

植物学报 ›› 2024, Vol. 59 ›› Issue (5): 783-791.DOI: 10.11983/CBB24063 cstr: 32102.14.CBB24063
收稿日期:2024-04-26
接受日期:2024-06-21
出版日期:2024-09-10
发布日期:2024-08-19
通讯作者:
覃思颖
基金资助:
Siying Qin1,*( ), Yan Luo1, He Zhang2, Jun Hu1, Jugou Liao3
), Yan Luo1, He Zhang2, Jun Hu1, Jugou Liao3
Received:2024-04-26
Accepted:2024-06-21
Online:2024-09-10
Published:2024-08-19
Contact:
Siying Qin
摘要: 原子力显微技术是研究植物细胞壁超微结构与力学性质的重要表征手段, 良好的样品制备是获得可靠数据的前提。花粉管作为微米级别的植物样品, 是研究细胞壁结构与功能的典型实验材料, 但是制样过程中活性生理状态下的花粉管与基底黏附不牢固, 难以获得活性生理状态下的花粉管原子力显微镜观测数据。该文以烟草(Nicotiana tabacum)花粉管为材料, 对花粉管原子力显微样品制备和观测方法进行优化, 采用固体培养基薄层作为花粉管与基底之间的黏附剂, 花粉管在萌发和生长的同时完成与基底的黏附。与常规的干燥-复水法相比, 优化的液下黏附法, 可在液体环境下直接观测, 无需经过干燥处理, 避免了干燥-复水过程对花粉管造成的形貌皱缩与力学性质改变, 能够获得活性生理状态下花粉管细胞壁原位的高分辨率原子力显微镜观测数据。该方法可应用于不同种属及不同尺寸花粉管的原子力显微镜观测。
中图分类号:
覃思颖, 罗燕, 张禾, 胡君, 廖菊够. 花粉管细胞壁原子力显微镜观测制样方法优化. 植物学报, 2024, 59(5): 783-791.
Siying Qin, Yan Luo, He Zhang, Jun Hu, Jugou Liao. Optimization of Preparation and Detection Methods for Pollen Tube Cell Wall by Atomic Force Microscopy. Chinese Bulletin of Botany, 2024, 59(5): 783-791.
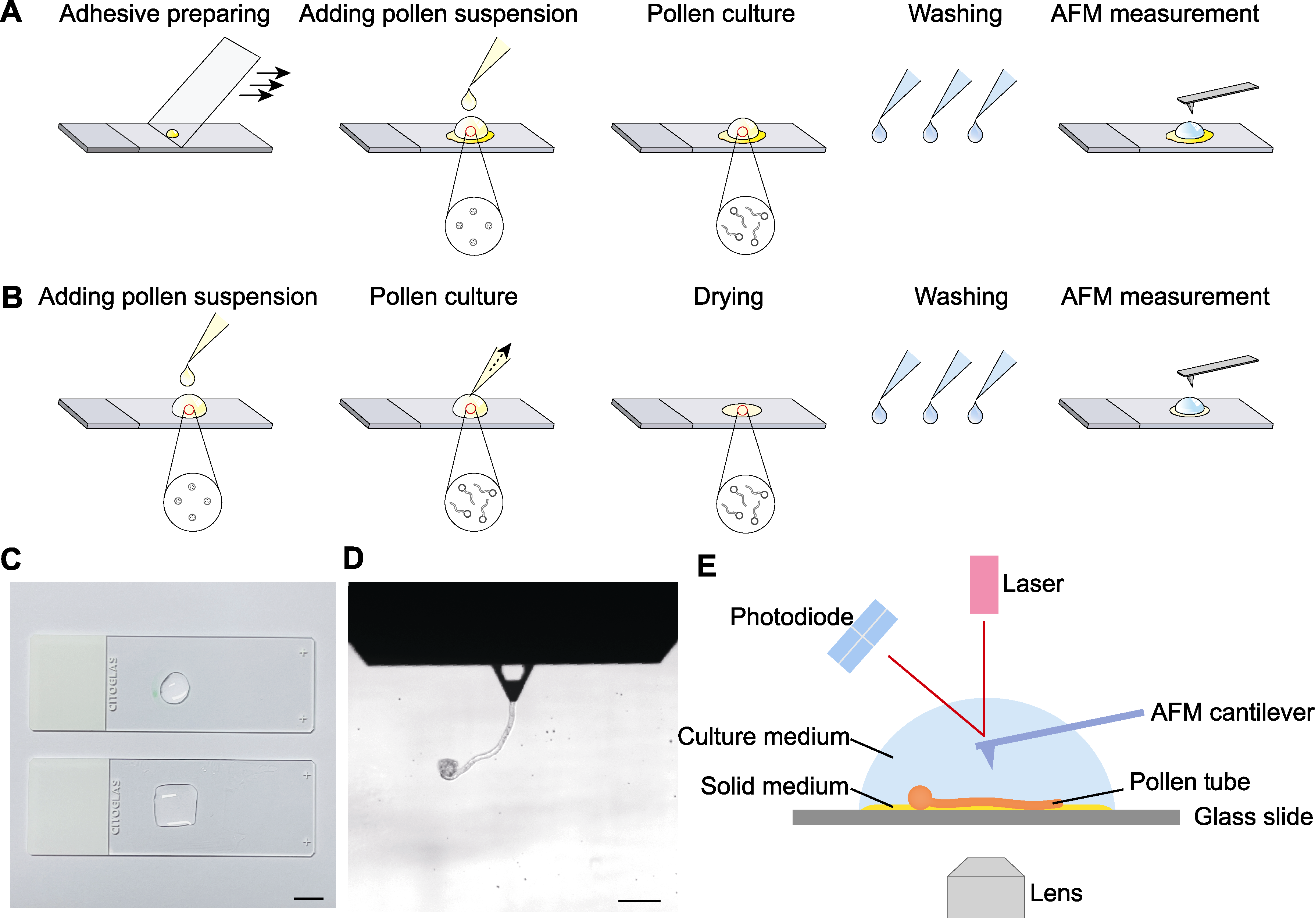
图1 花粉管原子力显微镜(AFM)制样及观测示意图 (A) 液下黏附法; (B) 干燥-复水法; (C) 载玻片待测区域(bar=1 cm); (D) AFM观测明场视野(bar=100 μm); (E) AFM液下观测
Figure 1 Schematic diagram of pollen tube atomic force microscope (AFM) preparation and detection (A) Liquid-attaching preparation method; (B) Drying-rehydration preparation method; (C) Test areas of glass slides (bar=1 cm); (D) Optical bright field image of AFM probe at work (bar=100 μm); (E) AFM detection under aqueous conditions
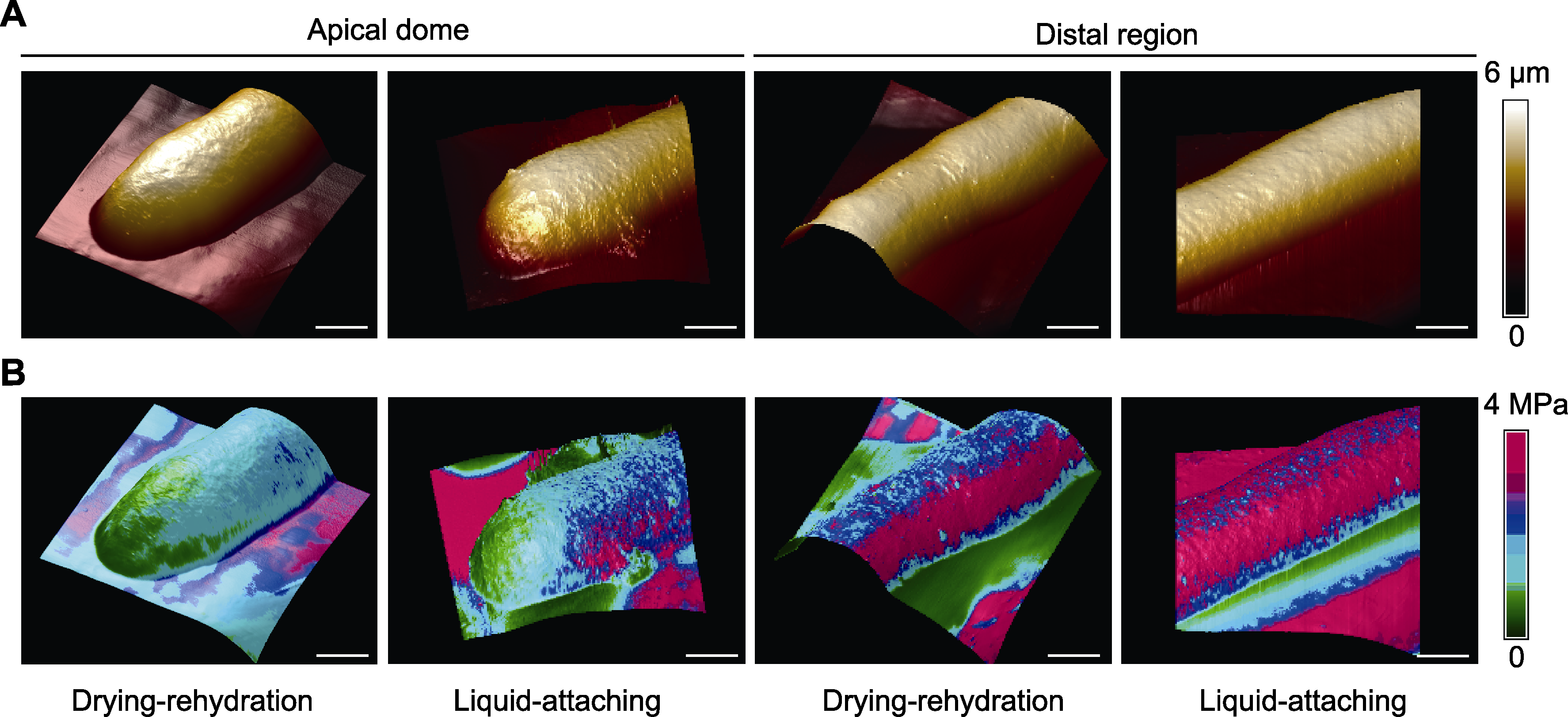
图2 干燥-复水法与液下黏附法的花粉管原子力显微镜(AFM)观测数据(顶端与中段部位) (A) 花粉管三维形貌图(颜色表示高度); (B) 叠加杨氏模量数据的花粉管三维形貌图(颜色表示杨氏模量)。Bars=3 μm
Figure 2 Pollen tube atomic force microscope (AFM) imaging data of drying-rehydration and liquid-attaching preparation methods (apical dome and distal region) (A) AFM-mapping of three-dimensional topography of pollen tubes (colors represent the height); (B) Three-dimensional topography of pollen tubes overlaid with Young’s modulus (colors represent the elasticity). Bars=3 μm
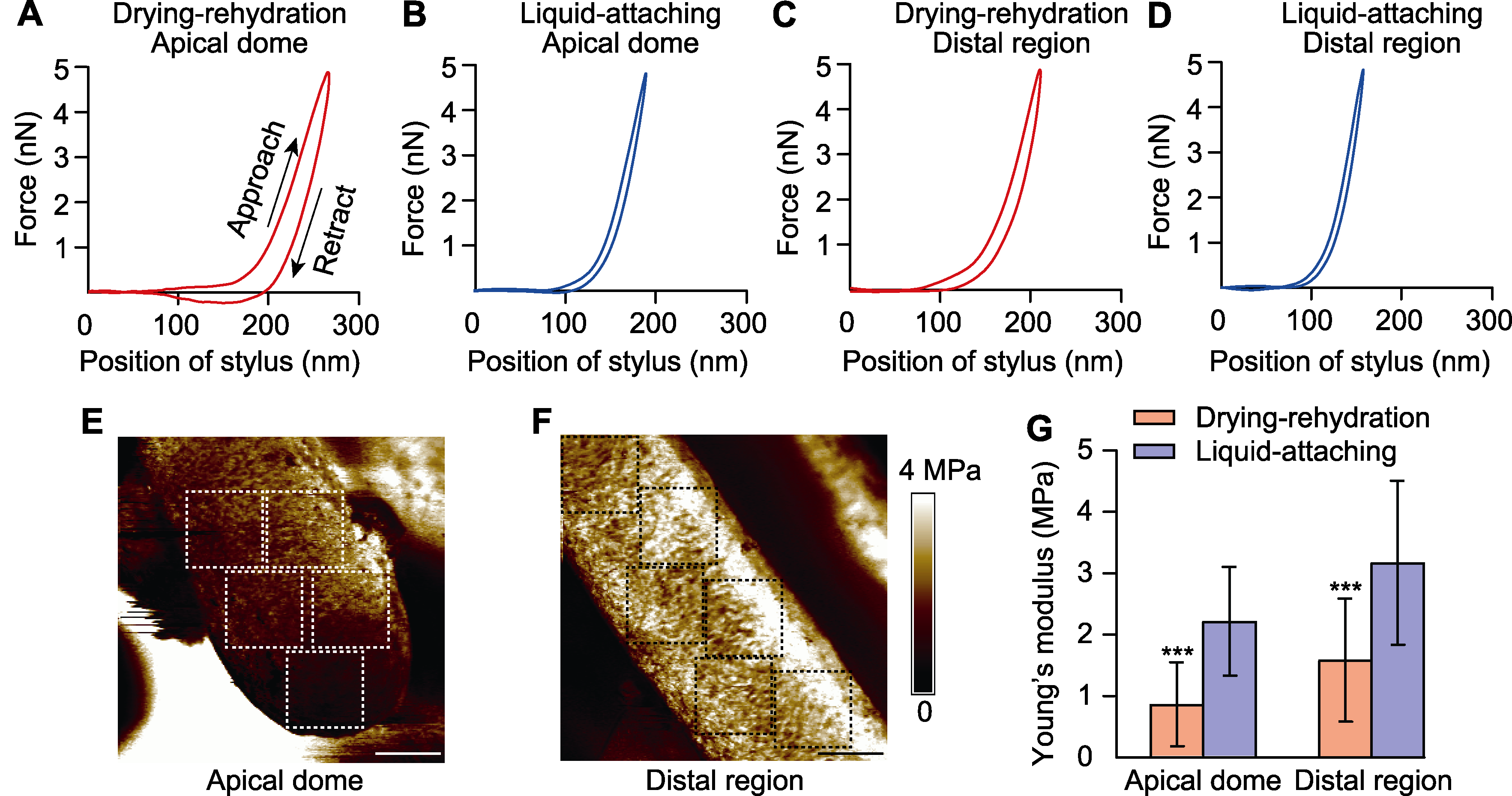
图3 不同黏附方法对花粉管顶端与中段杨氏模量的影响 (A)-(D) 干燥-复水法的花粉管顶端(A)、中段(C)与液下黏附法的花粉管顶端(B)、中段(D)的代表性力-距离曲线; (E) 花粉管顶端的杨氏模量图; (F) 花粉管中段的杨氏模量图(颜色表示杨氏模量, 虚线方框(边长3.5 μm)为统计杨氏模量平均值的区域); (G) 干燥-复水法与液下黏附法的花粉管顶端与中段的杨氏模量统计结果(***表示不同处理间在P<0.001水平差异极显著)。Bars=3 μm
Figure 3 Effects of different preparation methods on the Young’s modulus of pollen tube apical dome and distal region (A)-(D) Typical force-distance curves obtained from apical dome (A) and distal region (C) of drying-rehydration method, apical dome (B) and distal region (D) of liquid-attaching method; (E) Young’s modulus of pollen tube apical dome; (F) Young’s modulus of pollen tube distal region (colors represent the elasticity, dashed boxes (side length 3.5 μm) are the areas for calculating the average Young’s modulus); (G) The statistical results of Young’s modulus of pollen tube apical dome and distal region using drying-rehydration and liquid-attaching preparation methods (*** represent extremely significant differences among different treatments at P<0.001). Bars=3 μm
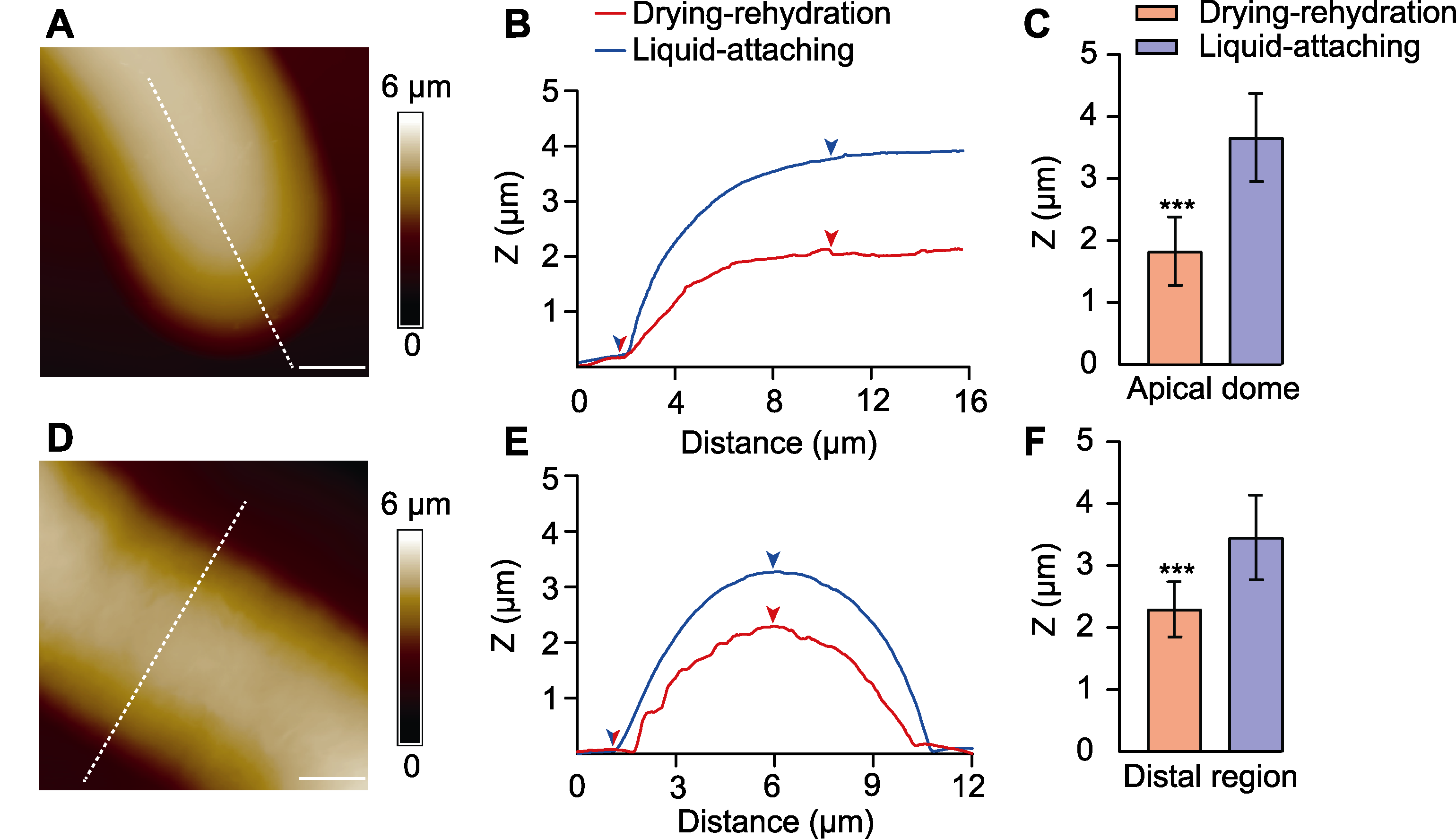
图4 不同黏附方法对花粉管顶端与中段截面高度的影响 (A), (D) 花粉管顶端(A)及中段(D)高度图(颜色表示高度, 虚线示截面位置); (B), (E) 不同黏附方法的花粉管顶端(B)及中段(E)截面高度图(箭头为截面高度差位置); (C), (F) 不同黏附方法的花粉管顶端(C)及中段(F)截面高度(Z)统计(***表示不同处理间在P<0.001水平差异极显著)。Bars=3 μm
Figure 4 Effects of different preparation methods on the cross-section height of pollen tube apical dome and distal region (A), (D) The height images of pollen tube apical dome (A) and distal region (D) (colors represent the height, and dashed lines represent the position of the cross-section); (B), (E) The cross-section height of pollen tube apical dome (B) and distal region (E) using different preparation methods (the arrows indicate the position of cross-section height); (C), (F) The statistical results of cross-section height (Z) of pollen tube apical dome (C) and distal region (F) using different preparation methods (*** represent extremely significant differences among different treatments at P<0.001). Bars=3 μm
| [1] | Adhikari PB, Liu XY, Kasahara RD (2020). Mechanics of pollen tube elongation: a perspective. Front Plant Sci 11, 589712. |
| [2] | Binnig G, Quate CF, Gerber C (1986). Atomic force microscope. Phys Rev Lett 56, 930-933. |
| [3] | Boudaoud A (2010). An introduction to the mechanics of morphogenesis for plant biologists. Trends Plant Sci 15, 353-360. |
| [4] | Burri JT, Vogler H, Munglani G, Läubli NF, Grossniklaus U, Nelson BJ (2019). A microrobotic system for simultaneous measurement of turgor pressure and cell-wall elasticity of individual growing plant cells. IEEE Robot Autom Lett 4, 641-646. |
| [5] | Cameron C, Geitmann A (2018). Cell mechanics of pollen tube growth. Curr Opin Genet Dev 51, 11-17. |
| [6] | Dufrêne YF, Ando T, Garcia R, Alsteens D, Martinez- Martin D, Engel A, Gerber C, Müller DJ (2017). Imaging modes of atomic force microscopy for application in molecular and cell biology. Nat Nanotechnol 12, 295-307. |
| [7] | Dumais J (2021). Mechanics and hydraulics of pollen tube growth. New Phytol 232, 1549-1565. |
| [8] | Gao BH, Xu L, Sun HJ, Xuan Y, Tang Y (2018). Research progress on atomic force microscopy in wood science. J Jiangsu For Sci Technol 45, 54-57. (in Chinese) |
| 高步红, 徐莉, 孙海军, 宣艳, 唐颖 (2018). 原子力显微镜在木材科学研究中的进展. 江苏林业科技 45, 54-57. | |
| [9] | Geitmann A, Parre E (2004). The local cytomechanical properties of growing pollen tubes correspond to the axial distribution of structural cellular elements. Sex Plant Reprod 17, 9-16. |
| [10] | Guan DS, Li HY, Tong PE (2020). Experimental methods and recent progress in biomechanics using atomic force microscopy. J Exp Fluid Mech 34(2), 57-66. (in Chinese) |
| 关东石, 李航宇, 童彭尔 (2020). 原子力显微镜的生物力学实验方法和研究进展. 实验流体力学 34(2), 57-66. | |
| [11] | Hu JR, Chen SB, Huang DD, Zhang Y, Lü SQ, Long M (2020). Global mapping of live cell mechanical features using PeakForce QNM AFM. Biophys Rep 6, 9-18. |
| [12] | Kozlova L, Petrova A, Ananchenko B, Gorshkova T (2019). Assessment of primary cell wall nanomechanical properties in internal cells of non-fixed maize roots. Plants 8, 172. |
| [13] | Krieg M, Fläschner G, Alsteens D, Gaub BM, Roos WH, Wuite GJL, Gaub HE, Gerber C, Dufrêne YF, Müller DJ (2019). Atomic force microscopy-based mechanobiology. Nat Rev Phys 1, 41-57. |
| [14] | Landrein B, Ingram G (2019). Connected through the force: mechanical signals in plant development. J Exp Bot 70, 3507-3519. |
| [15] | Leszczuk A, Kozioł A, Szczuka E, Zdunek A (2019). Analysis of AGP contribution to the dynamic assembly and mechanical properties of cell wall during pollen tube growth. Plant Sci 281, 9-18. |
| [16] | Li HY, Nie PC, Guan DS (2021). A hand of exploring the micro- and nano-scale world—atomic force microscope. Mech Eng 43, 806-811. (in Chinese) |
| 李航宇, 聂鹏程, 关东石 (2021). 探索微纳米世界的手——原子力显微镜. 力学与实践 43, 806-811. | |
| [17] | Li M, Xi N, Wang YC, Liu LQ (2018). Applications of multiparametric imaging atomic force microscopy in probing cellular and molecular mechanics. Prog Biochem Biophys 45, 1106-1114. (in Chinese) |
| 李密, 席宁, 王越超, 刘连庆 (2018). 基于多参数成像AFM的细胞及分子力学特性探测研究进展. 生物化学与生物物理进展 45, 1106-1114. | |
| [18] | Li TH, Wang T, Ren HY (2023). Advances in the study of cytoskeleton system regulating pollen tube development. Sci Sin Vitae 53, 763-774. (in Chinese) |
| 李彤辉, 王婷, 任海云 (2023). 细胞骨架系统调控花粉管发育的研究进展. 中国科学: 生命科学 53, 763-774. | |
| [19] | Majda M, Grones P, Sintorn IM, Vain T, Milani P, Krupinski P, Zagórska-Marek B, Viotti C, Jönsson H, Mellerowicz EJ, Hamant O, Robert S (2017). Mechanochemical polarization of contiguous cell walls shapes plant pavement cells. Dev Cell 43, 290-304. |
| [20] | Mirabet V, Das P, Boudaoud A, Hamant O (2011). The role of mechanical forces in plant morphogenesis. Annu Rev Plant Biol 62, 365-385. |
| [21] | Nezhad AS, Geitmann A (2013). The cellular mechanics of an invasive lifestyle. J Exp Bot 64, 4709-4728. |
| [22] | Qi JY, Wu BB, Feng SL, Lü SQ, Guan CM, Zhang X, Qiu DL, Hu YC, Zhou YH, Li CY, Long M, Jiao YL (2017). Mechanical regulation of organ asymmetry in leaves. Nat Plants 3, 724-733. |
| [23] | Qian L, Zhao HW (2018). Nanoindentation of soft biological materials. Micromachines (Basel) 9, 654. |
| [24] | Riglet L, Rozier F, Kodera C, Bovio S, Sechet J, Fobis-Loisy I, Gaude T (2020). KATANIN-dependent mechanical properties of the stigmatic cell wall mediate the pollen tube path in Arabidopsis. eLife 9, e57282. |
| [25] | Routier-Kierzkowska AL, Weber A, Kochova P, Felekis D, Nelson BJ, Kuhlemeier C, Smith RS (2012). Cellular force microscopy for in vivo measurements of plant tissue mechanics. Plant Physiol 158, 1514-1522. |
| [26] | Shamsudhin N, Laeubli N, Atakan HB, Vogler H, Hu CZ, Haeberle W, Sebastian A, Grossniklaus U, Nelson BJ (2016). Massively parallelized pollen tube guidance and mechanical measurements on a Lab-on-a-Chip platform. PLoS One 11, e0168138. |
| [27] | Uyttewaal M, Traas J, Hamant O (2010). Integrating physical stress, growth, and development. Curr Opin Plant Biol 13, 46-52. |
| [28] | Vaz Dias F, Serrazina S, Vitorino M, Marchese D, Heilmann I, Godinho M, Rodrigues M, Malhó R (2019). A role for diacylglycerol kinase 4 in signaling crosstalk during Arabidopsis pollen tube growth. New Phytol 222, 1434- 1446. |
| [29] | Vogler H, Draeger C, Weber A, Felekis D, Eichenberger C, Routier-Kierzkowska AL, Boisson-Dernier A, Ringli C, Nelson BJ, Smith RS, Grossniklaus U (2013). The pollen tube: a soft shell with a hard core. Plant J 73, 617- 627. |
| [30] | Wang MM, Zhu XP, Peng GQ, Liu ML, Zhang SQ, Chen MH, Liao ST, Wei XY, Xu P, Tan XY, Li FP, Li ZC, Deng L, Luo ZL, Zhu LY, Zhao S, Jiang DG, Li J, Liu ZL, Xie XR, Wang SK, Wu AM, Zhuang CX, Zhou H (2022). Methylesterification of cell-wall pectin controls the diurnal flower-opening times in rice. Mol Plant 15, 956-972. |
| [31] | Wu JZ (2011). Ultrastructure of pollen tube cell wall in Torenia fournieri L. observed by FESEM and AFM. J Anhui Agric Sci 39, 21802-21804. (in Chinese) |
| 吴娟子 (2011). 蓝猪耳花粉管细胞壁超微结构的FESEM和AFM比较研究. 安徽农业科学 39, 21802-21804. | |
| [32] | Wu QQ, Li Y, Lyu MH, Luo YW, Shi H, Zhong SW (2020). Touch-induced seedling morphological changes are determined by ethylene-regulated pectin degradation. Sci Adv 6, eabc9294. |
| [33] | Xiao L, Fang YY, Zhang H, Quan MY, Zhou JX, Li P, Wang D, Ji L, Ingvarsson PK, Wu HX, El-Kassaby YA, Du QZ, Zhang DQ (2023). Natural variation in the prolyl 4-hydroxylase gene PtoP4H9 contributes to perennial stem growth in Populus. Plant Cell 35, 4046-4065. |
| [34] | Zerzour R, Kroeger J, Geitmann A (2009). Polar growth in pollen tubes is associated with spatially confined dynamic changes in cell mechanical properties. Dev Biol 334, 437- 446. |
| [35] | Zhang BC, Gao YH, Zhang LJ, Zhou YH (2021a). The plant cell wall: biosynthesis, construction, and functions. J Integr Plant Biol 63, 251-272. |
| [36] | Zhang H, Guo ZL, Zhuang Y, Suo YZ, Du JM, Gao ZX, Pan JW, Li L, Wang TX, Xiao L, Qin GJ, Jiao YL, Cai HQ, Li L (2021b). MicroRNA775 regulates intrinsic leaf size and reduces cell wall pectin levels by targeting a galactosyltransferase gene in Arabidopsis. Plant Cell 33, 581- 602. |
| [37] | Zhang LJ, Gao CX, Mentink-Vigier F, Tang L, Zhang DM, Wang SG, Cao SX, Xu ZP, Liu XL, Wang T, Zhou YH, Zhang BC (2019). Arabinosyl deacetylase modulates the arabinoxylan acetylation profile and secondary wall formation. Plant Cell 31, 1113-1126. |
| [38] | Zhang M, Zhang YZ, He QZH, E YL, Li Y (2023). Advances in plant cell wall structure and imaging technology. Biotechnol Bull 39(7), 113-122. (in Chinese) |
| 张曼, 张叶卓, 何其邹洪, 鄂一岚, 李晔 (2023). 植物细胞壁结构及成像技术研究进展. 生物技术通报 39(7), 113- 122. | |
| [39] | Zhang Y, Yu JY, Wang X, Durachko DM, Zhang SL, Cosgrove DJ (2021c). Molecular insights into the complex mechanics of plant epidermal cell walls. Science 372, 706-711. |
| [40] | Zhang YG, Yuan XY, Zhang GF, Li YJ, Yin JH, Lin JX, Li XJ (2023). The application of click chemistry reactions in plant cell labeling. Chin Bull Bot 58, 956-965. (in Chinese) |
| 张御格, 袁笑妍, 张贵芳, 李雨健, 殷金环, 林金星, 李晓娟 (2023). 点击化学反应在植物细胞标记中的应用. 植物学报 58, 956-965. | |
| [41] | Zheng L, Chen YJ, Ding LP, Zhou Y, Xue SS, Li BY, Wei JH, Wang HZ (2023). The transcription factor MYB156 controls the polar stiffening of guard cell walls in poplar. Plant Cell 35, 3757-3781. |
| [42] | Zu YG, Zhang YL, Liu ZG, Wang YB, Liang HL, Liu HM (2006). Application of atomic force microscope in plant bio- logy research. Chin Bull Bot 23, 708-717. (in Chinese) |
| 祖元刚, 张宇亮, 刘志国, 王延兵, 梁慧丽, 刘红梅 (2006). 原子力显微镜在植物学研究中的应用. 植物学通报 23, 708-717. |
| [1] | 李虹茹, 杨桦, 陈伏生, 孙荣喜, 叶学敏. 氮磷添加对常绿阔叶林幼树与成树光合参数的分异影响[J]. 生物多样性, 2025, 33(4): 24435-. |
| [2] | 张御格, 袁笑妍, 张贵芳, 李雨健, 殷金环, 林金星, 李晓娟. 点击化学反应在植物细胞标记中的应用[J]. 植物学报, 2023, 58(6): 956-965. |
| [3] | 彭雄波, 孙蒙祥. 别开生面: 被子植物受精机制研究的新发现[J]. 植物学报, 2023, 58(4): 515-518. |
| [4] | 孙尚, 胡颖颖, 韩阳朔, 薛超, 龚志云. 水稻染色体双链寡核苷酸荧光原位杂交技术[J]. 植物学报, 2023, 58(3): 433-439. |
| [5] | 郭彦君, 陈枫, 罗敬文, 曾为, 许文亮. 植物细胞壁木聚糖的生物合成及其应用[J]. 植物学报, 2023, 58(2): 316-334. |
| [6] | 张凡凡, 邢新滢, 石文清, 沈懿, 程祝宽. 植物寡核苷酸荧光原位杂交技术方法[J]. 植物学报, 2023, 58(2): 274-284. |
| [7] | 冯旭飞, 雷长英, 张玉洁, 向导, 杨明凤, 张旺锋, 张亚黎. 棉花花铃期叶片氮分配对光合氮利用效率的影响[J]. 植物生态学报, 2023, 47(11): 1600-1610. |
| [8] | 熊映杰, 于果, 魏凯璐, 彭娟, 耿鸿儒, 杨冬梅, 彭国全. 天童山阔叶木本植物叶片大小与叶脉密度及单位叶脉长度细胞壁干质量的关系[J]. 植物生态学报, 2022, 46(2): 136-147. |
| [9] | 胡德美, 姚仁秀, 陈燕, 游贤松, 王顺雨, 汤晓辛, 王晓月. 青篱柴通过促进亲和花粉生长而提高传粉精确性[J]. 生物多样性, 2021, 29(7): 887-896. |
| [10] | 胡滨滨, 薛治慧, 张翠. 植物小RNA荧光原位杂交实验方法[J]. 植物学报, 2021, 56(3): 330-338. |
| [11] | 林梵宇, 尹希杰, 梁毓娜, 黄杰超. 微区XRF技术分析无机元素在植物中的原位分布[J]. 植物学报, 2020, 55(6): 733-739. |
| [12] | 李思佳, 张咏雪, 贾明生, 李莹, 戴绍军. 植物类LORELEI糖基磷脂酰肌醇锚定蛋白研究进展[J]. 植物学报, 2020, 55(5): 541-550. |
| [13] | 张雨, 赵明洁, 张蔚. 植物次生细胞壁生物合成的转录调控网络[J]. 植物学报, 2020, 55(3): 351-368. |
| [14] | 徐婉约,王应祥. 染色体展片法观察拟南芥雄性减数分裂过程中的染色体形态[J]. 植物学报, 2019, 54(5): 620-624. |
| [15] | 程新杰, 于恒秀, 程祝宽. 水稻减数分裂染色体分析方法[J]. 植物学报, 2019, 54(4): 503-508. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||