

植物学报 ›› 2016, Vol. 51 ›› Issue (1): 89-97.DOI: 10.11983/CBB14207 cstr: 32102.14.CBB14207
收稿日期:2014-12-08
接受日期:2015-05-30
出版日期:2016-01-01
发布日期:2016-02-01
通讯作者:
黄衡宇
作者简介:? 共同第一作者
基金资助:Meiping Lü1, Yuanzhong Wang2, Hengyu Huang1*
Received:2014-12-08
Accepted:2015-05-30
Online:2016-01-01
Published:2016-02-01
Contact:
Huang Hengyu
About author:? These authors contributed equally to this paper
摘要: 以地皮消(Pararuellia delavayana)无菌实生苗带叶茎尖和带节茎段为外植体, 探讨不同植物激素种类及组合对愈伤组织诱导、芽丛发生及植株再生的影响。结果表明, 地皮消组织培养和植株再生的适宜外植体为带节茎段, 其在MS+1.0 mg·L-1 6-BA+0.5 mg·L-1 NAA+0.1 mg·L-1 KT培养基中培养17天后, 约85.38%的外植体产生出分化能力较强的愈伤组织; 25天后约97.55%的愈伤组织开始分化出绿色芽丛; 30天后不定芽分化系数可达15.38。不定芽增殖继代6次后出现玻璃化现象, 且随着继代次数的增加, 玻璃化现象加重, 增殖率明显下降; 采用MS和B5培养基交替使用可改善试管苗玻璃化现象并保持较高的增殖率。不定芽生根的适宜培养基为MS+0.5 mg·L-1 NAA, 生根率可达100%。再生苗移栽成活率达95%以上。该研究建立了地皮消无性快速繁殖体系, 为保护地皮消野生资源及种苗繁育提供了有效途径, 也为其遗传转化研究奠定了基础。
吕美萍, 王元忠, 黄衡宇. 地皮消愈伤组织诱导及植株高效再生体系的建立. 植物学报, 2016, 51(1): 89-97.
Meiping Lü, Yuanzhong Wang, Hengyu Huang. Callus Induction and High Efficiency Plant Regeneration System Establishment of Pararuellia delavayana. Chinese Bulletin of Botany, 2016, 51(1): 89-97.
| Level | Factor | ||
|---|---|---|---|
| A (6-BA, mg·L -1) | B (NAA, mg·L -1) | C (KT, mg·L -1) | |
| 1 | 0.5 | 0.1 | 0.01 |
| 2 | 1.0 | 0.5 | 0.05 |
| 3 | 1.5 | 1.0 | 0.10 |
表1 地皮消愈伤组织诱导与芽丛发生L9 (34)正交设计
Table 1 L9 (34) orthogonal design for callus induction and bud induction of Pararuellia delavayana
| Level | Factor | ||
|---|---|---|---|
| A (6-BA, mg·L -1) | B (NAA, mg·L -1) | C (KT, mg·L -1) | |
| 1 | 0.5 | 0.1 | 0.01 |
| 2 | 1.0 | 0.5 | 0.05 |
| 3 | 1.5 | 1.0 | 0.10 |
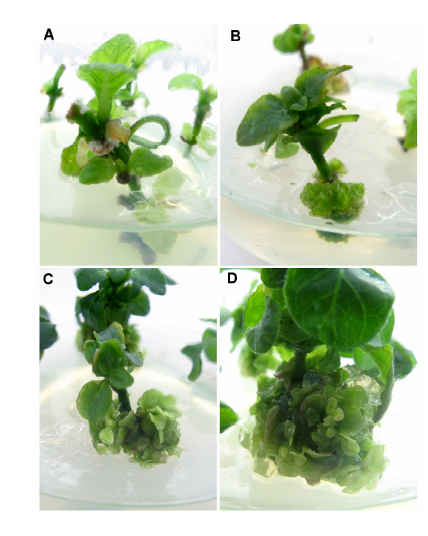
图1 地皮消愈伤组织诱导与芽丛发生 (A) 培养7天后腋芽迅速生长; (B) 培养17天, 接触培养基的外植体基部出现绿色愈伤组织; (C) 培养25天, 愈伤组织上出现绿色芽点; (D) 培养35天, 产生致密的芽丛
Figure 1 Callus induction and bud induction in Pararuellia delavayana(A) Axillary buds spring up quickly after 7 days; (B) Green callus deriving from the base of explants after 17 days; (C) Green buds from callus after 25 days; (D) Cluster buds from callus after 35 days
| No. | Hormone (mg·L-1) | Rate of callus induced (%) | Rate of buds induced (%) | ||||
|---|---|---|---|---|---|---|---|
| A (6-BA) | B (NAA) | C (KT) | D (Error) | ||||
| C1 | 0.5 | 0.1 | 0.01 | (1) | 25.12 | 53.03 | |
| C2 | 0.5 | 0.5 | 0.05 | (2) | 40.67 | 60.31 | |
| C3 | 0.5 | 1.0 | 0.10 | (3) | 49.23 | 64.80 | |
| C4 | 1.0 | 0.1 | 0.05 | (3) | 55.35 | 66.31 | |
| C5 | 1.0 | 0.5 | 0.10 | (1) | 85.38 | 97.55 | |
| C6 | 1.0 | 1.0 | 0.01 | (2) | 82.89 | 93.70 | |
| C7 | 1.5 | 0.1 | 0.10 | (2) | 58.51 | 89.51 | |
| C8 | 1.5 | 0.5 | 0.01 | (3) | 57.95 | 89.78 | |
| C9 | 1.5 | 1.0 | 0.05 | (1) | 50.97 | 63.62 | |
| Callus induced | K1 | 38.34 | 46.33 | 55.32 | 53.82 | ||
| K2 | 75.54 | 61.33 | 49.00 | 60.69 | |||
| K3 | 55.81 | 61.03 | 64.37 | 54.18 | |||
| R1 | 37.20 | 15.00 | 15.38 | 6.87 | |||
| Buds induced | K1 | 59.38 | 69.62 | 78.84 | 71.40 | ||
| K2 | 85.85 | 82.55 | 63.41 | 81.17 | |||
| K3 | 80.97 | 74.04 | 83.95 | 73.63 | |||
| R2 | 26.47 | 12.93 | 20.54 | 9.77 | |||
表2 地皮消愈伤组织诱导和芽丛发生L9 (34)正交实验设计与结果
Table 2 Results of callus induction and bud induction by L9 (34) orthogonal test in Pararuellia delavayana
| No. | Hormone (mg·L-1) | Rate of callus induced (%) | Rate of buds induced (%) | ||||
|---|---|---|---|---|---|---|---|
| A (6-BA) | B (NAA) | C (KT) | D (Error) | ||||
| C1 | 0.5 | 0.1 | 0.01 | (1) | 25.12 | 53.03 | |
| C2 | 0.5 | 0.5 | 0.05 | (2) | 40.67 | 60.31 | |
| C3 | 0.5 | 1.0 | 0.10 | (3) | 49.23 | 64.80 | |
| C4 | 1.0 | 0.1 | 0.05 | (3) | 55.35 | 66.31 | |
| C5 | 1.0 | 0.5 | 0.10 | (1) | 85.38 | 97.55 | |
| C6 | 1.0 | 1.0 | 0.01 | (2) | 82.89 | 93.70 | |
| C7 | 1.5 | 0.1 | 0.10 | (2) | 58.51 | 89.51 | |
| C8 | 1.5 | 0.5 | 0.01 | (3) | 57.95 | 89.78 | |
| C9 | 1.5 | 1.0 | 0.05 | (1) | 50.97 | 63.62 | |
| Callus induced | K1 | 38.34 | 46.33 | 55.32 | 53.82 | ||
| K2 | 75.54 | 61.33 | 49.00 | 60.69 | |||
| K3 | 55.81 | 61.03 | 64.37 | 54.18 | |||
| R1 | 37.20 | 15.00 | 15.38 | 6.87 | |||
| Buds induced | K1 | 59.38 | 69.62 | 78.84 | 71.40 | ||
| K2 | 85.85 | 82.55 | 63.41 | 81.17 | |||
| K3 | 80.97 | 74.04 | 83.95 | 73.63 | |||
| R2 | 26.47 | 12.93 | 20.54 | 9.77 | |||
| Source | Type III sum of square | df | Mean square | F | Significance |
|---|---|---|---|---|---|
| A (6-BA) | 1966.454 | 2 | 983.227 | 21.923 | P<0.05 |
| B (NAA) | 441.480 | 2 | 220.740 | 4.922 | P>0.05 |
| C (KT) | 358.389 | 2 | 179.195 | 3.995 | P>0.05 |
| Error | 89.699 | 2 | 44.850 |
表3 地皮消愈伤组织诱导方差分析结果
Table 3 Variance analysis of callus induction in Pararuellia delavayana
| Source | Type III sum of square | df | Mean square | F | Significance |
|---|---|---|---|---|---|
| A (6-BA) | 1966.454 | 2 | 983.227 | 21.923 | P<0.05 |
| B (NAA) | 441.480 | 2 | 220.740 | 4.922 | P>0.05 |
| C (KT) | 358.389 | 2 | 179.195 | 3.995 | P>0.05 |
| Error | 89.699 | 2 | 44.850 |
| Levels | Mean | 0.05 level |
|---|---|---|
| 2 | 75.54 | a |
| 3 | 55.81 | ab |
| 1 | 38.34 | b |
表4 6-BA 3水平Duncan检验
Table 4 Duncan’s test in three levels of 6-BA
| Levels | Mean | 0.05 level |
|---|---|---|
| 2 | 75.54 | a |
| 3 | 55.81 | ab |
| 1 | 38.34 | b |
| Source | Type III sum of square | df | Mean square | F | Significance |
|---|---|---|---|---|---|
| A (6-BA) | 1190.812 | 2 | 595.406 | 7.566 | P>0.05 |
| B (NAA) | 259.114 | 2 | 129.557 | 1.646 | P>0.05 |
| C (KT) | 685.951 | 2 | 342.976 | 4.358 | P>0.05 |
| Error | 157.393 | 2 | 78.696 |
表5 地皮消芽丛发生方差分析结果
Table 5 Variance analysis of bud induction in Pararuellia delavayana
| Source | Type III sum of square | df | Mean square | F | Significance |
|---|---|---|---|---|---|
| A (6-BA) | 1190.812 | 2 | 595.406 | 7.566 | P>0.05 |
| B (NAA) | 259.114 | 2 | 129.557 | 1.646 | P>0.05 |
| C (KT) | 685.951 | 2 | 342.976 | 4.358 | P>0.05 |
| Error | 157.393 | 2 | 78.696 |

图2 地皮消芽的增殖与生长 (A) 转接10天后分化出小芽点; (B) 20天后芽丛大量繁殖; (C) 培养30天的芽丛情况; (D) 培养40天丛生苗的生长情况
Figure 2 Proliferation and growth of adventitious shoots in Pararuellia delavayana(A) Small buds differentiation after 10 days; (B) Calluses blooming after 20 days; (C) Adventitious shoots after 30 days; (D) Adventitious seedlings after 40 days
| Media | Regeneration coefficient (%) | Growth condition |
|---|---|---|
| B5 | 11.05 | Adventitious shoots with lower induction rate were stronger, the vitrification was reduced obviously |
| N6 | 1.65 | Plantlets with no callus induction turned reddish, the vitrification was reduced obviously but the growth slowed |
| MS (1/2NH4NO3) | 7.93 | Weak adventitious shoots, the vitrification was reduced obviously |
表6 地皮消在不同基本培养基中的增殖及生长情况
Table 6 Results of proliferation and growth in Pararuellia delavayana in different basic medium
| Media | Regeneration coefficient (%) | Growth condition |
|---|---|---|
| B5 | 11.05 | Adventitious shoots with lower induction rate were stronger, the vitrification was reduced obviously |
| N6 | 1.65 | Plantlets with no callus induction turned reddish, the vitrification was reduced obviously but the growth slowed |
| MS (1/2NH4NO3) | 7.93 | Weak adventitious shoots, the vitrification was reduced obviously |
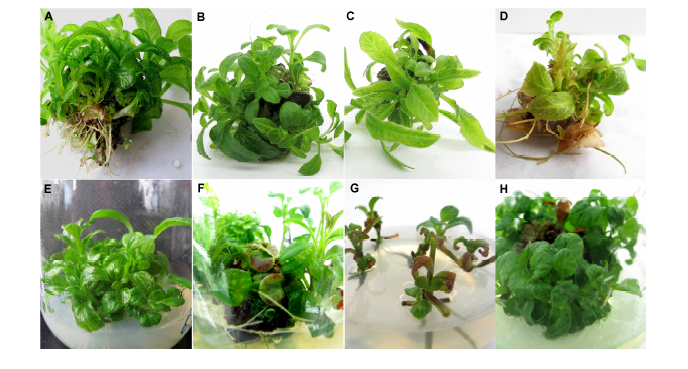
图3 地皮消玻璃化现象及改善情况 (A) 增殖第4代培养40天的试管苗; (B) 第4代试管苗; (C) 第5代试管苗; (D) 第6代玻璃化苗; (E) B5培养基中生长40天的试管苗; (F) MS (NH4NO3减半)培养基中生长40天后的试管苗; (G) N6培养基中培养30天的生长情况; (H) B5和MS交替使用2次后培养40天的试管苗
Figure 3 Phenomenon and improving results of vitrification in Pararuellia delavayana(A) Plantlets of the fourth generation after 40 days; (B) Plantlets of the fourth generation; (C) Plantlets of the fifth generation; (D) Vitrification plantlets of the sixth generation; (E) Proliferous plantlets in B5 medium after 40 days; (F) Proliferous plantlets in MS (NH4NO3 halved) medium after 40 days; (G) Proliferous plantlets in N6 medium after 30 days; (H) Proliferous plantlets of B5 and MS medium used interchangeably twice after 40 days
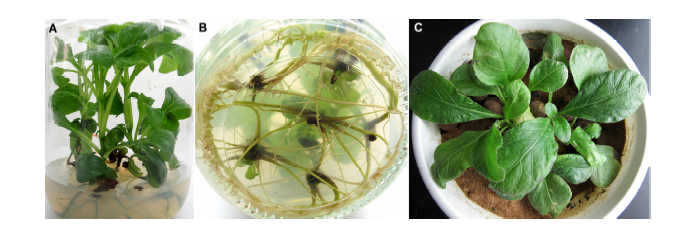
图4 地皮消生根培养及移栽 (A) MS培养基中培养45天时的生根苗; (B) MS培养基中培养45天时的根系; (C) 移栽苗
Figure 4 Rooting culture and transplanting of Pararuellia delavayana(A) Rooting plantlets in MS medium after 45 days; (B) Root system in MS medium after 45 days; (C) Transplanting plantlets
| 1 | 蔡能, 易自力, 李祥 (2003). 改善植物大规模组织培养条件的研究进展. 植物学通报 20, 745-751. |
| 2 | 蔡祖国, 徐小彪, 周会萍 (2005). 植物组织培养中的玻璃化现象及其预防. 生物技术通讯 16, 353-355. |
| 3 | 陈兵先, 黄宝灵, 吕成群, 杨来安, 陈文军, 任玲, 王劲松 (2011). 植物组织培养试管苗玻璃化现象研究进展. 林业科技开发 25, 1-5. |
| 4 | 陈雪, 张金柱, 潘兵兵, 桑成瑾, 马雪, 杨涛, 车代弟 (2011). 月季愈伤组织的诱导及植株再生. 植物学报 46, 569-574. |
| 5 | 冯欢, 易姝利, 谢佳恒, 雷梦琦, 黄萱 (2014). 微型月季愈伤组织诱导及植株再生研究. 植物学报 49, 595-602. |
| 6 | 国家中药管理局《中华本草》编委会 (1998). 中华本草(第7卷). 上海: 上海科学技术出版社. pp. 6478. |
| 7 | 胡峰, 施琼, 黄烈健 (2014). 黑木相思愈伤组织诱导及植株再生. 植物学报 49, 603-610. |
| 8 | 黄格 (2010). 药用植物穿心莲组培快繁及其影响因子的研究. 硕士论文. 南宁: 广西大学. pp. 6-9. |
| 9 | 姜北 (2001). 五种药用植物化学成分与生物活性研究. 硕士论文. 昆明: 中国科学院昆明植物研究所. pp. 9-16. |
| 10 | 江苏新医学院 (1977). 中药大词典(上册). 上海: 上海科学技术出版社. pp. 813. |
| 11 | 李红, 李永文 (2007). 金脉单药花栽培管理技术. 河北农业科技 6, 37. |
| 12 | 李林轩, 凌征柱, 李翠, 彭凌, 韦坤华 (2013). 珐菲亚组织培养条件的优化研究. 中草药 44, 1334-1337. |
| 13 | 李胜, 李唯, 杨德龙, 曹孜义 (2003). 植物试管苗玻璃化现象研究进展. 甘肃农业大学学报 38, 1-16. |
| 14 | 吕春 (2009). 红斑枪刀药组织培养体系研究. 硕士论文. 成都: 四川农业大学. pp. 8-12. |
| 15 | 苏钛, 黄宁珍, 付传明 (2009). 匙羹藤组织培养条件优化研究. 广西植物 29, 87-91. |
| 16 | 孙清荣, 孙洪雁, 张力思, 周广方, 刘庆忠 (2010). 酸枣叶片不定梢形成及玻璃化的影响因素. 西北植物学报 30, 1039-1044. |
| 17 | 吴征镒, 李锡文 (2010). 中国植物志(第70卷). 北京: 科学出版社. pp. 55. |
| 18 | 郗浩江, 葛素因, 王芳 (2013). 新疆紫草组织培养中试管苗玻璃化的控制与修复. 新疆师范大学学报 32, 21-25. |
| 19 | 张爱莲 (2005). 爵床,锥头麻和木姜冬青的化学成分研究. 硕士论文. 成都: 中国科学院成都有机化学研究所. pp. 6-18. |
| 20 | 张翠玉, 廖晴 (1991). 月季试管苗玻璃化原因及控制方法研究. 新疆农业科学 2, 76-78. |
| 21 | 张庆红, 汤丽云, 司徒少金, 王莎, 何国振 (2014). 药用植物栀子的组织培养. 植物学报 49, 331-336. |
| 22 | 周菊花, 林证明, 梁海曼 (1990). 控制瑞香试管苗玻璃化的研究. 园艺学报 17, 229-232. |
| 23 | Chakrabarty D, Park SY, Ali MB, Shin KS, Paek KY (2006). Hyperhydricity in apple: ultrastructure and physico- logical aspects.Tree Physiol 26, 377-388. |
| 24 | Ivanova M, Van SJ (2008). Effect of ammonium ions and cytokinins on hyperhydricity and multiplication rate of in vitro regenerated shoots of Aloe polyphylla.Plant Cell Tiss Org Cult 92, 227-231. |
| 25 | Kevers C, Fanck T, Strasser RJ, Dommes J, Gaspar T (2004). Hyperhydricity of micropropagated shoots: a typically stress-induced change of physiological state.Plant Cell Tiss Org Cult 77, 181-191. |
| 26 | Mayor ML, Nestares G, Zorzoli R, Picardi LA (2003). Reduction of hyperhydricity in sunfllower tissue culture.Plant Cell Tiss Org Cult 72, 99-103. |
| 27 | Piqueras A, Cortina M, Serna MD, Casas JL (2002). Polyamines and hyperhydricity in micropropagated carnation plants.Plant Sci 162, 671-678. |
| 28 | Saher S, Piqueras A, Hellin E, Olmos E (2004). Hyperhydricity in micro-propagated carnation shoot: the role of oxidative stress.Physiol Plant 120, 152-161. |
| 29 | Sreedhar RV, Venkatachalam L, Neelwarne B (2009). Hyperhydricity related morphologic and biochemical ch- anges in vanilla (Vanilla planifolia).J Plant Growth Regul 28, 46-57. |
| 30 | Wang YL, Wang XD, Zhao B, Wang YC (2007). Reduction of hyperhydricity in the culture of Lepidium meyenii shoots by the addition of rare earth elements.J Plant Growth Regul 52, 151-159. |
| 31 | Wu Z, Chen LJ, Long YJ (2009). Analysis of ultrastructure and reactive oxygen species of hyperhydric garlic (Allium sativum L.) shoot.In Vitro Cell Dev Biol-Plant 45, 483-490. |
| [1] | 郭政, 邵香君, 鲁海雯, 侯丹, 孔思梦, 李翔宇, 刘华倩, 林新春. 马来甜龙竹多倍体高效诱导及鉴定[J]. 植物学报, 2025, 60(2): 246-255. |
| [2] | 李宇琛, 赵海霞, 姜希萍, 黄馨田, 刘亚玲, 吴振映, 赵彦, 付春祥. 根癌农杆菌介导的蒙古冰草稳定遗传转化体系建立[J]. 植物学报, 2024, 59(4): 600-612. |
| [3] | 田旭平, 岳康杰, 王佳丽, 刘慧欣, 史子尹, 亢红伟. 毛建草愈伤组织诱导及植株再生[J]. 植物学报, 2024, 59(4): 613-625. |
| [4] | 曾浩, 李佩芳, 郭至辉, 刘春林, 阮颖. 银扇草再生体系的建立[J]. 植物学报, 2024, 59(3): 433-440. |
| [5] | 张尚文, 黄诗宇, 杨天为, 李婷, 张向军, 高曼熔. 基于正交实验的赤苍藤组培快繁体系建立[J]. 植物学报, 2024, 59(1): 99-109. |
| [6] | 刘小飞, 孙映波, 黄丽丽, 杨钰钗, 朱根发, 于波. 黑鹅绒海芋体细胞胚发生和植株再生[J]. 植物学报, 2023, 58(5): 750-759. |
| [7] | 刘叶飞, 赵海霞, 姜希萍, 邱锐, 周昕越, 赵彦, 付春祥. 野大麦高效组培快繁及农杆菌介导的愈伤侵染体系建立[J]. 植物学报, 2023, 58(3): 440-448. |
| [8] | 李楚然, 付羚, 刘云, 杨晓琴, 朱国磊, 解思达, 马焕成, 赵平. 樟叶越桔细胞悬浮培养条件的优化[J]. 植物学报, 2022, 57(2): 227-235. |
| [9] | 逯锦春, 曹丽娜, 佟冠杰, 王鑫颖, 张利英, 喻锌, 李荟芳, 李彦慧. 大花银莲花愈伤组织诱导及再生体系的建立[J]. 植物学报, 2022, 57(2): 217-226. |
| [10] | 赵青平, 梁雨萍, 周方园, 赵翔. 植物幼苗玻璃化发生机制研究进展[J]. 植物学报, 2022, 57(1): 90-97. |
| [11] | 李艳敏, 蒋卉, 符真珠, 张晶, 袁欣, 王慧娟, 高杰, 董晓宇, 王利民, 张和臣. 芍药花药愈伤组织诱导及体细胞胚发生[J]. 植物学报, 2021, 56(4): 443-450. |
| [12] | 罗钱, 张燕莎, 欧静. 郁金樱愈伤组织诱导及植株再生[J]. 植物学报, 2021, 56(4): 451-461. |
| [13] | 杜鹏飞, 王玉, 曹英萍, 杨松, 孙志超, 毛德才, 鄢家俊, 李达旭, 孙美贞, 付春祥, 白史且. 基因枪介导的老芒麦遗传转化体系的建立[J]. 植物学报, 2021, 56(1): 62-70. |
| [14] | 张冬瑞, 卜志刚, 陈玲玲, 常缨. 香鳞毛蕨的组织培养和快速繁殖体系构建[J]. 植物学报, 2020, 55(6): 760-767. |
| [15] | 王亚琴, 韦陆丹, 王文静, 刘宝骏, 张春玲, 张俊卫, 何燕红. 万寿菊再生体系的建立及优化[J]. 植物学报, 2020, 55(6): 749-759. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
