

植物学报 ›› 2021, Vol. 56 ›› Issue (3): 363-371.DOI: 10.11983/CBB20170 cstr: 32102.14.CBB20170
岳剑茹, 赫云建, 邱天麒, 郭南南, 韩雪萍, 王显玲*( )
)
收稿日期:2020-10-13
接受日期:2020-12-29
出版日期:2021-05-01
发布日期:2021-04-30
通讯作者:
王显玲
作者简介:*E-mail: wangxl100@syau.edu.cn† 共同第一作者。
基金资助:
Jianru Yue, Yunjian He, Tianqi Qiu, Nannan Guo, Xueping Han, Xianling Wang*( )
)
Received:2020-10-13
Accepted:2020-12-29
Online:2021-05-01
Published:2021-04-30
Contact:
Xianling Wang
About author:First author contact:† These authors contributed equally to this paper
摘要: 微管作为细胞骨架的重要成员, 在植物生长发育过程中起重要作用。下胚轴作为研究细胞伸长的模式系统之一, 其伸长受到多种信号的调节。该文综述了微管骨架在响应环境和生长发育信号调节下胚轴伸长过程中的作用及机制, 旨在帮助读者深入理解微管骨架响应上游信号在植物下胚轴伸长中的作用机理。
岳剑茹, 赫云建, 邱天麒, 郭南南, 韩雪萍, 王显玲. 植物微管骨架参与下胚轴伸长调节机制研究进展. 植物学报, 2021, 56(3): 363-371.
Jianru Yue, Yunjian He, Tianqi Qiu, Nannan Guo, Xueping Han, Xianling Wang. Research Advances in the Molecular Mechanisms of Plant Microtubules in Regulating Hypocotyl Elongation. Chinese Bulletin of Botany, 2021, 56(3): 363-371.
| 蛋白名称 | 对微管的调节 | 在下胚轴伸长中的作用 | 参考文献 |
|---|---|---|---|
| Katanin | 依赖ATP切割微管 | 通过蓝光刺激诱导, 在微管交叉部位切断微管, 产生新末端并迅速由横向变为纵向生长, 从而导致下胚轴细胞的生长方向改变 | |
| CLASP | 稳定微管正端 | 维持下胚轴正常生长, CLASP缺失突变体clasp-1下胚轴明显短于野生型 | |
| MDP25 | 解聚微管 | 作为下胚轴伸长的负调节因子, MDP25可直接与微管结合, 促进微管解聚, mdp25突变体的黄化下胚轴更长, 而MDP25过表达植株下胚轴较短 | |
| WDL3 | 稳定并重排微管 | 黑暗下, WDL3被26S蛋白酶体降解, 促进下胚轴伸长; 光调节微管重排过程中, WDL3通过调节下胚轴细胞中微管成束抑制下胚轴伸长 | |
| MDP60 | 去稳定并重排微管 | 通过PIF3介导光和乙烯信号, 协同调控微管去稳定蛋白, 促进下胚轴伸长 | |
| SPR1 | 稳定微管 | 下胚轴伸长的正向调节因子SPR1在快速生长的下胚轴中高表达, 通过调节微管动态促进黑暗下下胚轴伸长 | |
| WDL5 | 稳定并重排微管 | 乙烯激活EIN3, EIN3直接调控WDL5上调表达, WDL5通过维持微管纵向排列抑制黄化下胚轴伸长 | |
| MDP40 | 去稳定并重排微管 | 油菜素甾醇激活BZR1, BZR1直接结合到MDP40的启动子上并上调其表达, MDP40解聚微管使其变为横向排列, 从而促进黄化下胚轴伸长 |
表1 参与调节下胚轴伸长的微管相关蛋白
Table 1 Representative microtubule-associated proteins that are involved in the hypocotyl elongation
| 蛋白名称 | 对微管的调节 | 在下胚轴伸长中的作用 | 参考文献 |
|---|---|---|---|
| Katanin | 依赖ATP切割微管 | 通过蓝光刺激诱导, 在微管交叉部位切断微管, 产生新末端并迅速由横向变为纵向生长, 从而导致下胚轴细胞的生长方向改变 | |
| CLASP | 稳定微管正端 | 维持下胚轴正常生长, CLASP缺失突变体clasp-1下胚轴明显短于野生型 | |
| MDP25 | 解聚微管 | 作为下胚轴伸长的负调节因子, MDP25可直接与微管结合, 促进微管解聚, mdp25突变体的黄化下胚轴更长, 而MDP25过表达植株下胚轴较短 | |
| WDL3 | 稳定并重排微管 | 黑暗下, WDL3被26S蛋白酶体降解, 促进下胚轴伸长; 光调节微管重排过程中, WDL3通过调节下胚轴细胞中微管成束抑制下胚轴伸长 | |
| MDP60 | 去稳定并重排微管 | 通过PIF3介导光和乙烯信号, 协同调控微管去稳定蛋白, 促进下胚轴伸长 | |
| SPR1 | 稳定微管 | 下胚轴伸长的正向调节因子SPR1在快速生长的下胚轴中高表达, 通过调节微管动态促进黑暗下下胚轴伸长 | |
| WDL5 | 稳定并重排微管 | 乙烯激活EIN3, EIN3直接调控WDL5上调表达, WDL5通过维持微管纵向排列抑制黄化下胚轴伸长 | |
| MDP40 | 去稳定并重排微管 | 油菜素甾醇激活BZR1, BZR1直接结合到MDP40的启动子上并上调其表达, MDP40解聚微管使其变为横向排列, 从而促进黄化下胚轴伸长 |
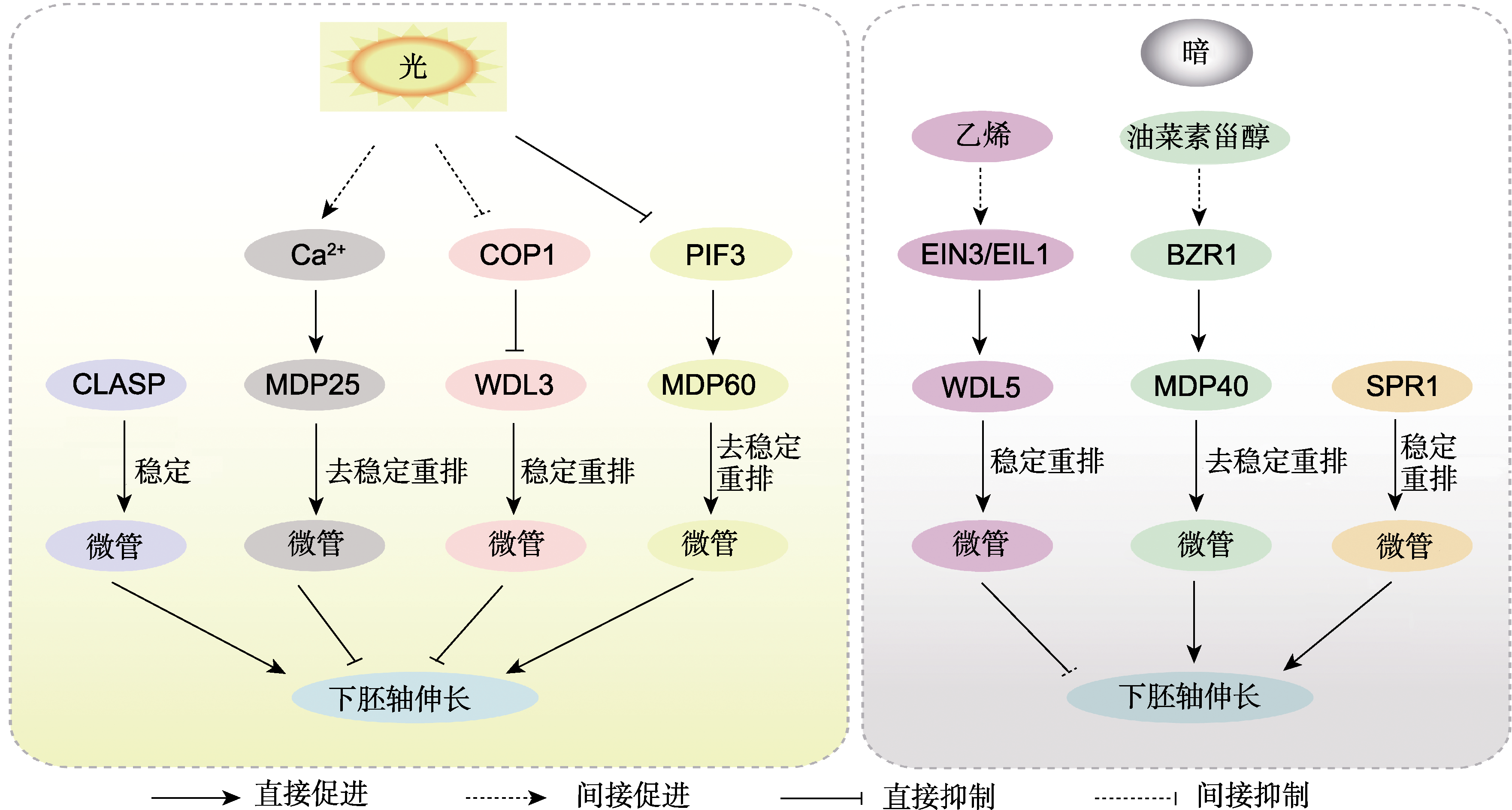
图1 响应光和激素信号调控下胚轴伸长的微管相关蛋白 COP1: 持续光形态建成1; EIN3/EIL1: 乙烯不敏感3/乙烯不敏感3类似1. CLASP、MDP25、WDL3、MDP60、SPR1、WDL5、MDP40、PIF3、EIN3和BZR1同表1。
Figure 1 Microtubule-associated proteins that are involved in hypocotyl elongation and regulated by light and phytohormones COP1: Constitutive photomorphogenic1; EIN3/EIL1: Ethylene-insensitive 3/EIN3 like 1. CLASP, MDP25, WDL3, MDP60, SPR1, WDL5, MDP40, PIF3, EIN3 and BZR1 see Table 1.
| 1 | 何群, 尤瑞麟 (2004). 应用Steedman’s wax切片法观察植物细胞微管骨架. 植物学通报 21, 547-555. |
| 2 | 李志刚, 张新成, 林丽, 李素丽, 杨丽涛, 李杨瑞 (2008). 甘蔗茎尖细胞有丝分裂过程中微管骨架的变化. 植物学通报 25, 276-283. |
| 3 |
Achard P, Liao LL, Jiang CF, Desnos T, Bartlett J, Fu XD, Harberd NP (2007). DELLAs contribute to plant photomorphogenesis. Plant Physiol 143, 1163-1172.
DOI URL |
| 4 |
Adamowski M, Li LX, Friml J (2019). Reorientation of cortical microtubule arrays in the hypocotyl of Arabidopsis thaliana is induced by the cell growth process and independent of auxin signaling. Int J Mol Sci 20, 3337.
DOI URL |
| 5 |
Alabadí D, Gallego-Bartolomé J, Orlando L, García- Cárcel L, Rubio V, Martínez C, Frigerio M, Iglesias-Pedraz JM, Espinosa A, Deng XW, Blázquez MA (2008). Gibberellins modulate light signaling pathways to prevent Arabidopsis seedling de-etiolation in darkness. Plant J 53, 324-335.
PMID |
| 6 |
Ambrose JC, Shoji T, Kotzer AM, Pighin JA, Wasteneys GO (2007). The Arabidopsis CLASP gene encodes a microtubule-associated protein involved in cell expansion and division. Plant Cell 19, 2763-2775.
DOI URL |
| 7 |
Baskin TI, Beemster GTS, Judy-March JE, Marga F (2004). Disorganization of cortical microtubules stimulates tangential expansion and reduces the uniformity of cellulose microfibril alignment among cells in the root of Ara- bidopsis. Plant Physiol 135, 2279-2290.
DOI URL |
| 8 |
Bleecker AB, Estelle MA, Somerville C, Kende H (1988). Insensitivity to ethylene conferred by a dominant mutation in Arabidopsis thaliana. Science 241, 1086-1089.
DOI URL |
| 9 | Chen X, Grandont L, Li HJ, Hauschild R, Paque S, Abuzeineh A, Rakusová H, Benkova E, Perrot-Re- chenmann C, Friml J (2014). Inhibition of cell expansion by rapid ABP1-mediated auxin effect on microtubules. Na- ture 516, 90-93. |
| 10 |
Chory J, Nagpal P, Peto CA (1991). Phenotypic and genetic analysis of det2, a new mutant that affects light- regulated seedling development in Arabidopsis. Plant Cell 3, 445-459.
DOI URL |
| 11 |
Clouse SD (2011). Brassinosteroid signal transduction: from receptor kinase activation to transcriptional networks regulating plant development. Plant Cell 23, 1219-1230.
DOI URL |
| 12 |
Cowling RJ, Harberd NP (1999). Gibberellins control Arabidopsis hypocotyl growth via regulation of cellular elongation. J Exp Bot 50, 1351-1357.
DOI URL |
| 13 |
de Grauwe L, Vandenbussche F, Tietz O, Palme K, van der Straeten D (2005). Auxin, ethylene and brassinos-teroids: tripartite control of growth in theArabidopsis hy- pocotyl. Plant Cell Physiol 46, 827-836.
DOI URL |
| 14 |
De Lucas M, Davière JM, Rodríguez-Falcón M, Pontin M, Iglesias-Pedraz JM, Lorrain S, Fankhauser C, Blázquez MA, Titarenko E, Prat S (2008). A molecular framework for light and gibberellin control of cell elongation. Nature 451, 480-484.
DOI URL |
| 15 |
Duek PD, Fankhauser C (2005). bHLH class transcription factors take centre stage in phytochrome signaling. Trends Plant Sci 10, 51-54.
DOI URL |
| 16 |
Ehrhardt DW, Shaw SL (2006). Microtubule dynamics and organization in the plant cortical array. Annu Rev Plant Biol 57, 859-875.
PMID |
| 17 |
Feng SH, Martinez C, Gusmaroli G, Wang Y, Zhou JL, Wang F, Chen LY, Yu L, Iglesias-Pedraz JM, Kircher S, Schäfer E, Fu XD, Fan LM, Deng XW (2008). Coordi-nated regulation ofArabidopsis thaliana development by light and gibberellins. Nature 451, 475-479.
DOI URL |
| 18 |
Fischer K, Schopfer P (1997). Interaction of auxin, light, and mechanical stress in orienting microtubules in relation to tropic curvature in the epidermis of maize coleoptiles. Protoplasma 196, 108-116.
DOI URL |
| 19 |
Friml J, Wiśniewska J, Benková E, Mendgen K, Palme K (2002). Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature 415, 806-809.
PMID |
| 20 | Furutani I, Watanabe Y, Prieto R, Masukawa M, Suzuki K, Naoi K, Thitamadee S, Shikanai T, Hashimoto T (2000). The SPIRAL genes are required for directional control of cell elongation in Arabidopsis thaliana. Deve- lopment 127, 4443-4453. |
| 21 |
Gendreau E, Traas J, Desnos T, Grandjean O, Caboche M, Höfte H (1997). Cellular basis of hypocotyl growth in Arabidopsis thaliana. Plant Physiol 114, 295-305.
PMID |
| 22 |
Gray WM, Ostin A, Sandberg G, Romano CP, Estelle M (1998). High temperature promotes auxin-mediated hy-pocotyl elongation in Arabidopsis. Proc Natl Acad Sci USA 95, 7197-7202.
DOI URL |
| 23 |
Gudesblat GE, Russinova E (2011). Plants grow on bras-sinosteroids. Curr Opin Plant Biol 14, 530-537.
DOI URL |
| 24 |
Harberd NP, Belfield E, Yasumura Y (2009). The angio- sperm gibberellin-GID1-DELLA growth regulatory mecha-nism: how an “inhibitor of an inhibitor” enables flexible response to fluctuating environments. Plant Cell 21, 1328-1339.
DOI URL |
| 26 |
Hashimoto T (2003). Dynamics and regulation of plant interphase microtubules: a comparative view. Curr Opin Plant Biol 6, 568-576.
PMID |
| 27 |
Hashimoto T, Kato T (2006). Cortical control of plant microtubules. Curr Opin Plant Biol 9, 5-11.
PMID |
| 28 |
Jensen PJ, Hangarter RP, Estelle M (1998). Auxin transport is required for hypocotyl elongation in light-grown but not dark-grownArabidopsis. Plant Physiol 116, 455-462.
PMID |
| 29 | Kim SY, Kim BH, Lim CJ, Lim CO, Nam KH (2010). Constitutive activation of stress-inducible genes in a brassinosteroid-insensitive 1(bri1) mutant results in higher tolerance to cold. Physiol Plant 138, 191-204. |
| 30 |
Kim TW, Wang ZY (2010). Brassinosteroid signal transduc-tion from receptor kinases to transcription factors. Annu Rev Plant Biol 61, 681-704.
DOI URL |
| 31 |
Kost B, Chua NH (2002). The plant cytoskeleton: vacuoles and cell walls make the difference. Cell 108, 9-12.
DOI URL |
| 32 |
Le J, Vandenbussche F, de Cnodder T, van der Straeten D, Verbelen JP (2005). Cell elongation and microtubule behavior in the Arabidopsis hypocotyl: responses to ethy- lene and auxin. J Plant Growth Regul 24, 166-178.
DOI URL |
| 33 |
Li JJ, Wang XL, Qin T, Zhang Y, Liu XM, Sun JB, Zhou Y, Zhu L, Zhang ZD, Yuan M, Mao TL (2011). MDP25, a novel calcium regulatory protein, mediates hypocotyl cell elongation by destabilizing cortical microtubules in Ara- bidopsis. Plant Cell 23, 4411-4427.
DOI URL |
| 34 |
Li L, Ye HX, Guo HQ, Yin YH (2010). Arabidopsis IWS1 interacts with transcription factor BES1 and is involved in plant steroid hormone brassinosteroid regulated gene expression. Proc Natl Acad Sci USA 107, 3918-3923.
DOI URL |
| 35 |
Lian N, Liu XM, Wang XH, Zhou YY, Li H, Li JG, Mao TL (2017). COP1 mediates dark-specific degradation of micro- tubule-associated protein WDL3 in regulating Arabidopsis hypocotyl elongation. Proc Natl Acad Sci USA 114, 12321-12326.
DOI URL |
| 36 |
Lindeboom JJ, Nakamura M, Hibbel A, Shundyak K, Gutierrez R, Ketelaar T, Emons AMC, Mulder BM, Kirik V, Ehrhardt DW (2013). A mechanism for reorientation of cortical microtubule arrays driven by microtubule seve- ring. Science 342, 1245533.
DOI URL |
| 37 |
Lindeboom JJ, Nakamura M, Saltini M, Hibbel A, Walia A, Ketelaar T, Emons AMC, Sedbrook JC, Kirik V, Mulder BM, Ehrhardt DW (2019). CLASP stabilization of plus ends created by severing promotes microtubule creation and reorientation. J Cell Biol 218, 190-205.
DOI |
| 38 |
Liu XM, Qin T, Ma QQ, Sun JB, Liu ZQ, Yuan M, Mao TL (2013). Light-regulated hypocotyl elongation involves proteasome-dependent degradation of the microtubule regulatory protein WDL3 in Arabidopsis. Plant Cell 25, 1740-1755.
DOI URL |
| 39 | Lloyd C, Chan J (2004). Microtubules and the shape of plants to come. Nat Rev Mol Cell Biol 5, 13-23. |
| 40 | Ma QQ, Wang XH, Sun JB, Mao TL (2018). Coordinated regulation of hypocotyl cell elongation by light and ethy- lene through a microtubule destabilizing protein. Plant Phy- siol 176, 678-690. |
| 41 |
Margolis RL, Wilson L (1981). Microtubule treadmills— possible molecular machinery. Nature 293, 705-711.
PMID |
| 42 |
Mitchison T, Kirschner M (1984). Dynamic instability of microtubule growth. Nature 312, 237-242.
PMID |
| 43 |
Nakajima K, Furutani I, Tachimoto H, Matsubara H, Hashimoto T (2004). SPIRAL1 encodes a plant-specific microtubule-localized protein required for directional control of rapidly expanding Arabidopsis cells. Plant Cell 16, 1178-1190.
PMID |
| 44 |
Nakajima K, Kawamura T, Hashimoto T (2006). Role of the SPIRAL1 gene family in anisotropic growth of Arabi-dopsis thaliana. Plant Cell Physiol 47, 513-522.
PMID |
| 45 |
Peng JR, Carol P, Richards DE, King KE, Cowling RJ, Murphy GP, Harberd NP (1997). The Arabidopsis GAI gene defines a signaling pathway that negatively regulates gibberellin responses. Genes Dev 11, 3194-3205.
DOI URL |
| 46 |
Reid JB, Botwright NA, Smith JJ, O’Neill DP, Kerckhoffs LHJ (2002). Control of gibberellin levels and gene expres- sion during de-etiolation in pea. Plant Physiol 128, 734-741.
DOI URL |
| 47 |
Romano CP, Robson PRH, Smith H, Estelle M, Klee H (1995). Transgene-mediated auxin overproduction in Ara- bidopsis: hypocotyl elongation phenotype and interactions with the hy6-1 hypocotyl elongation and axr1 auxin-resis tant mutants. Plant Mol Biol 27, 1071-1083.
PMID |
| 48 |
Sambade A, Pratap A, Buschmann H, Morris RJ, Lloyd C (2012). The influence of light on microtubule dynamics and alignment in the Arabidopsis hypocotyl. Plant Cell 24, 192-201.
DOI URL |
| 49 |
Sauret-Güeto S, Calder G, Harberd NP (2012). Transient gibberellin application promotes Arabidopsis thaliana hypocotyl cell elongation without maintaining transverse o- rientation of microtubules on the outer tangential wall of epidermal cells. Plant J 69, 628-639.
DOI URL |
| 50 | Shibaoka H (1974). Involvement of wall microtubules in gibberellin promotion and kinetin inhibition of stem elongation. Plant Cell Physiol 15, 255-263. |
| 51 | Shibaoka H (1993). Regulation by gibberellins of the orientation of cortical microtubules in plant cells. Aust J Plant Physiol 20, 461-470. |
| 52 |
Silverstone AL, Ciampaglio CN, Sun TP (1998). The Arabidopsis RGA gene encodes a transcriptional regulator repressing the gibberellin signal transduction pathway. Plant Cell 10, 155-169.
PMID |
| 53 |
Smalle J, Haegman M, Kurepa J, van Montagu M, Straeten DVD (1997). Ethylene can stimulate Arabidopsis hypocotyl elongation in the light. Proc Natl Acad Sci USA 94, 2756-2761.
DOI URL |
| 54 |
Soga K, Yamaguchi A, Kotake T, Wakabayashi K, Hoson T (2010). Transient increase in the levels of γ-tubulin complex and katanin are responsible for reorientation by ethylene and hypergravity of cortical microtubules. Plant Signal Behav 5, 1480-1482.
DOI URL |
| 55 | Sun JB, Ma QQ, Mao TL (2015). Ethylene regulates the Arabidopsis microtubule-associated protein WAVE-DAM- PENED2-LIKE5 in etiolated hypocotyl elongation. Plant Physiol 169, 325-337. |
| 56 |
Tang WQ, Kim TW, Oses-Prieto JA, Sun Y, Deng ZP, Zhu SW, Wang RJ, Burlingame AL, Wang ZY (2008). BSKs mediate signal transduction from the receptor kinase BRI1 in Arabidopsis. Science 321, 557-560.
DOI URL |
| 57 |
Thitamadee S, Tuchihara K, Hashimoto T (2002). Microtubule basis for left-handed helical growth inArabidopsis. Nature 417, 193-196.
PMID |
| 58 |
True JH, Shaw SL (2020). Exogenous auxin induces trans- verse microtubule arrays through TRANSPORT INHIBI-TOR RESPONSE1/AUXIN SIGNALING F-BOX receptors. Plant Physiol 182, 892-907.
DOI URL |
| 59 |
van der Graaff E, Nussbaumer C, Keller B (2003). The Arabidopsis thaliana rlp mutations revert the ectopic leaf blade formation conferred by activation tagging of the LEP gene. Mol Genet Genomics 270, 243-252.
PMID |
| 60 | Vineyard L, Elliott A, Dhingra S, Lucas JR, Shaw SL (2013). Progressive transverse microtubule array organization in hormone-induced Arabidopsis hypocotyl cells. Plant Cell 25, 662-676. |
| 61 |
Wang CF, Liu WW, Wang GD, Li J, Dong L, Han LB, Wang Q, Tian J, Yu YJ, Gao CX, Kong ZS (2017). KTN80 confers precision to microtubule severing by specific targeting of Katanin complexes in plant cells. EMBO J 36, 3435-3447.
DOI URL |
| 62 |
Wang XF, Mao TL (2019). Understanding the functions and mechanisms of plant cytoskeleton in response to environmental signals. Curr Opin Plant Biol 52, 86-96.
DOI URL |
| 63 |
Wang XL, Zhang J, Yuan M, Ehrhardt DW, Wang ZY, Mao TL (2012). Arabidopsis microtubule destabilizing protein40 is involved in brassinosteroid regulation of hypocotyl elongation. Plant Cell 24, 4012-4025.
DOI URL |
| 64 |
Wang ZY, Seto H, Fujioka S, Yoshida S, Chory J (2001). BRI1 is a critical component of a plasma-membrane receptor for plant steroids. Nature 410, 380-383.
PMID |
| 65 |
Ye HX, Li L, Yin YH (2011). Recent advances in the regulation of brassinosteroid signaling and biosynthesis pathways. J Integr Plant Biol 53, 455-468.
DOI URL |
| 66 | Yu YW, Wang J, Zhang ZJ, Quan RD, Zhang HW, Deng XW, Ma LG, Huang RF (2013). Ethylene promotes hypocotyl growth and HY5 degradation by enhancing the movement of COP1 to the nucleus in the light. PLoS Ge- net 9, e1004025. |
| 67 |
Zhao YD, Christensen SK, Fankhauser C, Cashman JR, Cohen JD, Weigel D, Chory J (2001). A role for flavin monooxygenase-like enzymes in auxin biosynthesis. Science 291, 306-309.
DOI URL |
| 68 |
Zhong SW, Shi H, Xue C, Wang L, Xi YP, Li JG, Quail PH, Deng XW, Guo HW (2012). A molecular framework of light-controlled phytohormone action in Arabidopsis. Curr Biol 22, 1530-1535.
DOI URL |
| [1] | 景艳军, 林荣呈. 蓝光受体CRY2化身“暗黑舞者”[J]. 植物学报, 2024, 59(6): 878-882. |
| [2] | 王韫慧, 王一帆, 蔺佳雨, 李金红, 姚士恩, 冯湘池, 曹振林, 王俊, 李美娜. 植物驱动蛋白: 从微管阵列到生理活动调控[J]. 植物学报, 2022, 57(3): 358-374. |
| [3] | 蔡芳芳, 邵长生, 孙玉强. 可变剪切在植物成花转换中的作用[J]. 植物学报, 2022, 57(1): 69-79. |
| [4] | 范业赓,丘立杭,黄杏,周慧文,甘崇琨,李杨瑞,杨荣仲,吴建明,陈荣发. 甘蔗节间伸长过程赤霉素生物合成关键基因的表达及相关植物激素动态变化[J]. 植物学报, 2019, 54(4): 486-496. |
| [5] | 沈锦波, 姜里文. 中国科学家在植物细胞骨架研究领域取得突破性进展[J]. 植物学报, 2018, 53(6): 741-744. |
| [6] | 王红飞, 尚庆茂. 被子植物下胚轴细胞伸长的分子机理[J]. 植物学报, 2018, 53(2): 276-287. |
| [7] | 刘广超, 丁兆军. 生长素介导环境信号调控植物的生长发育[J]. 植物学报, 2018, 53(1): 17-26. |
| [8] | 帅海威, 孟永杰, 陈锋, 周文冠, 罗晓峰, 杨文钰, 舒凯. 植物荫蔽胁迫的激素信号响应[J]. 植物学报, 2018, 53(1): 139-148. |
| [9] | 李凌飞, 彭建宗, 王小菁. 非洲菊微管相关蛋白基因GMAP65-1功能分析[J]. 植物学报, 2015, 50(1): 12-21. |
| [10] | 赵翔, 王琳丹, 李园园, 赵青平, 张骁. PHOT2介导拟南芥下胚轴向光弯曲调节子的筛选与鉴定[J]. 植物学报, 2014, 49(3): 254-261. |
| [11] | 许德成, 王小菁. 红厚壳茎段丛生芽诱导与植株再生[J]. 植物学报, 2014, 49(2): 167-172. |
| [12] | 王文婧, 刘婷, 郭磊, 刘春明. SLC/AGO1基因控制拟南芥细胞分裂与定向伸长[J]. 植物学报, 2011, 46(4): 370-378. |
| [13] | 黄刚, 赵学勇, 黄迎新, 李玉霖, 苏延桂. 两种生境条件下差不嘎蒿细根寿命[J]. 植物生态学报, 2009, 33(4): 755-763. |
| [14] | 李志刚;张新成;林丽;李素丽;杨丽涛;李杨瑞. 甘蔗茎尖细胞有丝分裂过程中微管骨架的变化[J]. 植物学报, 2008, 25(03): 276-283. |
| [15] | 黄刚, 赵学勇, 苏延桂. 科尔沁沙地3种草本植物根系生长动态[J]. 植物生态学报, 2007, 31(6): 1161-1167. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||