProtoc.2003668. (in Chinese) |
| 马玉颖, 褚海燕 (2021). 小麦相关微生物的野外采样与样品保存. Bio-101 e2003668. doi: 10.21769/BioProtoc.2003668. | |
| [24] |
Morano KA, Grant CM, Moye-Rowley WS (2012). The response to heat shock and oxidative stress in Saccharomyces cerevisiae. Genetics 190, 1157-1195.
DOI PMID |
| [25] | Murty MG (1983). Nitrogen fixation (acetylene reduction) in the phyllosphere of some economically important plants. Plant Soil 73, 151-153. |
| [26] | Naveed M, Mitter B, Reichenauer TG, Wieczorek K, Sessitsch A (2014). Increased drought stress resilience of maize through endophytic colonization by Burkholderia phytofirmans PsJN and Enterobacter sp. FD17. Environ Exp Bot 97, 30-39. |
| [27] |
Rastogi G, Coaker GL, Leveau JHJ (2013). New insights into the structure and function of phyllosphere microbiota through high-throughput molecular approaches. FEMS Microbiol Lett 348, 1-10.
DOI PMID |
| [28] |
Redford AJ, Bowers RM, Knight R, Linhart Y, Fierer N (2010). The ecology of the phyllosphere: geographic and phylogenetic variability in the distribution of bacteria on tree leaves. Environ Microbiol 12, 2885-2893.
DOI PMID |
| [29] | Ren GD, Zhu CW, Alam MS, Tokida T, Sakai H, Nakamura H, Usui Y, Zhu JG, Hasegawa T, Jia ZJ (2015). Response of soil, leaf endosphere and phyllosphere bacterial communities to elevated CO2 and soil temperature in a rice paddy. Plant Soil 392, 27-44. |
| [30] | Rogers HJ (2017). Leaf senescence. Encycl Appl Plant Sci (Second Ed) 1, 308-314. |
| [31] | Song H, Feng BL, Gao XL, Gao JF, Wang PK, Chai Y, Zhang PP (2010). Leaf senescence and reactive oxygen metabolism in different adzuki bean cultivars (lines). Acta Agron Sin 36, 347-353. (in Chinese) |
|
宋慧, 冯佰利, 高小丽, 高金锋, 王鹏科, 柴岩, 张盼盼 (2010). 不同小豆品种(系)叶片衰老与活性氧代谢. 作物学报 36, 347-353.
DOI |
|
| [32] | Song Y, Chen HH, Cui X, Lu ZF, Liao SP, Zhang YY, Li XK, Cong RH, Ren T, Lu JW (2024). Potassium nutrient status-mediated leaf growth of oilseed rape (Brassica napus) and its effect on phyllosphere microorganism. Chin Bull Bot 59, 54-65. (in Chinese) |
|
宋毅, 陈航航, 崔鑫, 陆志峰, 廖世鹏, 张洋洋, 李小坤, 丛日环, 任涛, 鲁剑巍 (2024). 钾营养状况介导的油菜叶片生长及其对叶际微生物的影响. 植物学报 59, 54-65.
DOI |
|
| [33] | Su P, Tan XQ, Li CG, Zhang DY, Cheng J, Zhang SB, Zhou XG, Yan QP, Peng J, Zhang Z, Liu Y, Lu XY (2017). Photosynthetic bacterium Rhodopseudomonas palustris GJ-22 induces systemic resistance against viruses. Microb Biotechnol 10, 612-624. |
| [34] | Su P, Zhang DY, Zhang Z, Chen A, Cheng JE, Zeng J, Tan SY, Dai JP, Liu Y (2021). Dissection on the agronomical functions of photosynthetic bacteria. Chin J Biol Control 37, 30-37. (in Chinese) |
|
苏品, 张德咏, 张卓, 陈昂, 程菊娥, 曾军, 谭石勇, 戴建平, 刘勇 (2021). 光合细菌的农用微生物功能解读. 中国生物防治学报 37, 30-37.
DOI |
|
| [35] | Tong SY, Song FB, Xu HW (2009). Differences of morphological senescence of leaves in various maize varieties during mature period of seed. Acta Agric Boreali-Sin 24, 11-15. (in Chinese) |
|
童淑媛, 宋凤斌, 徐洪文 (2009). 不同品种玉米籽粒成熟期间叶片形态衰老的差异. 华北农学报 24, 11-15.
DOI |
|
| [36] | Velikova V, Yordanov I, Edreva A (2000). Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Sci 151, 59-66. |
| [37] |
Vorholt JA (2012). Microbial life in the phyllosphere. Nat Rev Microbiol 10, 828-840.
DOI PMID |
| [38] | Wang HZ, Zhao HL, Feng YX, Jiang L, Ning DK, Xie LY, Lin ED (2014). Response of soluble substances content in flag leaves during late growth stage and plant productivity of rice to elevated CO2 in North China. Acta Agron Sin 40, 320-328. (in Chinese) |
|
王惠贞, 赵洪亮, 冯永祥, 姜乐, 宁大可, 谢立勇, 林而达 (2014). 北方水稻生育后期剑叶可溶性物质含量及植株生产力对CO2浓度增高的响应. 作物学报 40, 320-328.
DOI |
|
| [39] | Wang P (2015). Regulation of Leaf Senescence by Exogenous Melatonin and Functional Analysis of Related Autophagy Genes in Malus. PhD dissertation. Yangling: Northwest A&F University. pp. 16-23. (in Chinese) |
| 王平 (2015). 外源褪黑素对苹果叶片衰老的调控及相关自噬基因的功能分析. 博士论文. 杨凌: 西北农林科技大学. pp. 16-23. | |
| [40] |
Wang YJ, Lü YJ, Liu HT, Bian SF, Wang LC (2019). Integrated management of high-yielding and high nutrient efficient spring maize in Northeast China. Sci Agric Sin 52, 3533-3535. (in Chinese)
DOI |
|
王永军, 吕艳杰, 刘慧涛, 边少锋, 王立春 (2019). 东北春玉米高产与养分高效综合管理. 中国农业科学 52, 3533-3535.
DOI |
|
| [41] | Xiong C, He JZ, Zhang LM (2021). DNA extraction, amplification and source-tracking analysis for plant microbiomes. Bio-101 e2003695. doi: 10.21769/BioProtoc.2003695. (in Chinese) |
| 熊超, 贺纪正, 张丽梅 (2021). 植物微生物组DNA提取扩增及溯源分析. Bio-101 e2003695. doi: 10.21769/Bio-Protoc.2003695. | |
| [42] |
Xiong C, Singh BK, He JZ, Han YL, Li PP, Wan LH, Meng GZ, Liu SY, Wang JT, Wu CF, Ge AH, Zhang LM (2021). Plant developmental stage drives the differentiation in ecological role of the maize microbiome. Microbiome 9, 171.
DOI PMID |
| [43] | Yan L, Zhang YZ, Qing Y, Fang ZR, Lai XJ (2020). Community rhythms of rhizosphere microbiome during the whole life cycle of potato. Acta Microbiol Sin 60, 246-260. (in Chinese) |
| 颜朗, 张义正, 清源, 方志荣, 赖先军 (2020). 马铃薯全生育期内根际微生物组变化规律. 微生物学报 60, 246-260. | |
| [44] | Yutthammo C, Thongthammachat N, Pinphanichakarn P, Luepromchai E (2010). Diversity and activity of PAH- degrading bacteria in the phyllosphere of ornamental plants. Microb Ecol 59, 357-368. |
| [45] |
Zhang JY, Zhang N, Liu YX, Zhang XN, Hu B, Qin Y, Xu HR, Wang H, Guo XX, Qian JM, Wang W, Zhang PF, Jin T, Chu CC, Bai Y (2018). Root microbiota shift in rice correlates with resident time in the field and developmental stage. Sci China Life Sci 61, 613-621.
DOI PMID |
| [46] | Zhang K, Li Z, Zheng Y, Ma HX, Liu MH, Ding HJ, Wang Y, Liu L, Xia XC (2020). Biodiversity of culturable moderate halophilic bacteria of rock salt in Yexian county, Henan province. Microbiol China 47, 3987-3997. (in Chinese) |
| 张科, 李臻, 郑瑶, 麻红星, 刘梦含, 丁慧杰, 王瑜, 刘丽, 夏西超 (2020). 河南叶县岩盐可培养中度嗜盐菌的多样性. 微生物学通报 47, 3987-3997. | |
| [47] | Zhang ZL, Qu WJ (2003). The Experimental Guide for Plant Physiology, 3rd edn. Beijing: Higher Education Press. pp. 67-159. (in Chinese) |
| 张志良, 瞿伟菁 (2003). 植物生理学实验指导(第3版). 北京: 高等教育出版社. pp. 67-159. | |
| [48] | Zhao GS, Zhang DY, Liu Y, Su P (2018). Application prospect of photosynthetic bacteria in inducing plant system resistance. Guizhou Agric Sci 46(11), 53-56. (in Chinese) |
| 赵国盛, 张德咏, 刘勇, 苏品 (2018). 光合细菌在植物诱导系统抗性中的应用前景. 贵州农业科学 46(11), 53-56. | |
| [49] |
Zhou M, Zhang JJ, Luo Y (2023). The mechanism, current status and prospects of microbial fertilizers. Chin Agric Sci Bull 39(33), 68-75. (in Chinese)
DOI |
|
周萌, 张嘉俊, 罗洋 (2023). 微生物肥料的作用机理、现状及展望. 中国农学通报 39 (33), 68-75.
DOI |
| [1] | Zhiyu Liu, Xin Ji, Guohui Sui, Ding Yang, Xuankun Li. Invertebrate diversity in buffalo grass and weedy lawns at Beijing Capital International Airport [J]. Biodiv Sci, 2025, 33(4): 24456-. |
| [2] | WANG Juan, ZHANG Deng-Shan, XIAO Yuan-Ming, PEI Quan-Bang, WANG Bo, FAN Bo, ZHOU Guo-Ying. Relationships between the characteristics of root exudate and environmental factors in the alpine steppe following long-term grazing exclusion [J]. Chin J Plant Ecol, 2025, 49(4): 596-609. |
| [3] | Li Hualiang, Zhang Mingjun, Zhang Xibin, Tan Rong, Li Shichuan, Feng Erhui, Lin Xueyun, Chen Min, Yan enbo, Zeng Zhigao. Composition and influencing factors of the amphibian community in Hainan Dongzhaigang National Nature Reserve [J]. Biodiv Sci, 2025, 33(2): 24350-. |
| [4] | Yuan Li, Kaijian Fan, Tai An, Cong Li, Junxia Jiang, Hao Niu, Weiwei Zeng, Yanfang Heng, Hu Li, Junjie Fu, Huihui Li, Liang Li. Study on Multi-environment Genome-wide Prediction of Inbred Agronomic Traits in Maize Natural Populations [J]. Chinese Bulletin of Botany, 2024, 59(6): 1041-1053. |
| [5] | Tao Wang, Jinglei Feng, Cui Zhang. Research Progress on Molecular Mechanisms of Heat Stress Affecting the Growth and Development of Maize [J]. Chinese Bulletin of Botany, 2024, 59(6): 963-977. |
| [6] | Juan Yang, Yuelei Zhao, Xiaoyuan Chen, Baobao Wang, Haiyang Wang. Regulation Mechanism and Breeding Application of Flowering Time in Maize [J]. Chinese Bulletin of Botany, 2024, 59(6): 912-931. |
| [7] | Hengyu Yan, Zhaoxia Li, Yubin Li. Research Progress on Heat Stress Impact on Maize Growth and Heat-Tolerant Maize Screening in China [J]. Chinese Bulletin of Botany, 2024, 59(6): 1007-1023. |
| [8] | Qingguo Du, Wenxue Li. Research Progress in the Regulation of Development and Stress Responses by Long Non-coding RNAs in Maize [J]. Chinese Bulletin of Botany, 2024, 59(6): 950-962. |
| [9] | Ziyang Wang, Shengxue Liu, Zhirui Yang, Feng Qin. Genetic Dissection of Drought Resistance in Maize [J]. Chinese Bulletin of Botany, 2024, 59(6): 883-902. |
| [10] | Suowei Wu, Xueli An, Xiangyuan Wan. Molecular Mechanisms of Male Sterility and their Applications in Biotechnology-based Male-sterility Hybrid Seed Production in Maize [J]. Chinese Bulletin of Botany, 2024, 59(6): 932-949. |
| [11] | Mingmin Zheng, Qiang Huang, Peng Zhang, Xiaowei Liu, Zhuofan Zhao, Hongyang Yi, Tingzhao Rong, Moju Cao. Research Progress on Cytoplasmic Male Sterility and Fertility Restoration in Maize [J]. Chinese Bulletin of Botany, 2024, 59(6): 999-1006. |
| [12] | Qiang Zhang, Zhenyu Zhao, Pinghua Li. Research Progress of Gene Editing Technology in Maize [J]. Chinese Bulletin of Botany, 2024, 59(6): 978-998. |
| [13] | CHEN Ke-Yu, XING Sen, TANG Yu, SUN Jia-Hui, REN Shi-Jie, ZHANG Jing, JI Bao-Ming. Arbuscular mycorrhizal fungal community characteristics and driving factors in different grassland types [J]. Chin J Plant Ecol, 2024, 48(5): 660-674. |
| [14] | CHENG Ke-Xin, DU Yao, LI Kai-Hang, WANG Hao-Chen, YANG Yan, JIN Yi, HE Xiao-Qing. Genetic mechanism of interaction between maize and phyllospheric microbiome [J]. Chin J Plant Ecol, 2024, 48(2): 215-228. |
| [15] | WANG Liang, ZHAO Xue-Chao, YANG Shao-Bo, WANG Qing-Kui. Priming effect of soil organic carbon decomposition induced by Cunninghamia lanceolate leaf litter and fine root and its response to nitrogen addition in subtropical forests [J]. Chin J Plant Ecol, 2024, 48(11): 1434-1444. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||