

Chinese Bulletin of Botany ›› 2023, Vol. 58 ›› Issue (4): 573-589.DOI: 10.11983/CBB23006 cstr: 32102.14.CBB23006
• EXPERIMENTAL COMMUNICATIONS • Previous Articles Next Articles
Xuanwen Mao1, Zhichao Wang1, Xinyi Ruan1, Jingfei Sun1, Yating Zhang1, Jinhao Lu1, Tiantian Shao1, Xian Wang1, Jiamin Xiao1, Li Xiao1, Mengyao Ye1, Yuhuan Wu2,3, Peng Liu1( )
)
Received:2023-01-15
Accepted:2023-03-08
Online:2023-07-01
Published:2023-03-10
Contact:
*E-mail: sky79@zjnu.cn
Xuanwen Mao, Zhichao Wang, Xinyi Ruan, Jingfei Sun, Yating Zhang, Jinhao Lu, Tiantian Shao, Xian Wang, Jiamin Xiao, Li Xiao, Mengyao Ye, Yuhuan Wu, Peng Liu. Regulatory Effects of Exogenous Organic Acids on the Physiological Responses of Helianthus tuberosus Under Aluminium Stress[J]. Chinese Bulletin of Botany, 2023, 58(4): 573-589.
| Group | Aluminium concentration (μmol?L-1) | Compound organic acid (OA) concentration (μmol?L-1) |
|---|---|---|
| 1 | 0 | 0 |
| 2 | 0 | 30 |
| 3 | 0 | 60 |
| 4 | 0 | 90 |
| 5 | 350 | 0 |
| 6 | 350 | 30 |
| 7 | 350 | 60 |
| 8 | 350 | 90 |
| 9 | 700 | 0 |
| 10 | 700 | 30 |
| 11 | 700 | 60 |
| 12 | 700 | 90 |
Table 1 Experimental design in this study
| Group | Aluminium concentration (μmol?L-1) | Compound organic acid (OA) concentration (μmol?L-1) |
|---|---|---|
| 1 | 0 | 0 |
| 2 | 0 | 30 |
| 3 | 0 | 60 |
| 4 | 0 | 90 |
| 5 | 350 | 0 |
| 6 | 350 | 30 |
| 7 | 350 | 60 |
| 8 | 350 | 90 |
| 9 | 700 | 0 |
| 10 | 700 | 30 |
| 11 | 700 | 60 |
| 12 | 700 | 90 |
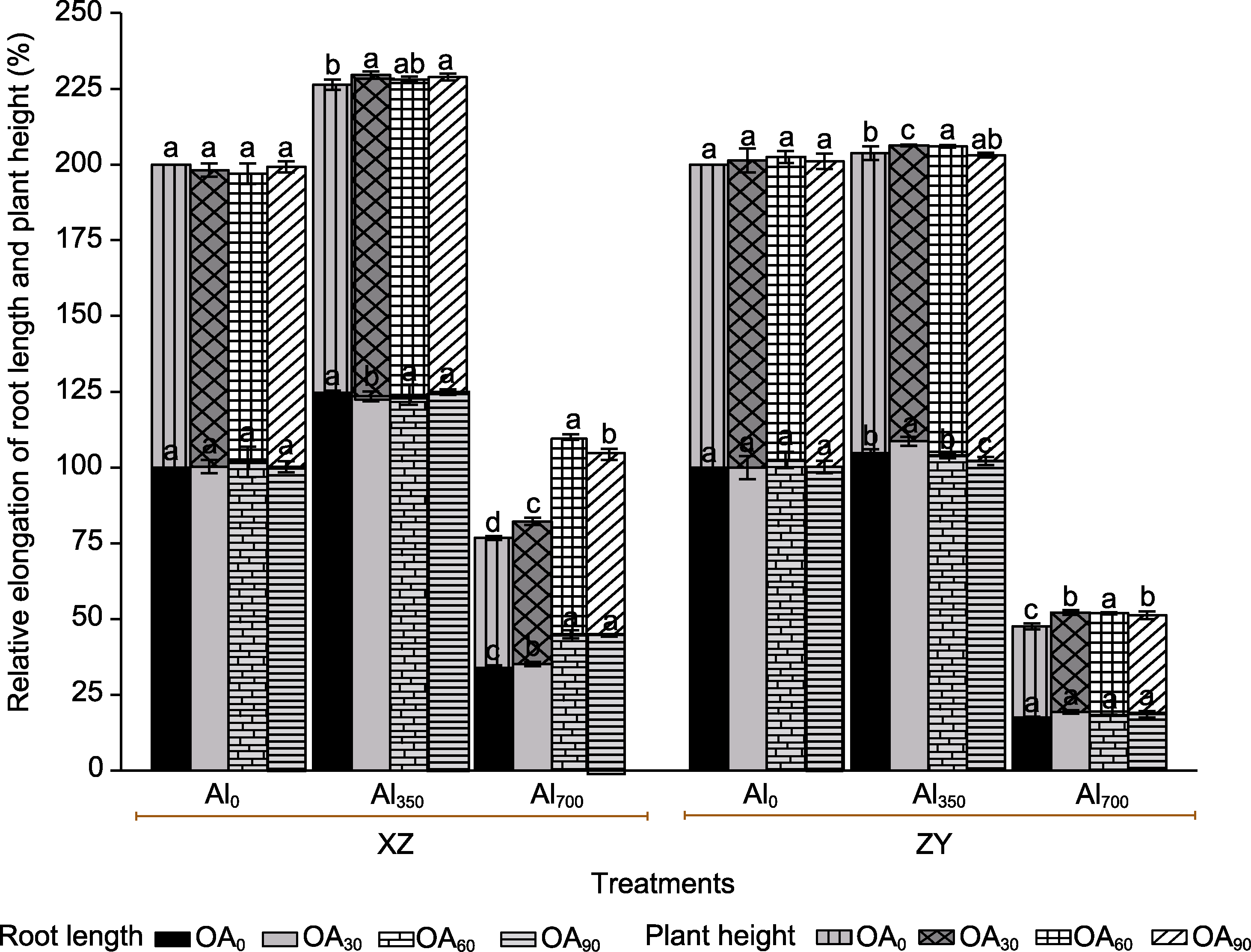
Figure 1 The effect of exogenous compound organic acid (OA) on the root length and plant height of Helianthus tuberosus under aluminum (Al) stress Al0, Al350, and Al700 indicating concentrations of Al are 0, 350, and 700 μmol?L-1, respectively; OA0, OA30, OA60, and OA90 indicating concentrations of OA are 0, 30, 60, and 90 μmol?L-1, respectively. XZ: H. tuberosus cv. ‘Xuzhou’; ZY: H. tuberosus cv. ‘Ziyang’. Different lowercase letters indicate significant differences among treatment groups at the same period (P<0.05).
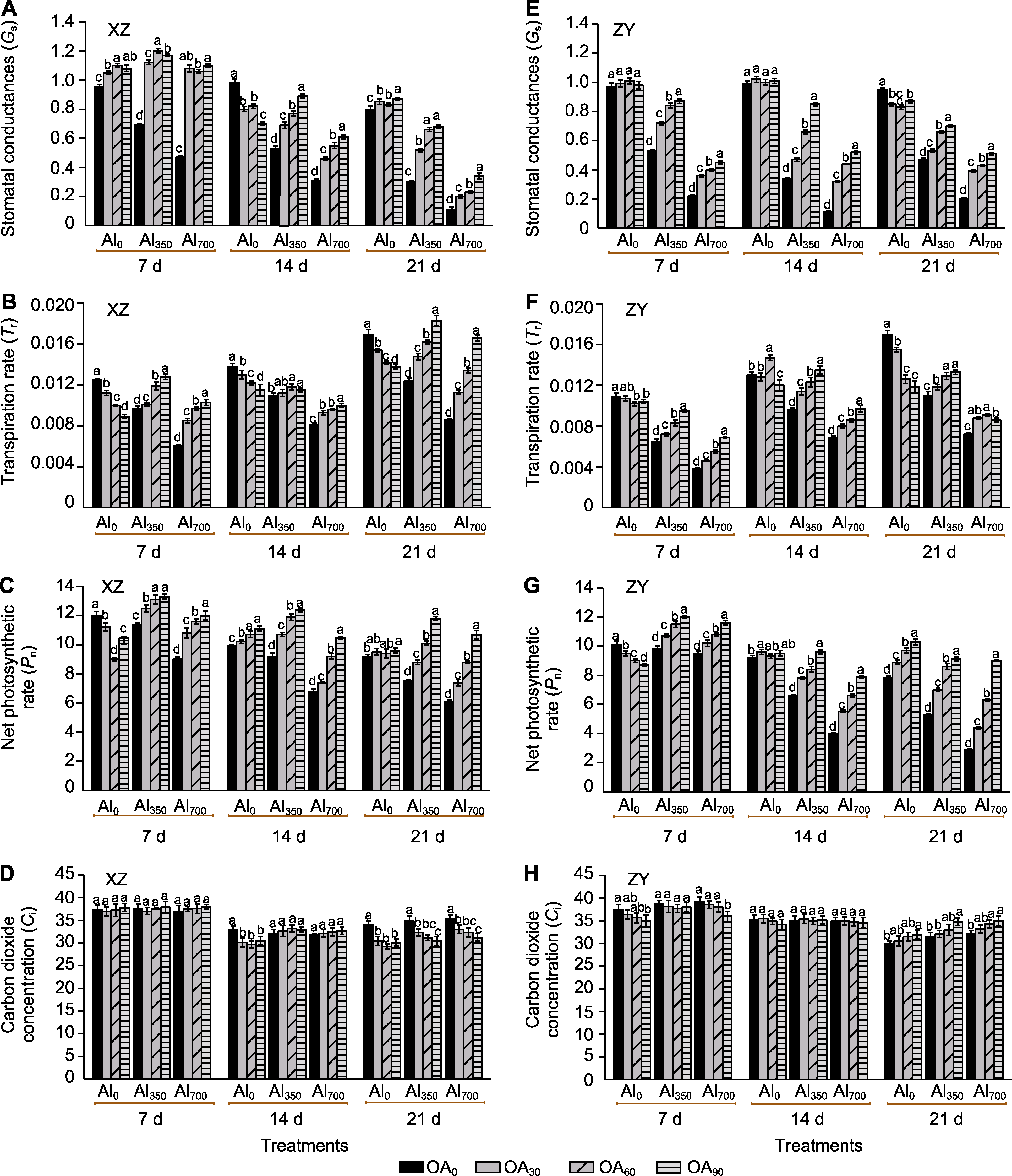
Figure 2 The effect of exogenous compound organic acid (OA) on the stomatal conductance (Gs) (A, E), transpiration rate (Tr) (B, F), net photosynthetic rate (Pn) (C, G), and intercellular CO2 concentration (Ci) (D, H) of Helianthus tuberosus leaves under aluminum (Al) stress Al0, Al350, Al700, OA0, OA30, OA60, and OA90 are the same as shown in Figure 1. XZ and ZY are the same as shown in Figure 1. Different lowercase letters indicate significant differences among different treatment groups at the same period (P<0.05).
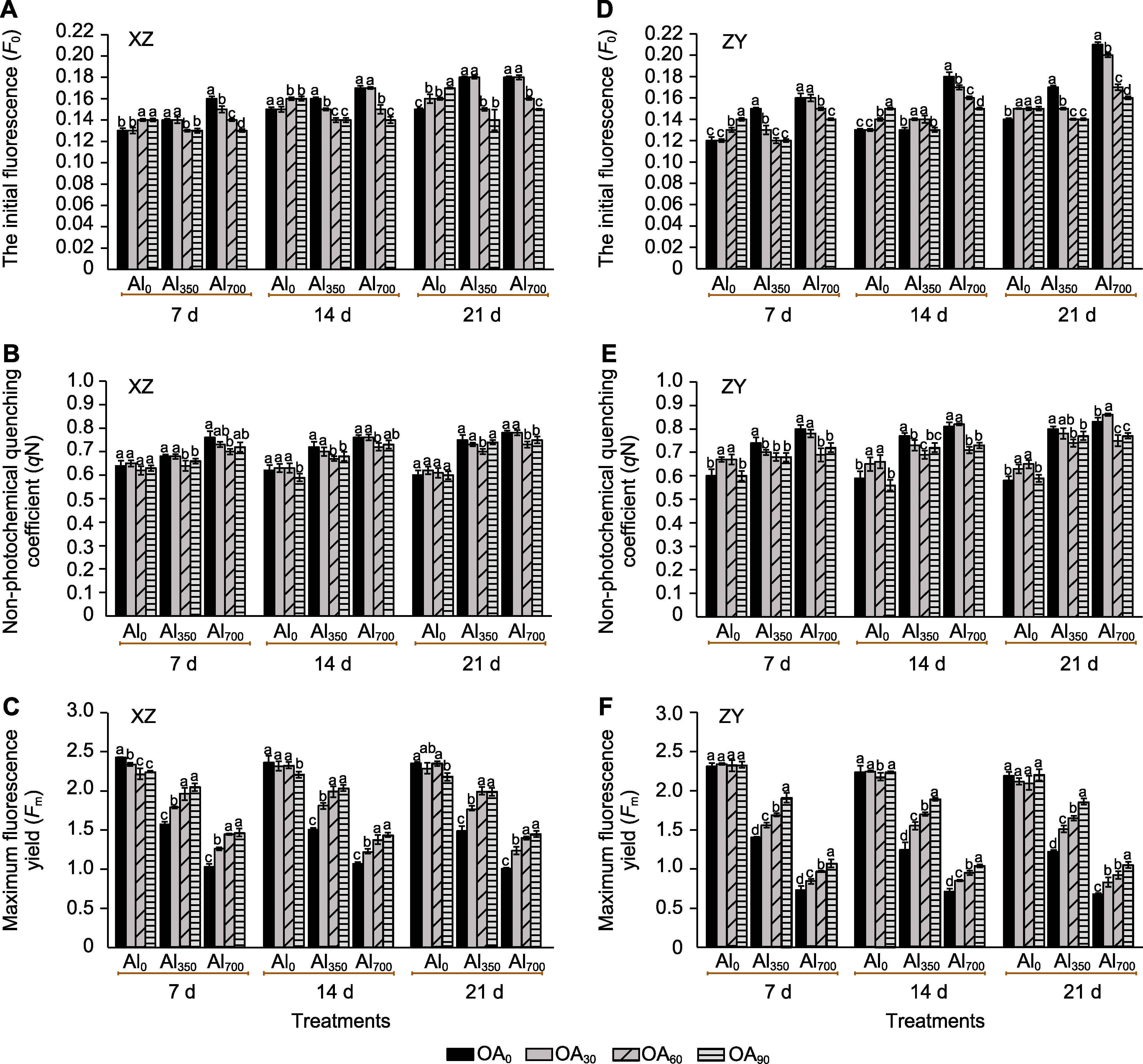
Figure 3 The effect of exogenous compound organic acid (OA) on the initial fluorescence (F0) (A, D), non-photochemical quenching (qN) (B, E) and maximal fluorescence (Fm) (C, F) of Helianthus tuberosus leaves under aluminum (Al) stress Al0, Al350, Al700, OA0, OA30, OA60, and OA90 are the same as shown in Figure 1. XZ and ZY are the same as shown in Figure 1. Different lowercase letters indicate significant differences among different treatment groups at the same period (P<0.05).
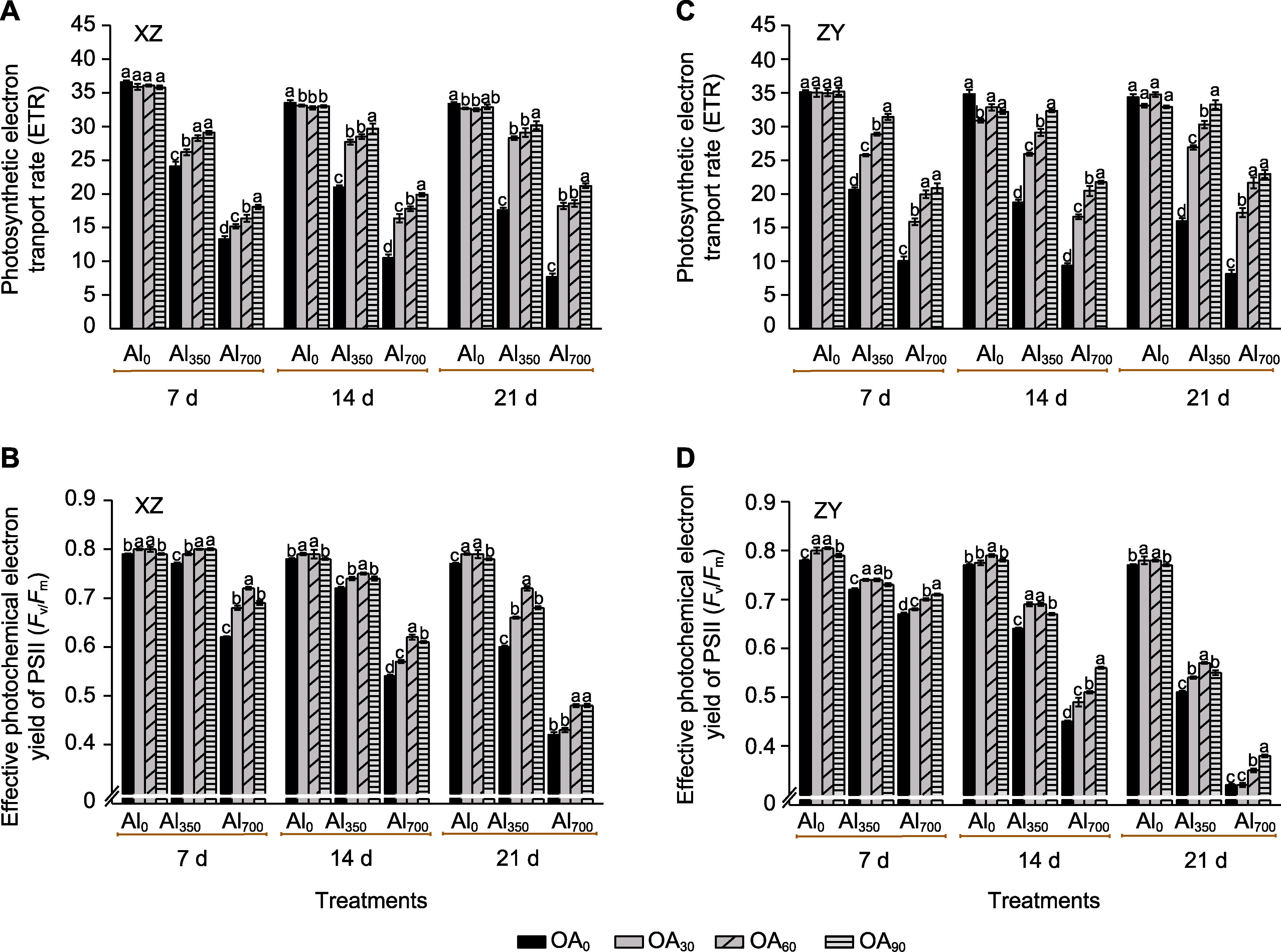
Figure 4 The effect of exogenous compound organic acid (OA) on the electron transport rate (ETR) (A, C) and maximum photochemical quantum yield of PSII (Fv/Fm) (B, D) of Helianthus tuberosus leaves under aluminum (Al) stress Al0, Al350, Al700, OA0, OA30, OA60, and OA90 are the same as shown in Figure 1. XZ and ZY are the same as shown in Figure 1. Different lowercase letters indicate significant differences among different treatment groups at the same period (P<0.05).
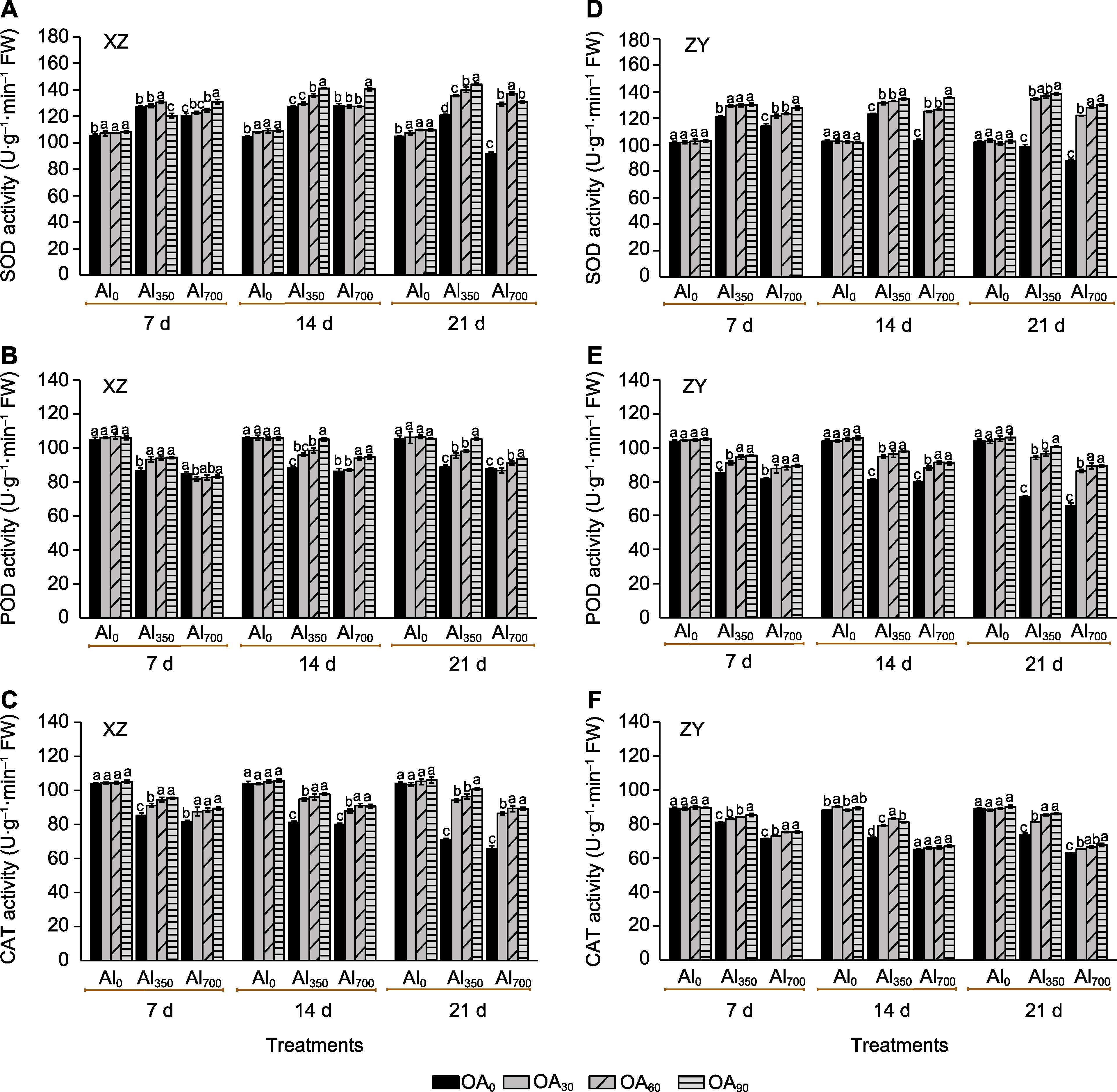
Figure 5 The effect of exogenous compound organic acid (OA) on the superoxide dismutase (SOD) (A, D), peroxidase (POD) (B, E) and catalase (CAT) (C, F) activities of Helianthus tuberosus leaves under aluminum (Al) stress Al0, Al350, Al700, OA0, OA30, OA60, and OA90 are the same as shown in Figure 1. XZ and ZY are the same as shown in Figure 1. Different lowercase letters indicate significant differences among different treatment groups at the same period (P<0.05).
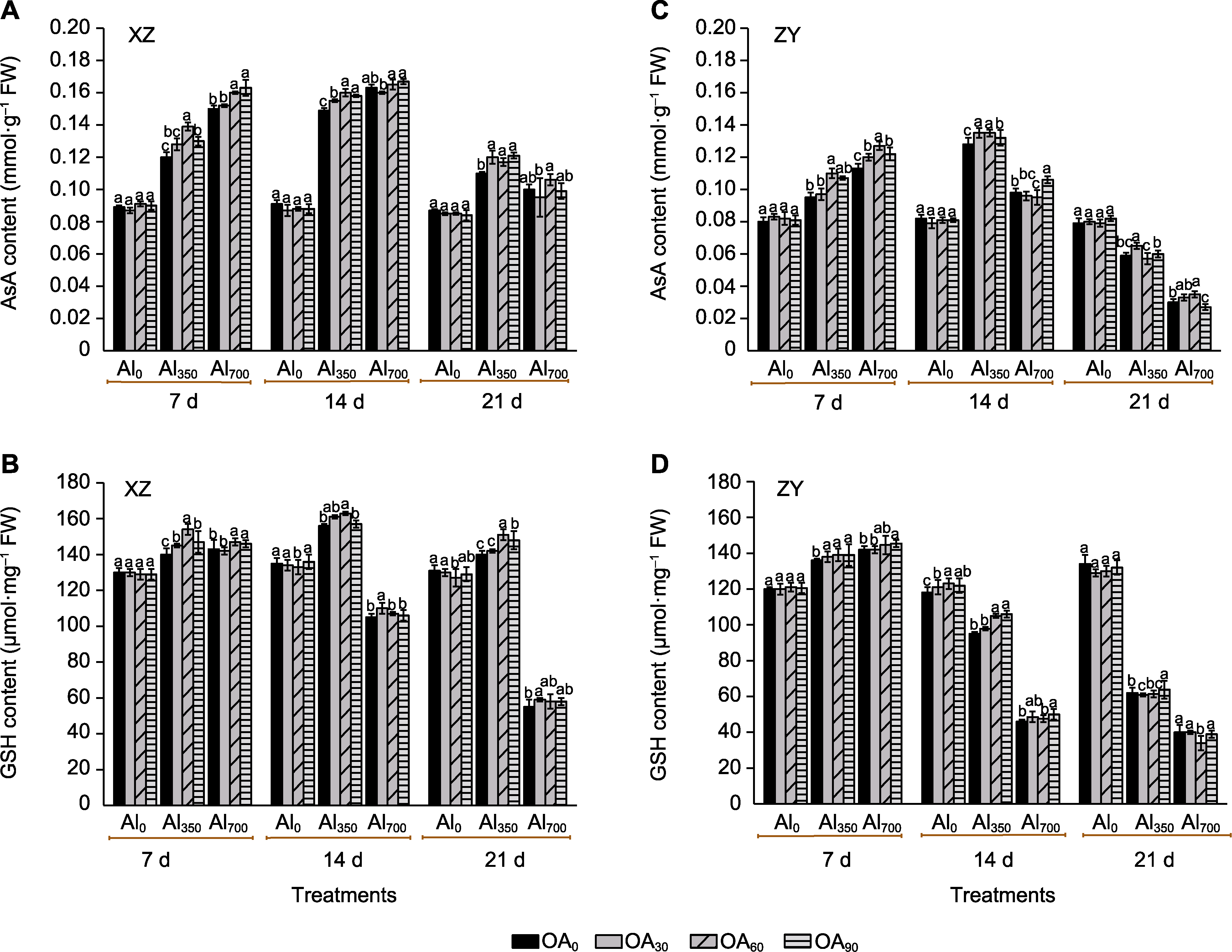
Figure 6 The effect of exogenous compound organic acid (OA) on the ascorbic acid (AsA) (A, C) and glutathione (GSH) (B, D) contents of Helianthus tuberosus leaves under aluminum (Al) stress Al0, Al350, Al700, OA0, OA30, OA60, and OA90 are the same as shown in Figure 1. XZ and ZY are the same as shown in Figure 1. Different lowercase letters indicate significant differences among different treatment groups at the same period (P<0.05).
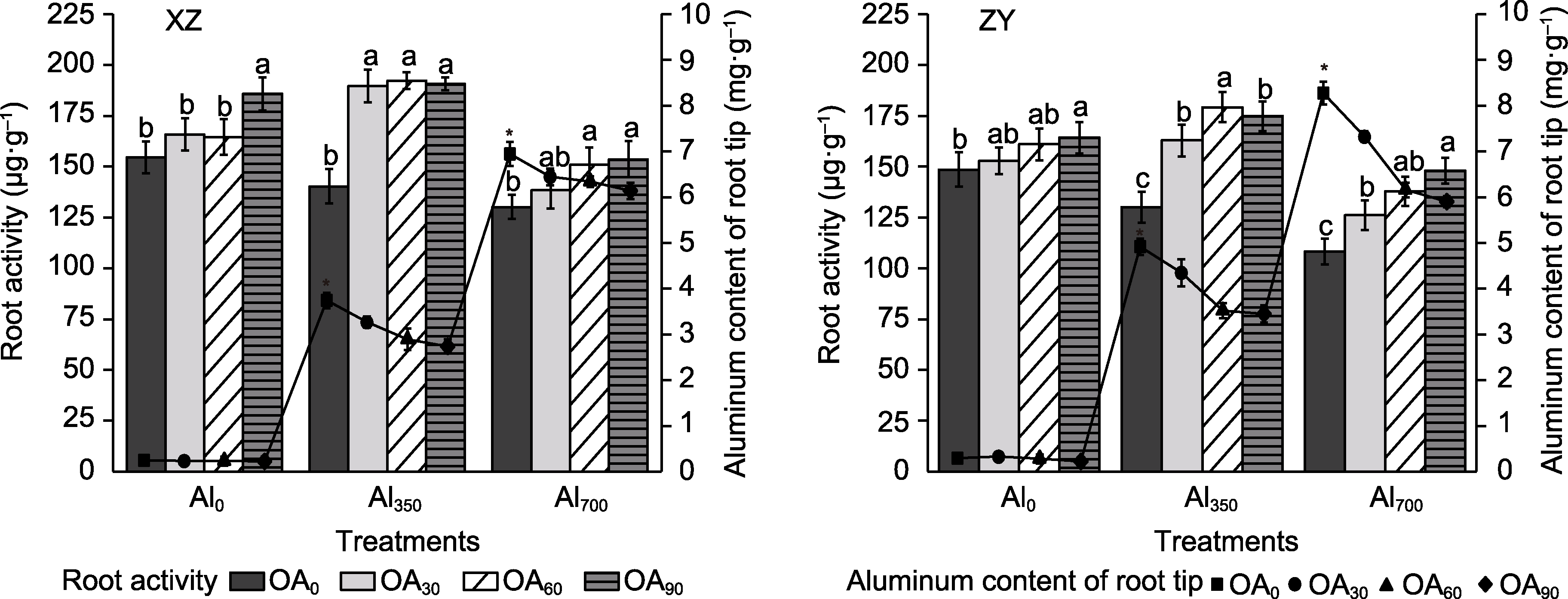
Figure 7 Effect of exogenous compound organic acid (OA) on the aluminum (Al) content of root tip and root activity of Helianthus tuberosus under aluminum stress Al0, Al350, Al700, OA0, OA30, OA60, and OA90 are the same as shown in Figure 1. XZ and ZY are the same as shown in Figure 1. Different lowercase letters and * indicate significant differences among different treatment groups at the same period (P<0.05).
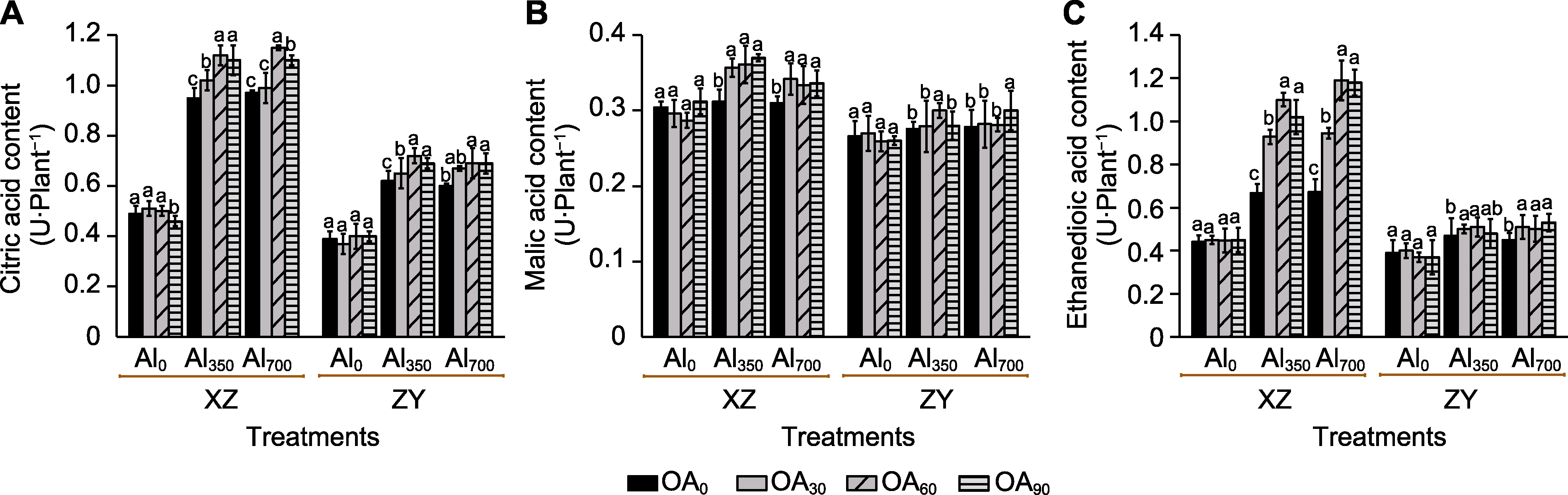
Figure 8 The effect of exogenous compound organic acid (OA) on the content of citric acid (CA) (A), malic acid (MA) (B) and ethanedioic acid (EA) (C) in root exudates of Helianthus tuberosus under aluminum (Al) stress Al0, Al350, Al700, OA0, OA30, OA60, and OA90 are the same as shown in Figure 1. XZ and ZY are the same as shown in Figure 1. Different lowercase letters indicate significant differences among different treatment groups at the same period (P<0.05).
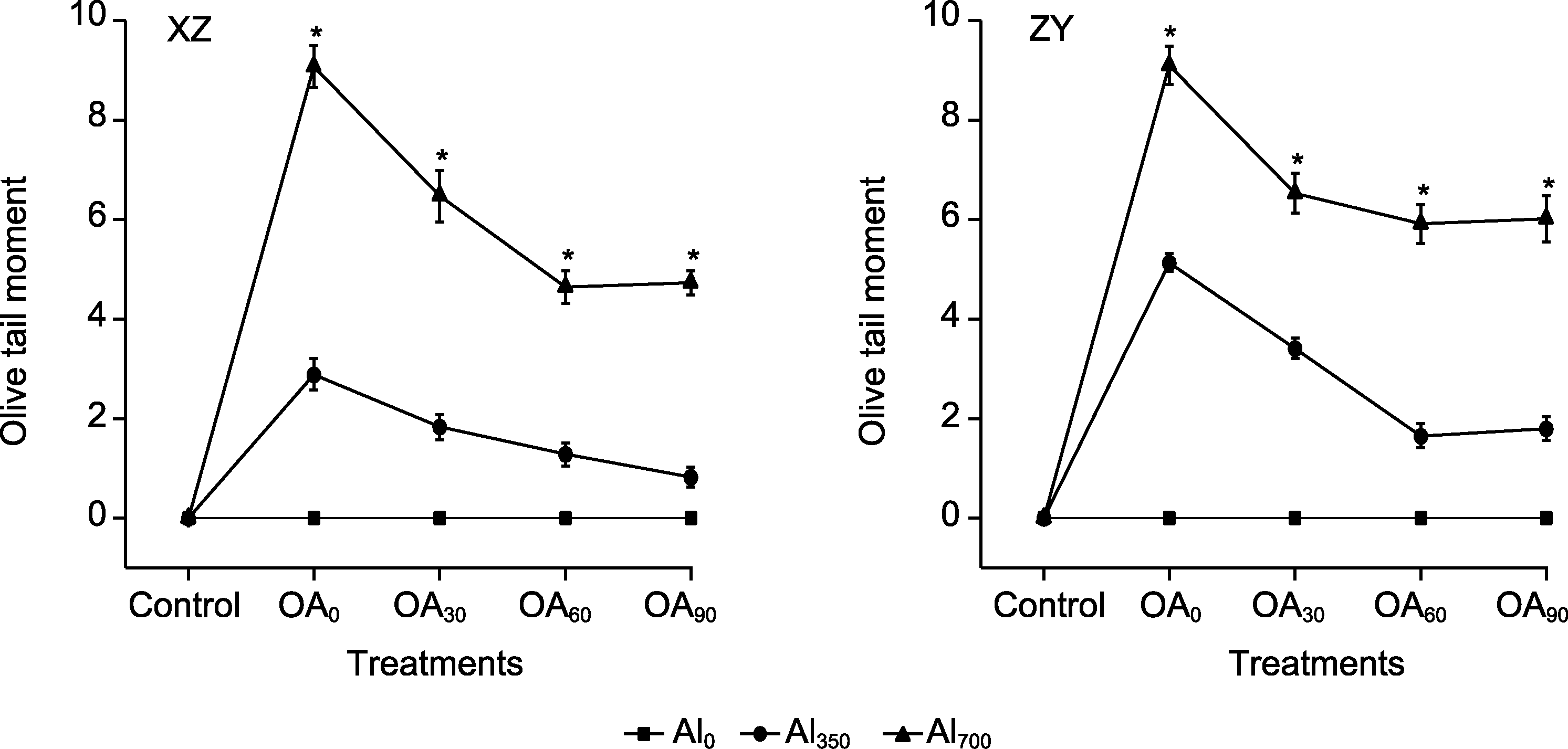
Figure 9 The effect of exogenous compound organic acid (OA) on oliver tail moment (OTM) of root cells of Helianthus tuberosus under aluminum (Al) stress Al0, Al350, Al700, OA0, OA30, OA60, and OA90 are the same as shown in Figure 1. XZ and ZY are the same as shown in Figure 1. * indicate significant differences among different treatment groups at the same period (P<0.05).
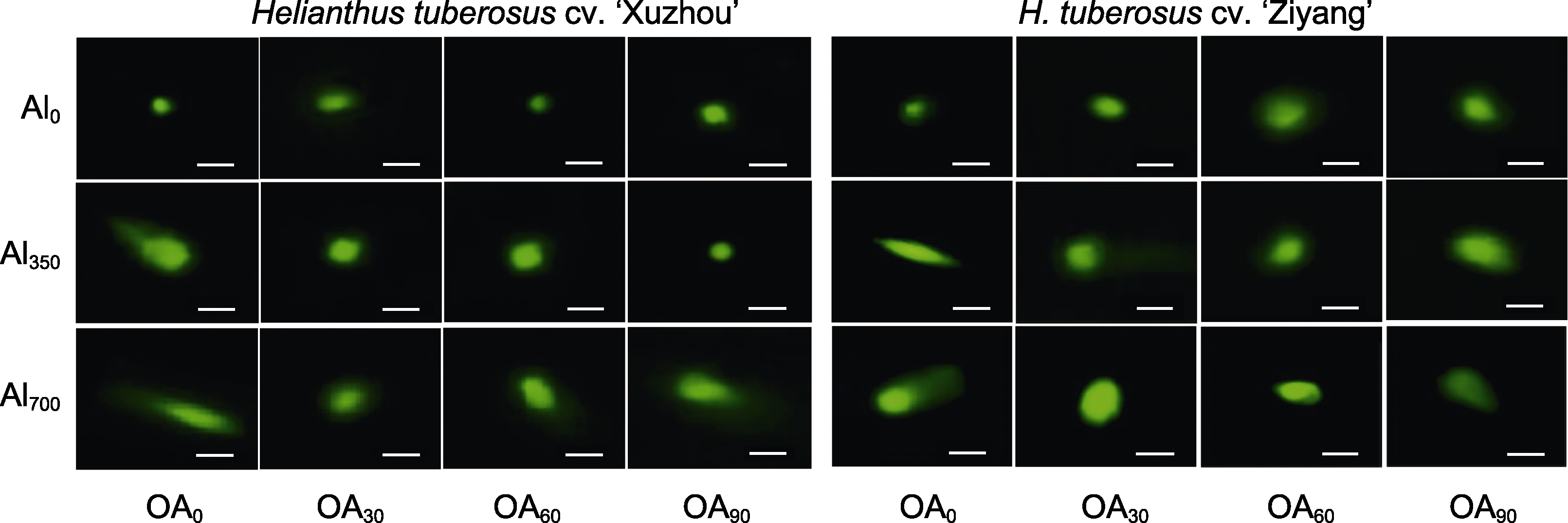
Figure 10 DNA damage of Helianthus tuberosus root under different concentration of compound organic acid (OA) and aluminum (Al) treatments Al0, Al350, Al700, OA0, OA30, OA60, and OA90 are the same as shown in Figure 1. Bars=20 μm
| [1] | 曹林, 吴玉环, 章艺, 郭怡, 肖有铁, 郦枫, 马丽, 徐根娣, 刘鹏 (2015). 外源水杨酸对铝胁迫下菊芋光合特性及耐铝性的影响. 水土保持学报 29, 260-266. |
| [2] | 陈昌, 宋颖华, 高晓强 (2010). 干法消解与湿法消解测定紫菜中铝含量. 食品安全质量检测学报 27(3), 118-123. |
| [3] | 程晓晴, 方婷玉, 李凤杰, 安渊, 周鹏 (2020). 喷施水杨酸对铝胁迫紫花苜蓿幼苗光合作用和光化学系统的影响. 中国草地学报 42(4), 42-49. |
| [4] | 邓晓霞, 李月明, 姚堃姝, 乔婧文, 王竞红, 蔺吉祥 (2022). 植物适应酸铝胁迫机理的研究进展. 生物工程学报 38, 2754-2766. |
| [5] | 郜红建, 常江, 张自立, 丁士明, 魏俊岭 (2003). 研究植物根系分泌物的方法. 植物生理学通讯 39, 56-60. |
| [6] |
郭书亚, 艾金祥, 陈虹宇, 邵烨瑶, 汪妍, 王倩, 叶怡彤, 张雅婷, 丁哲晓, 吴昊辰, 吴玉环, 张建新, 饶米德, 刘鹏 (2022). 基于主成分-聚类-逐步回归分析构建番茄苗期耐铝性综合评价体系. 植物学报 57, 479-489.
DOI |
| [7] | 郭炜 (2008). 紫外辐照导致植物细胞DNA损伤的彗星电泳检测及生理指标的测定. 硕士论文. 济南: 山东大学. pp. 1-89. |
| [8] | 胡文海, 胡雪华, 闫小红, 周升团 (2021). 低温胁迫及恢复对番茄快速叶绿素荧光诱导动力学特征的影响. 中国农业气象 42, 859-869. |
| [9] |
胡文海, 叶子飘, 闫小红, 杨旭升 (2017). 越冬期广玉兰阳生叶和阴生叶PSII功能及捕光色素分子内禀特性的比较研究. 植物研究 37, 281-287.
DOI |
| [10] | 李合生 (2000). 植物生理生化实验原理和技术. 北京: 高等教育出版社. pp. 195-197. |
| [11] | 李青容, 陈建军, 祖艳群, 何永美, 湛方栋, 李博, 李元 (2022). 酸性农田土壤改良效果综合评价指标体系的构建及验证. 农业环境科学学报 41, 547-558. |
| [12] |
刘晓龙, 季平, 杨洪涛, 丁永电, 付佳玲, 梁江霞, 余聪聪 (2022). 脱落酸对水稻抽穗开花期高温胁迫的诱抗效应. 植物学报 57, 596-610.
DOI |
| [13] | 钱莲文, 李清彪, 孙境蔚, 冯莹 (2018). 铝胁迫下常绿杨根系有机酸和氨基酸的分泌. 厦门大学学报(自然科学版) 57, 221-227. |
| [14] | 沈仁芳, 赵学强 (2019). 酸性土壤可持续利用. 农学学报 9(3), 16-20. |
| [15] | 孙琴, 倪吾钟, 杨肖娥 (2002). 有机酸在植物解铝毒中的作用及生理机制. 植物学通报 19, 496-503. |
| [16] | 唐宏亮, 申建波, 张福锁, Rengel Z (2013). 磷和外源生长素对白羽扇豆(Lupinus albus L.)根形态和生理特性的影响. 中国科学: 生命科学 43, 201-212. |
| [17] |
王浩, 王明, 梁婷, 姚玉新, 杜远鹏, 高振 (2022). 气温和根区温度对葡萄叶片光合荧光特性的影响. 植物学报 57, 209-216.
DOI |
| [18] | 王亚校, 王子岚, 孙然, 杜克久 (2019). Aroclor1242暴露对荻组培苗不定根分化的影响. 林业与生态科学 34, 308-313. |
| [19] |
魏志琴, 陈志勇, 秦蓉, 王宇涛, 李韶山 (2013). Cu2+对拟南芥根的局部毒性及诱导DNA损伤和细胞死亡. 植物学报 48, 303-312.
DOI |
| [20] |
伍自力, 余孟瑶, 陈露, 魏静, 王晓琴, 胡勇, 闫妍, 万平 (2015). 小立碗藓对重金属镉胁迫的应答特征. 植物学报 50, 171-179.
DOI |
| [21] |
熊洁, 丁戈, 李书宇, 陈伦林, 宋来强 (2020). 铝胁迫对不同耐铝油菜品种苗期生长发育和养分吸收的影响. 华北农学报 35(6), 165-171.
DOI |
| [22] |
许馨露, 李丹丹, 马元丹, 翟建云, 孙建飞, 高岩, 张汝民 (2018). 四季桂抗氧化防御系统对干旱、高温及协同胁迫的响应. 植物学报 53, 72-81.
DOI |
| [23] | 余倩, 段雷, 郝吉明 (2021). 中国酸沉降: 来源、影响与控制. 环境科学学报 41, 731-746. |
| [24] | 张婷婷, 刘子凡, 安锋, 谢贵水 (2020). 铝胁迫造成橡胶苗死亡的机制研究. 热带作物学报 41, 2439-2445. |
| [25] |
张云, 王丹媚, 王孝源, 任晴雯, 唐可, 张丽宇, 吴玉环, 刘鹏 (2021). 外源茉莉酸对菊芋镉胁迫下光合特性及镉积累的影响. 作物学报 47, 2490-2500.
DOI |
| [26] | 郑开敏, 肖家昶, 马俊英, 贺茂林, 格桑, 郑阳霞 (2022). 柠檬酸对铝胁迫下豆瓣菜生长及生理的影响. 江苏农业学报 38, 476-485. |
| [27] | 周谷, 李秧秧, 樊军 (2023). 利用植物气体交换参数确定萎蔫系数的方法. 土壤学报 60, 776-786. |
| [28] | 周蜜, 吴玉环, 刘星星, 陈娇, 郑婷, 章嗣瑶, 李江雯, 李润桥, 刘鹏 (2019). 镉胁迫对菊芋生理变化及镉富集的影响. 水土保持学报 33, 323-330. |
| [29] | 周小华, 李昆志, 赵峥, 张小玲, 程霞, 冯庆 (2021). 外源抗坏血酸对水稻抗铝生理指标的影响. 热带作物学报 42, 769-776. |
| [30] |
Alasfar RH, Isaifan RJ (2021). Aluminum environmental pollution: the silent killer. Environ Sci Pollut Res Int 28, 44587-44597.
DOI |
| [31] |
Chen ZC, Liao H (2016). Organic acid anions: an effective defensive weapon for plants against aluminum toxicity and phosphorus deficiency in acidic soils. J Genet Genomics 43, 631-638.
DOI PMID |
| [32] |
Diarra I, Kotra KK, Prasad S (2022). Application of phytoremediation for heavy metal contaminated sites in the South Pacific: strategies, current challenges and future prospects. Appl Spectrosc Rev 57, 490-512.
DOI URL |
| [33] |
Dos Reis AR, Lisboa LAM, Reis HPG, De Queiroz Barcelos JP, Santos EF, Santini JMK, Meyer-Sand BRV, Putti FF, Galindo FS, Kaneko FH, Barbosa JZ, Paixão AP, Junior EF, De Figueiredo PAM, Lavres J (2018). Depicting the physiological and ultrastructural responses of soybean plants to Al stress conditions. Plant Physiol Biochem 130, 377-390.
DOI URL |
| [34] |
Guo MX, Zhang XT, Liu JJ, Hou LL, Liu HX, Zhao XS (2020). OsProDH negatively regulates thermotolerance in rice by modulating proline metabolism and reactive oxygen species scavenging. Rice 13, 61.
DOI |
| [35] |
Guo P, Qi YP, Cai YT, Yang TY, Yang LT, Huang ZR, Chen LS (2018). Aluminum effects on photosynthesis, reactive oxygen species and methylglyoxal detoxification in two Citrus species differing in aluminum tolerance. Tree Physiol 38, 1548-1565.
DOI URL |
| [36] |
Hartmann H, Link RM, Schuldt B (2021). A whole-plant perspective of isohydry: stem-level support for leaf-level plant water regulation. Tree Physiol 41, 901-905.
DOI PMID |
| [37] |
Igamberdiev AU, Bykova NV (2018). Role of organic acids in the integration of cellular redox metabolism and mediation of redox signaling in photosynthetic tissues of higher plants. Free Radical Biol Med 122, 74-85.
DOI URL |
| [38] |
Jaskulak M, Grobelak A, Grosser A, Vandenbulcke F (2019). Gene expression, DNA damage and other stress markers in Sinapis alba L. exposed to heavy metals with special reference to sewage sludge application on contaminated sites. Ecotoxicol Environ Saf 181, 508-517.
DOI URL |
| [39] | Koppen G, Verschaeve L (1996). The alkaline comet test on plant cells: a new genotoxicity test for DNA strand breaks in Vicia faba root cells. Mutat Res 360, 193-200. |
| [40] |
Liu WJ, Xu FJ, Lv T, Zhou WW, Chen Y, Jin CW, Li LL, Lin XY (2018). Spatial responses of antioxidative system to aluminum stress in roots of wheat (Triticum aestivum L.) plants. Sci Total Environ 627, 462-469.
DOI URL |
| [41] |
Luo J, Qi SH, Gu XWS, Wang JL, Xie XM (2016). An evaluation of EDTA additions for improving the phytoremediation efficiency of different plants under various cultivation systems. Ecotoxicology 25, 646-654.
DOI PMID |
| [42] |
Park YM, Yeon KM, Park CH (2020). Silica treatment technologies in reverse osmosis for industrial desalination: a review. Environ Eng Res 25, 819-829.
DOI URL |
| [43] | Pattanayak A, Pfukrei K (2013). Aluminium toxicity tolerance in crop plants: present status of research. Afr J Biotechnol 12, 3752-3757. |
| [44] |
Pimenta LS, Mariano EDA, Gazaffi R, Carneiro MS (2020). Crescimento radicular e resposta de enzimas antioxidantes ao estresse por alumínio em cana-de-açúcar. Semina: Ciências Agrárias 41(Supl),3449-3456.
DOI URL |
| [45] |
Rahman R, Upadhyaya H (2021). Aluminium toxicity and its tolerance in plant: a review. J Plant Biol 64, 101-121.
DOI |
| [46] |
Rao LY, Li SY, Cui X (2021). Leaf morphology and chlorophyll fluorescence characteristics of mulberry seedlings under waterlogging stress. Sci Rep 11, 13379.
DOI PMID |
| [47] |
Riaz M, Yan L, Wu XW, Hussain S, Aziz O, Jiang CC (2018). Mechanisms of organic acids and boron induced tolerance of aluminum toxicity: a review. Ecotoxicol Environ Saf 165, 25-35.
DOI URL |
| [48] |
Singh S, Tripathi DK, Singh S, Sharma S, Dubey NK, Chauhan DK, Vaculík M (2017). Toxicity of aluminium on various levels of plant cells and organism: a review. Environ Exp Bot 137, 177-193.
DOI URL |
| [49] |
Tripathi DK, Singh S, Singh VP, Prasad SM, Dubey NK, Chauhan DK (2017). Silicon nanoparticles more effectively alleviated UV-B stress than silicon in wheat (Triticum aestivum) seedlings. Plant Physiol Biochem 110, 70-81.
DOI URL |
| [50] |
Wu WQ, Ueda H, Löbmann K, Rades T, Grohganz H (2018). Organic acids as co-formers for co-amorphous systems—influence of variation in molar ratio on the physicochemical properties of the co-amorphous systems. Eur J Pharm Biopharm 131, 25-32.
DOI URL |
| [51] |
Yang Y, Ma L, Zeng H, Chen LY, Zheng Y, Li CX, Yang ZP, Wu N, Mu X, Dai CY, Guan HL, Cui XM, Liu Y (2018). iTRAQ-based proteomics screen for potential regulators of wheat (Triticum aestivum L.) root cell wall component response to Al stress. Gene 675, 301-311.
DOI PMID |
| [52] |
Yun T, An F, Li WZ, Sun Y, Cao L, Xue LF (2016). A novel approach for retrieving tree leaf area from ground-based LiDAR. Remote Sens 8, 942.
DOI URL |
| [53] |
Zhou Y, Liu ZY, Yao MD, Chen J, Xiao YN, Han GY, Shen JR, Wang FJ (2022). Elucidating the molecular mechanism of dynamic photodamage of photosystem II membrane protein complex by integrated proteomics strategy. CCS Chem 4, 182-193.
DOI URL |
| [1] | LU Zhen, XIE Guang-Jie, Qaisar KHAN, QIN Ying, HUANG Yu-Yan, GUO Dao-Jun, YANG Ting-Ting, YANG Li-Tao, XING Yong-Xiu, LI Yang-Rui, WANG Zhen. Burkholderia strains enhance the tolerance of sugarcane to aluminum stress by improving the physiological adaptability and regulating the expression of aluminum responsive genes [J]. Chin J Plant Ecol, 2025, 49(3): 475-487. |
| [2] | Guo Shuya, Ai Jinxiang, Chen Hongyu, Shao Yeyao, Wang Yan, Wang Qian, Ye Yitong, Zhang Yating, Ding Zhexiao, Wu Haochen, Wu Yuhuan, Zhang Jianxin, Rao Mide, Liu Peng. Establishment of a Comprehensive Evaluation System for Aluminum Tolerance in Tomato Seedlings Based on Principal Component Analysis-Clustering Analysis-Stepwise Regression Analysis [J]. Chinese Bulletin of Botany, 2022, 57(4): 479-489. |
| [3] | Jinxiang Ai, Jiayi Song, Zhenan Yan, Zhichao Wang, Wenqian Chen, Yuhuan Wu, Yanyan Wang, Leilei Pan, Yutao Xu, Peng Liu. Effects of Exogenous Melatonin on Physiological Response and DNA Damage of Ardisia mamillata and A. crenata Under Lead Stress [J]. Chinese Bulletin of Botany, 2022, 57(2): 171-181. |
| [4] | Cheng Chen, Aiwu Dong, Wei Su. Histone Chaperone AtHIRA is Involved in Somatic Homologous Recombination and Salinity Response in Arabidopsis [J]. Chinese Bulletin of Botany, 2018, 53(1): 42-50. |
| [5] | WU Qi-Mei,ZHOU Qi-Xing. Eco-physiological responses of Polytrichum commune to soil contamination by polychlorinated biphenyls [J]. Chin J Plan Ecolo, 2015, 39(3): 275-282. |
| [6] | ZHAO Ha-Lin, QU Hao, ZHOU Rui-Lian, LI Jin, PAN Cheng-Chen, WANG Jin. Effects of sand burial on growth in two psammophyte seedlings and differences in their physiological responses [J]. Chin J Plant Ecol, 2013, 37(9): 830-838. |
| [7] | LÜ Jin-Hui,REN Lei,LI Yan-Feng,WANG Xuan,ZHAO Xia-Lu,ZHANG Chun-Lai. Responses to salt stress among different genotypes of tea Chrysanthemum [J]. Chin J Plant Ecol, 2013, 37(7): 656-664. |
| [8] | WANG Hai-Cui, HU Lin-Lin, LI Min, CHEN Wei-Feng, WANG Ying, ZHOU Jia-Jia. Growth effects and accumulations of polycyclic aromatic hydrocarbons (PAHs) in rape [J]. Chin J Plant Ecol, 2013, 37(12): 1123-1131. |
| [9] | XU Hao, LI Yan, XIE Jing-Xia, CHENG Lei, ZHAO Yan, LIU Ran. Influence of solar radiation and groundwater table on carbon balance of phreatophytic desert shrub Tamarix [J]. Chin J Plant Ecol, 2010, 34(4): 375-386. |
| [10] | Jianfeng Ning, Qingsong Zheng, Xianzhong Zou, Lili Sun, Yao Yao, Yong Chen, Jinlong Wu, Lan Wei. Physiological Responses of Apocynum venetum to Different Levels of Salt Stress [J]. Chinese Bulletin of Botany, 2010, 45(06): 689-697. |
| [11] | LI Yang, HUANG Jian-Hui. PHOTOSYNTHETIC PHYSIOLOGICAL RESPONSES OF GLYCYRRHIZA URALENSISUNDER DIFFERENT WATER AND NUTRIENT SUPPLIES IN KUBUQI DESERT, CHINA [J]. Chin J Plant Ecol, 2009, 33(6): 1112-1124. |
| [12] | LIU Bin-Yang, LIU Wei-Qiu, LEI Chun-Yi, ZHANG Yi-Shun. PHYSIOLOGICAL RESPONSES OF THREE BRYOPHYTE SPECIES OF SOUTH CHINA TO SIMULATED NITROGEN DEPOSITION [J]. Chin J Plant Ecol, 2009, 33(1): 141-149. |
| [13] | Zhigang Nie;Yan Wang;Shaoshan Li. Heavy Metal-induced DNA Damage in Arabidopsis thaliana Protoplasts Measured by Single-cell Gel Electrophoresis [J]. Chinese Bulletin of Botany, 2009, 44(01): 117-123. |
| [14] | XUE Yan-Feng, LIU Zhao-Pu. EFFECTS OF NaCl AND Na2CO3 STRESSES ON PHOTOSYNTHESIS AND PARAMETERS OF CHLOROPHYLL FLUORESCENCE IN HELIANTHUS TUBEROSUS SEEDLINGS [J]. Chin J Plant Ecol, 2008, 32(1): 161-167. |
| [15] | Jing Wang;Lei Jiang;Yan Wang;Shaoshan Li. Sensitivity of Plant Leaves at Different Developmental Stages to UV-induced DNA Damage [J]. Chinese Bulletin of Botany, 2007, 24(02): 189-193. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||