

植物学报 ›› 2024, Vol. 59 ›› Issue (4): 585-599.DOI: 10.11983/CBB23145 cstr: 32102.14.CBB23145
廖星鑫,†, 牛祎,†, 多兴武, 阿克也得力·居玛哈孜, 买热哈巴·阿不都克尤木, 热孜瓦尼姑丽·胡甫尔, 兰海燕, 曹婧*( )
)
收稿日期:2023-10-29
接受日期:2024-05-27
出版日期:2024-07-10
发布日期:2024-07-10
通讯作者:
曹婧
作者简介:第一联系人:† 共同第一作者
基金资助:
Xingxin Liao,†, Yi Niu,†, Xingwu Duo, Akeyedeli Jumahazi, Marhaba Abdukuyum, Rizwangul Hufur, Haiyan Lan, Jing Cao*( )
)
Received:2023-10-29
Accepted:2024-05-27
Online:2024-07-10
Published:2024-07-10
Contact:
Jing Cao
About author:First author contact:† These authors contributed equally to this paper
摘要: 磷酸烯醇式丙酮酸羧化酶(PEPC)是C4光合关键酶, 有助于植物在非生物胁迫下抵御逆境。异子蓬(Suaeda aralocaspica)是一种无须Kranz结构即可在单细胞中高效执行C4光合作用的荒漠盐生植物, 在C3作物遗传改良方面具有天然优势。以转异子蓬SaPEPC2基因烟草(Nicotiana tabacum)为材料, 探讨了其抗旱功能和光合性能。结果表明, 过表达SaPEPC2提高了烟草叶片持水能力, 可保持叶绿素稳定; 积累更多的渗透调节物质, 增强了抗氧化酶活性, 进而降低了植株体内的活性氧水平, 减轻膜损伤程度; 同时还增强了烟草抗旱相关基因和内源光合基因的表达, 提高了PEPC活性和净光合速率, 可能是促进了烟草体内的“类C4微循环”途径所致。研究结果为进一步利用异子蓬单细胞C4途径PEPC基因培育高光效抗逆农作物品种奠定了基础。
廖星鑫, 牛祎, 多兴武, 阿克也得力·居玛哈孜, 买热哈巴·阿不都克尤木, 热孜瓦尼姑丽·胡甫尔, 兰海燕, 曹婧. 异源表达异子蓬SaPEPC2基因提高烟草抗旱性和光合特性(长英文摘要). 植物学报, 2024, 59(4): 585-599.
Xingxin Liao, Yi Niu, Xingwu Duo, Akeyedeli Jumahazi, Marhaba Abdukuyum, Rizwangul Hufur, Haiyan Lan, Jing Cao. Heterologous Expression of Suaeda aralocaspica SaPEPC2 Gene Improves Drought Resistance and Photosynthesis in Transgenic Tobacco. Chinese Bulletin of Botany, 2024, 59(4): 585-599.
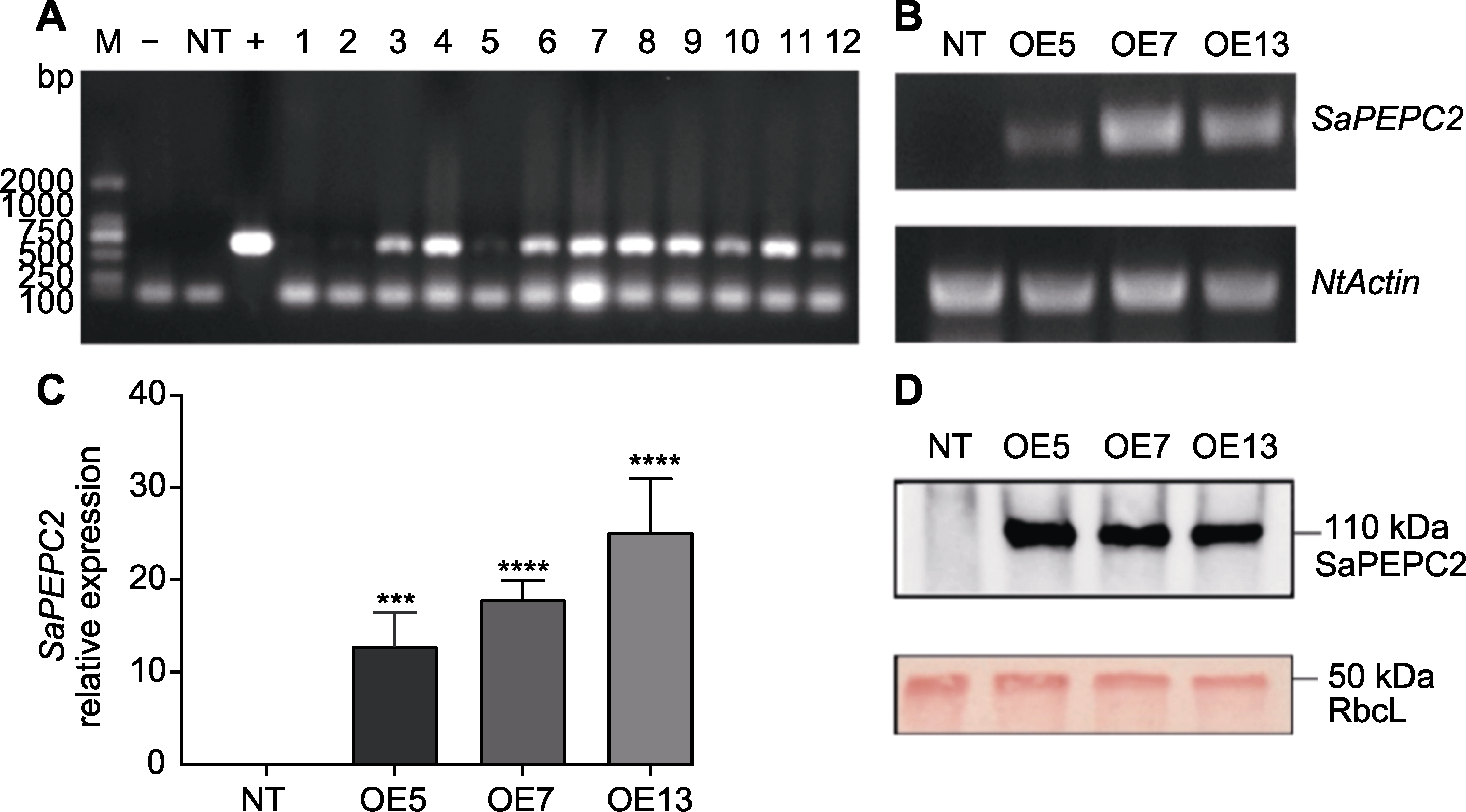
图1 SaPEPC2转基因烟草基因组DNA PCR (A)、RT-PCR (B)、qRT-PCR (C)和Western blot (D)鉴定 M: DL2000分子量标准; -: 阴性对照; +: 阳性对照; NT: NC89非转基因烟草; 1-12: 转基因株系。*** P<0.001; **** P<0.0001 (Student’s t test)
Figure 1 Identification of SaPEPC2 in transgenic tobacco by genomic DNA PCR (A), RT-PCR (B), qRT-PCR (C) and Western blot (D) M: DL2000 marker; -: Negative control; +: Positive control; NT: NC89 non-transgenic tobacco; 1-12: Transgenic lines. *** P<0.001; **** P<0.0001 (Student’s t test)

图2 自然干旱条件下过表达SaPEPC2转基因烟草表型(A)、叶片存活数(B)、失水率(C)和相对含水量(D) 不同小写字母表示不同株系在P<0.05水平差异显著。* P<0.05; ** P<0.01 (Student’s t检验)。NT: 非转基因。OE5、OE7、OE13为不同转基因株系。Bars=2 cm
Figure 2 Phenotype (A), leaf survival number (B), water loss rate (C) and relative water content (D) in SaPEPC2 overexpression transgenic tobacco lines under natural drought conditions Different lowercase letters indicate significant differences among different lines at P<0.05 level. * P<0.05; ** P<0.01 (Student’s t test). NT: Non-transgenic. OE5, OE7 and OE13 are different transgenic individuals. Bars=2 cm
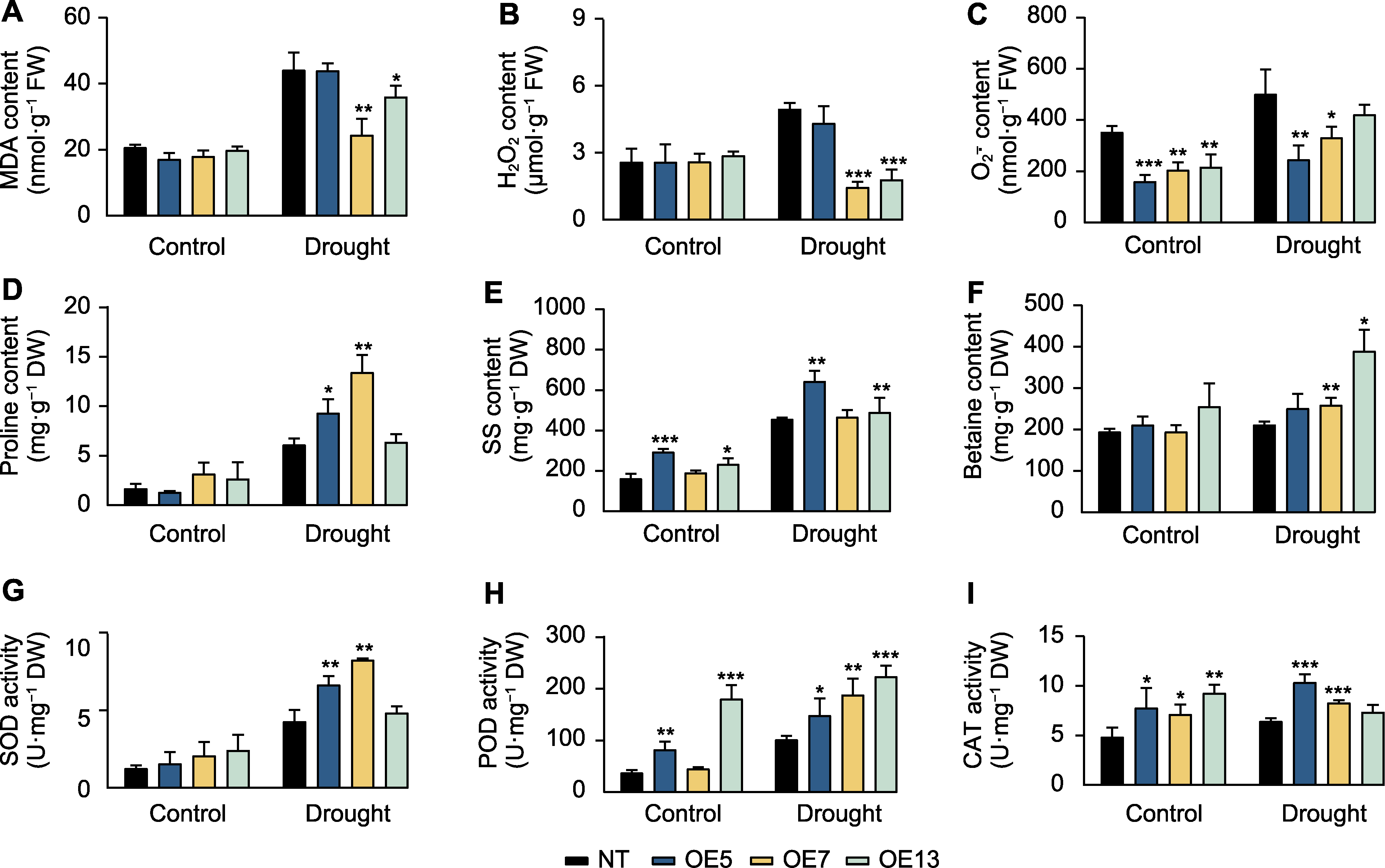
图3 干旱胁迫下过表达SaPEPC2转基因烟草的生理指标 (A) 丙二醛(MDA)含量; (B) H2O2含量; (C) O2-.含量; (D) 脯氨酸含量; (E) 可溶性糖(SS)含量; (F) 甜菜碱含量; (G) 超氧化物歧化酶(SOD)活性; (H) 过氧化物酶(POD)活性; (I) 过氧化氢酶(CAT)活性。*、**和***分别表示转基因植株与非转基因(NT)植株在0.05、0.01和0.001水平存在显著差异(Student’s t检验)。OE5、OE7和OE13同图2。
Figure 3 Physiological indicators in SaPEPC2 overexpression transgenic tobacco lines under drought stress (A) Malondialdehyde (MDA) content; (B) H2O2 content; (C) O2-. content; (D) Proline content; (E) Soluble sugar (SS) content; (F) Betaine content; (G) Superoxide dismutase (SOD) activity; (H) Peroxidase (POD) activity; (I) Catalase (CAT) activity. *, **, and *** indicate significant differences existing between the transgenic line and non-transgenic (NT) plant at 0.05, 0.01, and 0.001 levels, respectively (Student’s t test). OE5, OE7 and OE13 are the same as shownin Figure 2.
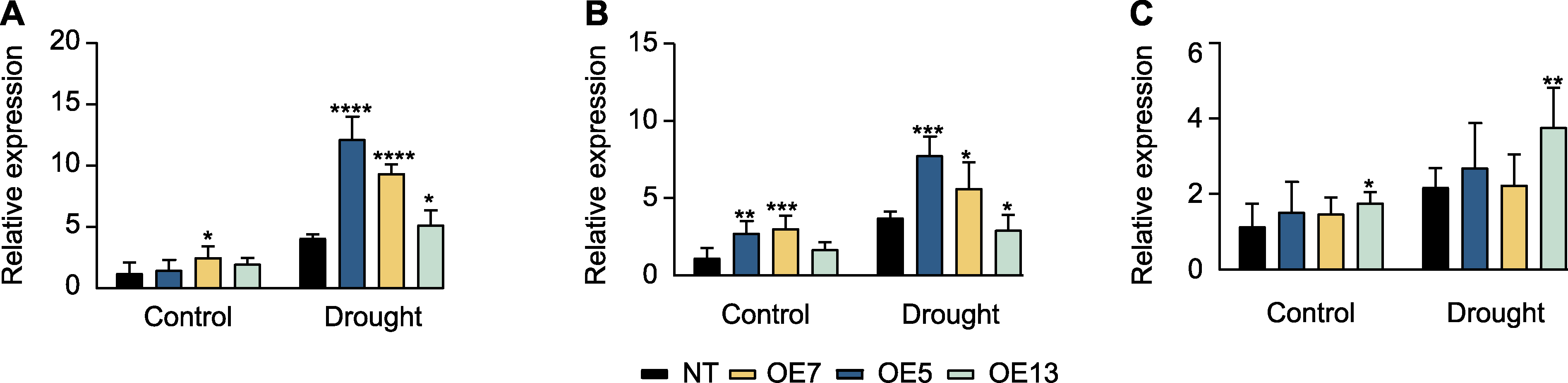
图4 干旱胁迫下过表达SaPEPC2转基因烟草干旱胁迫响应基因的表达模式 (A) NtP5CS (编码吡咯烷-5-羧酸合酶); (B) NtERD (脱水诱导早期应答基因); (C) NtDREB1 (编码脱水响应元件结合蛋白)。*、**、***和****分别表示转基因植株与非转基因(NT)植株在0.05、0.01、0.001和0.0001水平存在显著差异(Student’s t检验)。
Figure 4 Expression patterns of drought stress-responsible genes in SaPEPC2 overexpression transgenic tobacco lines under drought stress (A) NtP5CS (encoding pyrroline-5-carboxylate synthase); (B) NtERD (early responsive to dehydration); (C) NtDREB1 (encoding dehydration responsive element binding protein). *, **, ***, and **** indicate significant differences existing between the transgenic line and non-transgenic (NT) plant at 0.05, 0.01, 0.001, and 0.0001 levels, respectively (Student’s t test).
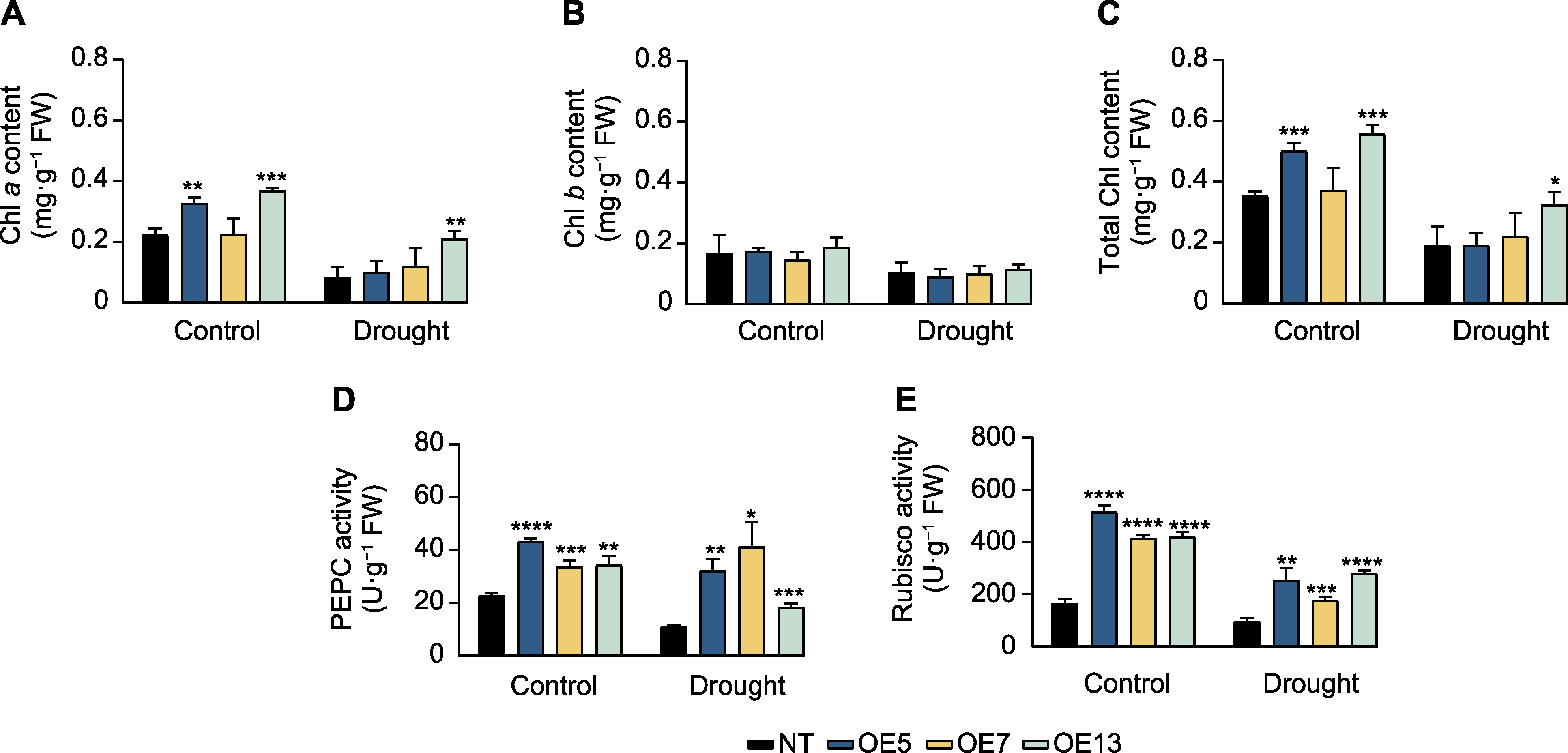
图5 干旱胁迫下过表达SaPEPC2转基因烟草叶绿素含量和光合酶活性 (A) 叶绿素a含量; (B) 叶绿素b含量; (C) 总叶绿素含量; (D) 磷酸烯醇式丙酮酸羧化酶(PEPC)活性; (E) Rubisco酶活性。*、**、***和****分别表示转基因植株与非转基因(NT)植株在0.05、0.01、0.001和0.0001水平存在显著差异(Student’s t检验)。
Figure 5 Chlorophyll content and photosynthetic enzyme activity in SaPEPC2 overexpression transgenic tobacco lines under drought stress (A) Chlorophyll a content; (B) Chlorophyll b content; (C) Total chlorophyll content; (D) Phosphoenolpyruvate carboxylase (PEPC) activity; (E) Rubisco activity. *, **, ***, and **** indicate significant differences existing between the transgenic line and non- transgenic (NT) plant at 0.05, 0.01, 0.001, and 0.0001 levels, respectively (Student’s t test).
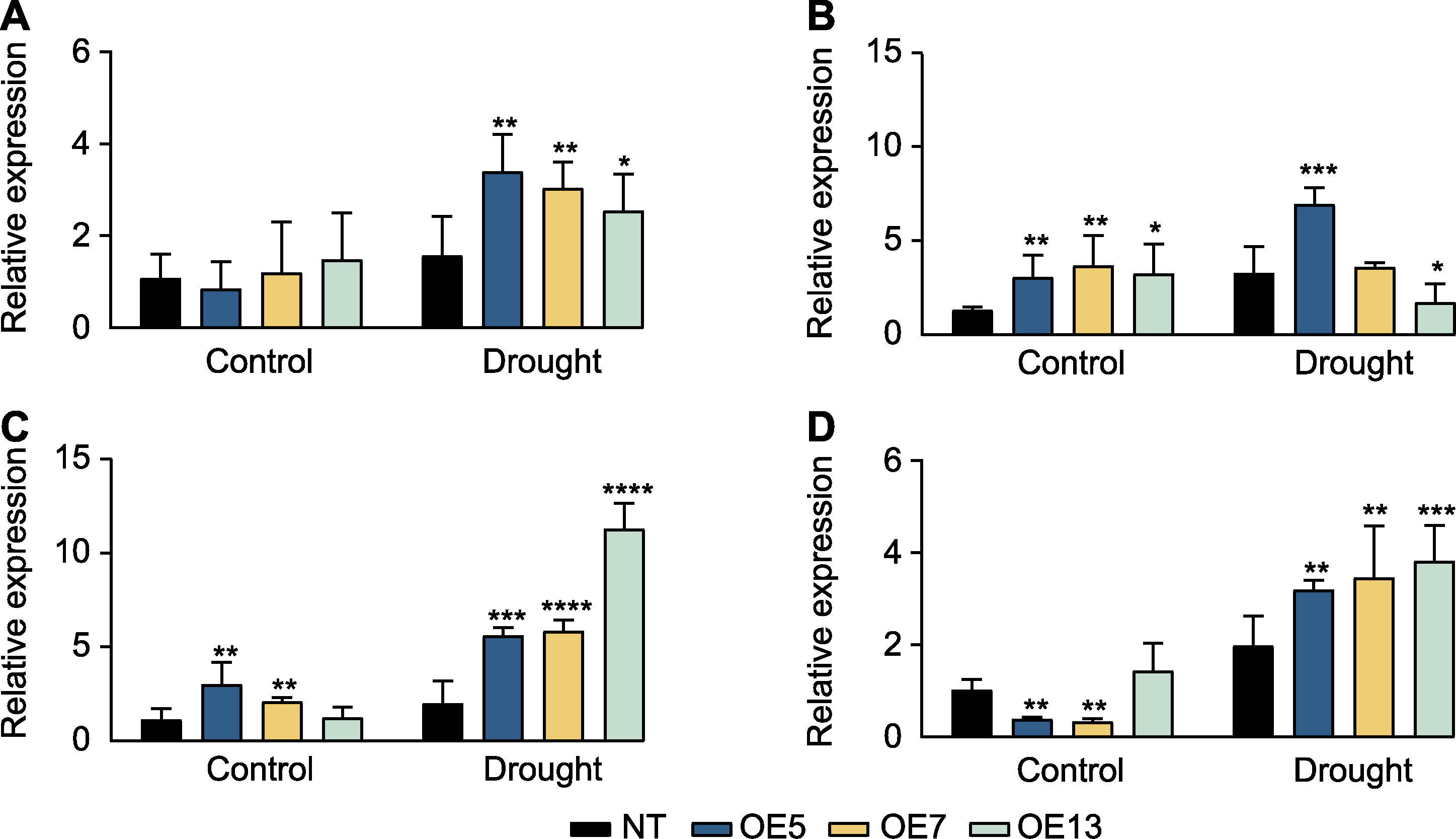
图6 干旱胁迫下过表达SaPEPC2转基因烟草内源光合基因的表达模式 (A) NtPEPC; (B) NtCA (编码磷酸酐酶); (C) NtFBP (编码果糖-1,6-二磷酸酶); (D) NtTPT (编码磷酸丙糖转运器)。*、**、***和****分别表示转基因植株与非转基因(NT)植株在0.05、0.01、0.001和0.0001水平存在显著差异(Student’s t检验)。
Figure 6 Expression patterns of endogenous photosynthetic genes in SaPEPC2 overexpression transgenic tobacco lines under drought stress (A) NtPEPC; (B) NtCA (encoding carbonic anhydrase); (C) NtFBP (encoding fructose-1,6-bisphosphatase); (D) NtTPT (encoding triose phosphate translocator). *, **, ***, and **** indicate significant differences existing between the transgenic line and non-transgenic (NT) plant at 0.05, 0.01, 0.001, and 0.0001 levels, respectively (Student’s t test).
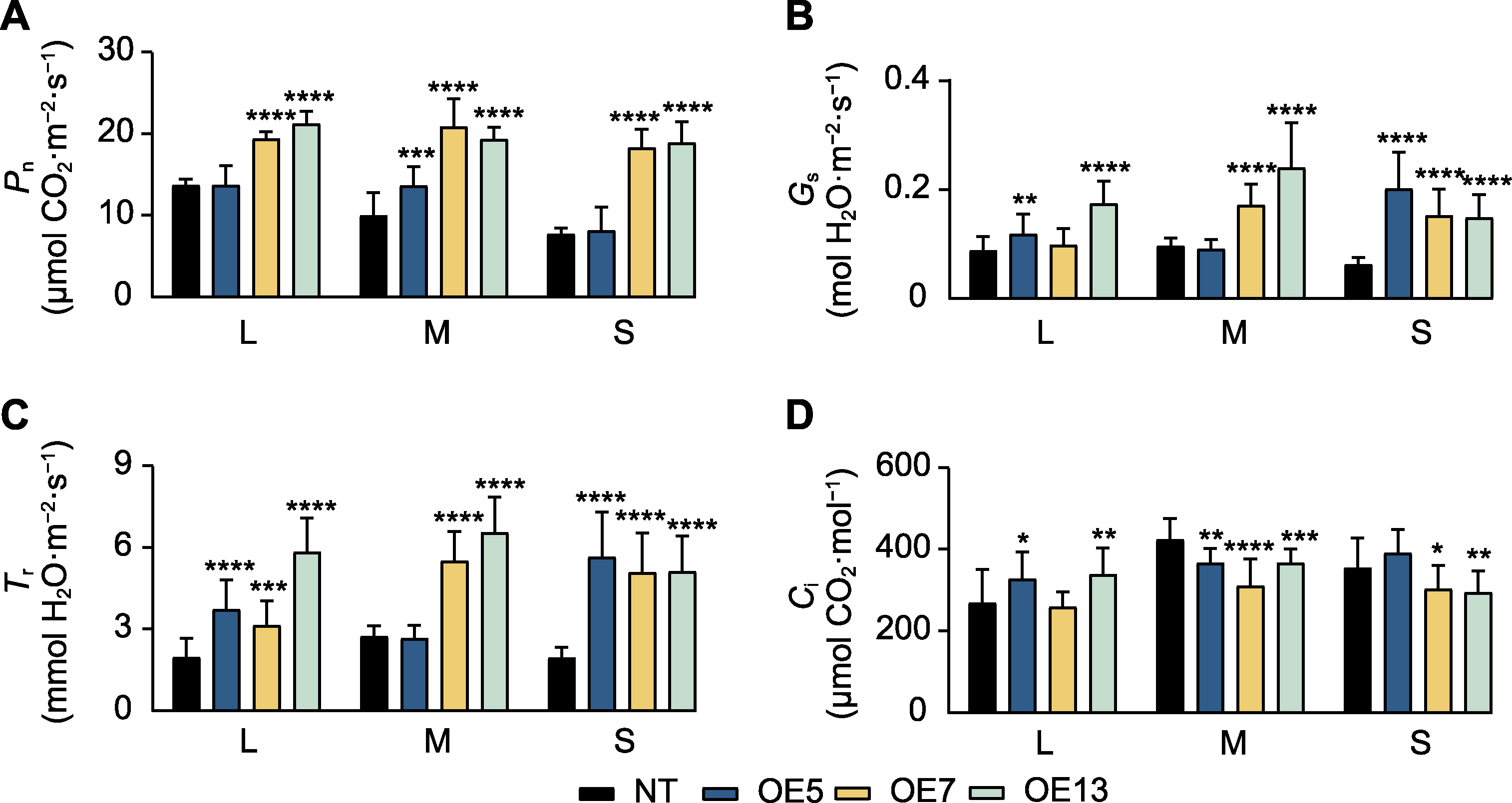
图7 过表达SaPEPC2转基因烟草光合指标 (A) 净光合速率(Pn); (B) 气孔导度(Gs); (C) 蒸腾速率(Tr); (D) 胞间CO2浓度(Ci)。L: 大(7-8叶期)植株; M: 中(6-7叶期)植株; S: 小(5-6叶期)植株。*、**、***和****分别表示转基因植株与非转基因(NT)植株在0.05、0.01、0.001和0.0001水平存在显著差异(Student’s t检验)。
Figure 7 Photosynthetic indicators in SaPEPC2 overexpression transgenic tobacco lines (A) Net photosynthetic rate (Pn); (B) Stomatal conductance (Gs); (C) Transpiration rate (Tr); (D) Intercellular CO2 concentration (Ci). L: Large (7-8 leaf stage) plants; M: Medium (6-7 leaf stage) plants; S: Small (5-6 leaf stage) plants. *, **, ***, and **** indicate significant differences existing between the transgenic line and non-transgenic (NT) plant at 0.05, 0.01, 0.001, and 0.0001 levels, respectively (Student’s t test).
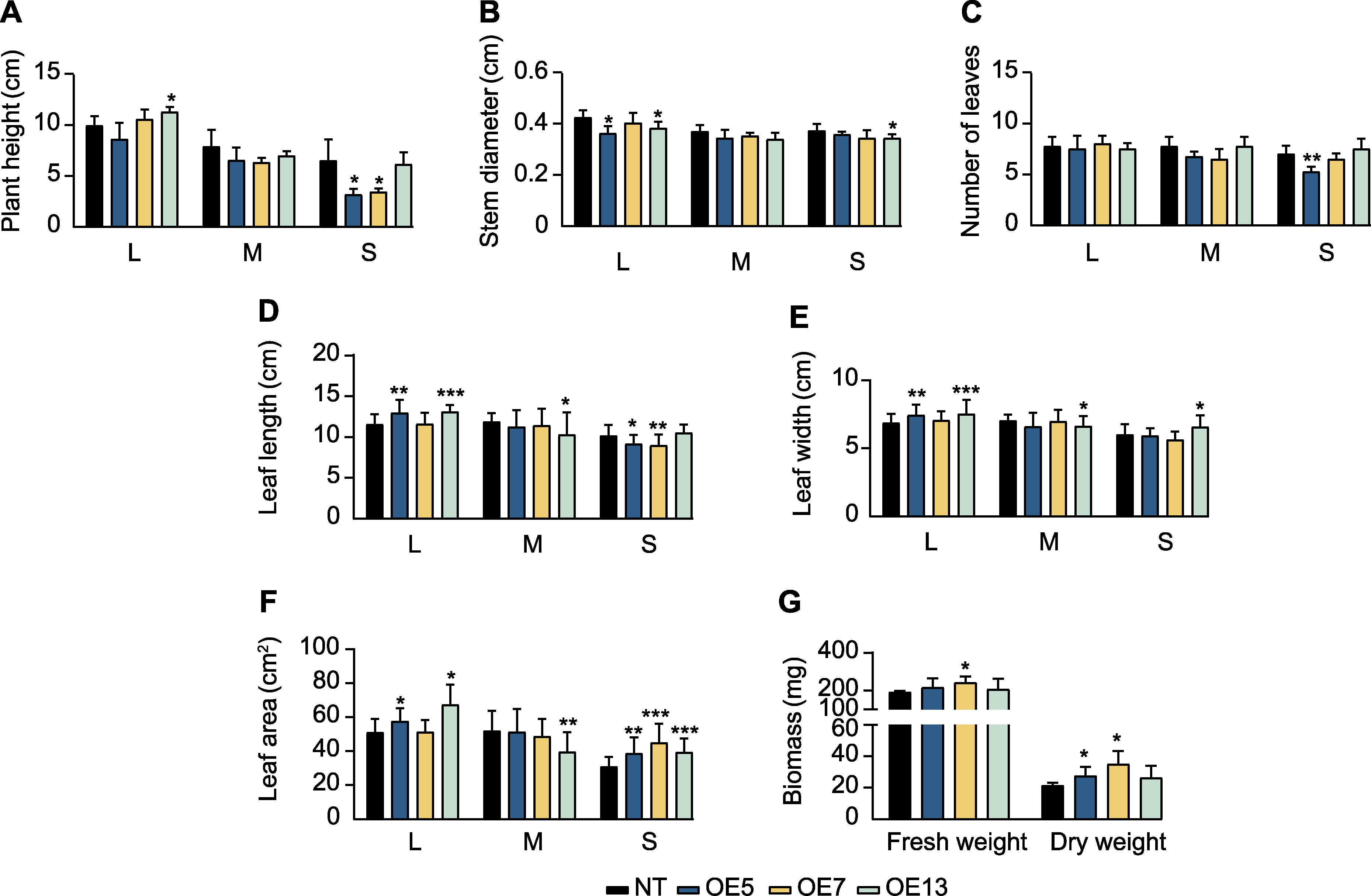
图8 过表达SaPEPC2转基因烟草生长指标 (A) 株高; (B) 茎粗; (C) 叶片数; (D) 叶长; (E) 叶宽; (F) 叶面积; (G) 生物量。L、M和S同图7。*、**和***分别表示转基因株系与非转基因(NT)植株在0.05、0.01和0.001水平存在显著差异(Student’s t检验)。
Figure 8 Growth indexes of SaPEPC2 overexpression transgenic tobacco lines (A) Plant height; (B) Stem diameter; (C) Number of leaves; (D) Leaf length; (E) Leaf width; (F) Leaf area; (G) Biomass. L, M, and S are the same as shownin Figure 7. *, **, and *** indicate significant differences existing between the transgenic line and non-transgenic (NT) plant at 0.05, 0.01, and 0.001 levels, respectively (Student’s t test).
| [1] | Bandyopadhyay A, Datta K, Zhang J, Yang W, Raychaudhuri S, Miyao M, Datta SK (2007). Enhanced photosynthesis rate in genetically engineered indica rice expressing pepc gene cloned from maize. Plant Sci 172, 1204- 1209. |
| [2] | Bowes G (2010). Single-cell C4 photosynthesis in aquatic plants. In: Raghavendra AS, Sage RF, eds. C4 Photosynthesis and Related CO2 Concentrating Mechanisms. Dordrecht: Springer. pp. 63-80. |
| [3] | Cao J, Cheng G, Wang L, Maimaitijiang T, Lan HY (2021). Genome-wide identification and analysis of the phosphoenolpyruvate carboxylase gene family in Suaeda aralocaspica, an annual halophyte with single-cellular C4 anatomy. Front Plant Sci 12, 665279. |
| [4] | Cao J, Lv XY, Chen L, Xing JJ, Lan HY (2015). Effects of salinity on the growth, physiology and relevant gene expression of an annual halophyte grown from heteromorphic seeds. AoB Plants 7, plv112. |
| [5] |
Casati P, Lara MV, Andreo CS (2000). Induction of a C4-like mechanism of CO2 fixation in Egeria densa, a submersed aquatic species. Plant Physiol 123, 1611-1622.
DOI PMID |
| [6] | Chen Z, Xu JY, Fan YK, Huang WX, Wang PW, Wen XY, Xu ZC (2016). Response of morphological structure and photosynthetic parameters to water deficit in four flue-cured tobacco cultivar seedlings. Chin J Eco-Agric 24, 1508- 1520. (in Chinese) |
| 陈征, 许嘉阳, 范艺宽, 黄五星, 王佩雯, 温心怡, 许自成 (2016). 不同烤烟品种幼苗形态结构及光合参数对干旱胁迫响应机制的差异. 中国生态农业学报 24, 1508-1520. | |
| [7] | Chen ZX, Spreitzer RJ (1992). How various factors influence the CO2/O2specificity of ribulose-1,5-bisphosphate carboxylase/oxygenase. Photosynth Res 31, 157-164. |
| [8] | Cheng G (2017). Enzymatic Activities of Two PEPC Isozymes Recombinant in Prokaryote and the Expression Patterns in Plant of Suaeda aralocaspica, a Desert C4 Species without Kranz Anatomy in Chenopodiaceae. PhD disseration. Urumqi: Xinjiang University. pp. I-VII. (in Chinese) |
| 程刚 (2017). 荒漠藜科无花环结构C4植物异子蓬两种PEPC同工酶的体外活性及植物体内表达特性研究. 博士论文. 乌鲁木齐: 新疆大学. pp. I-VII. | |
| [9] | Cheng G, Wang L, Lan HY (2016). Cloning of PEPC-1 from a C4 halophyte Suaeda aralocaspica without Kranz anatomy and its recombinant enzymatic activity in responses to abiotic stresses. Enzyme Microb Technol 83, 57-67. |
| [10] | Chollet R, Vidal J, O’Leary MH (1996). PHOSPHOENOLPYRUVATE CARBOXYLASE: a ubiquitous, highly regulated enzyme in plants. Annu Rev Plant Physiol Plant Mol Biol 47, 273-298. |
| [11] | Ding ZS, Huang SH, Zhou BY, Sun XF, Zhao M (2013). Over-expression of phosphoenolpyruvate carboxylase cDNA from C4 millet (Seteria italica) increase rice photosynthesis and yield under upland condition but not in wetland fields. Plant Biotechnol Rep 7, 155-163. |
| [12] | Ding ZS, Zhou BY, Sun XF, Zhao M (2012). High light tolerance is enhanced by overexpressed PEPC in rice under drought stress. Acta Agron Sin 38, 285-292. (in Chinese) |
|
丁在松, 周宝元, 孙雪芳, 赵明 (2012). 干旱胁迫下PEPC过表达增强水稻的耐强光能力. 作物学报 38, 285-292.
DOI |
|
| [13] |
Doubnerová V, Ryšlavá H (2011). What can enzymes of C4 photosynthesis do for C3 plants under stress? Plant Sci 180, 575-583.
DOI PMID |
| [14] |
Driever SM, Kromdijk J (2013). Will C3 crops enhanced with the C4 CO2-concentrating mechanism live up to their full potential (yield)? J Exp Bot 64, 3925-3935.
DOI PMID |
| [15] |
Edwards GE, Franceschi VR, Voznesenskaya EV (2004). Single-cell C4 photosynthesis versus the dual-cell (Kranz) paradigm. Annu Rev Plant Biol 55, 173-196.
PMID |
| [16] | Edwards GE, Voznesenskaya EV, Smith ME, Koteyeva NK, Park YI, Park JH, Kiirats O, Okita TW, Chuong SDX (2008). Breaking the Kranz paradigm in terrestrial C4 plants: does it hold promise for C4 rice? In: Sheehy JE, Mitchell PL, Hardy B, eds. Charting New Pathways to C4 Rice. New Jersey: World Scientific. pp. 249-273. |
| [17] | Giuliani R, Karki S, Covshoff S, Lin HC, Coe RA, Koteyeva NK, Evans MA, Quick WP, von Caemmerer S, Furbank RT, Hibberd JM, Edwards GE, Cousins AB (2019). Transgenic maize phosphoenolpyruvate carboxylase alters leaf-atmosphere CO2 and 13CO2 exchanges in Oryza sativa. Photosynth Res 142, 153-167. |
| [18] | Guo LN (2013). Cloning of Brassica napus L. Gene BnSBPase and BncyFBPase and Function Analysis in Tobacco. Master’s thesis. Beijing: Chinese Academy of Agricultural Sciences. pp. 5-9. (in Chinese) |
| 郭利娜 (2013). 油菜BnSBPase和BncyFBPase基因克隆及在烟草中的功能分析. 硕士论文. 北京: 中国农业科学院. pp. 5-9. | |
| [19] |
Holaday AS, Bowes G (1980). C4 acid metabolism and dark CO2 fixation in a submersed aquatic macrophyte (Hydrilla verticillata). Plant Physiol 65, 331-335.
DOI PMID |
| [20] | Huppe HC, Turpin DH (1994). Integration of carbon and nitrogen metabolism in plant and algal cells. Annu Rev Plant Physiol Plant Mol Biol 45, 577-607. |
| [21] |
Izui K, Matsumura H, Furumoto T, Kai Y (2004). PHOSPHOENOLPYRUVATE CARBOXYLASE: a new era of structural biology. Annu Rev Plant Biol 55, 69-84.
PMID |
| [22] |
Jeanneau M, Gerentes D, Foueillassar X, Zivy M, Vidal J, Toppan A, Perez P (2002). Improvement of drought tolerance in maize: towards the functional validation of the Zm-Asr1gene and increase of water use efficiency by over-expressing C4-PEPC. Biochimie 84, 1127-1135.
PMID |
| [23] | Kandoi D, Mohanty S, Govindjee, Tripathy BC (2016). Towards efficient photosynthesis: overexpression of Zea mays phosphoenolpyruvate carboxylase in Arabidopsis thaliana. Photosynth Res 130, 47-72. |
| [24] |
Keeley JE (1998). C4 photosynthetic modifications in the evolutionary transition from land to water in aquatic grasses. Oecologia 116, 85-97.
DOI PMID |
| [25] |
Ku MSB, Kano-Murakami Y, Matsuoka M (1996). Evolution and expression of C4 photosynthesis genes. Plant Physiol 111, 949-957.
PMID |
| [26] | Li JX, Li X, Xie YF (2021). Mechanism on drought tolerance enhanced by exogenous trehalose in C4-PEPC Rice. Chin Bull Bot 56, 296-314. (in Chinese) |
| 李佳馨, 李霞, 谢寅峰 (2021). 外源海藻糖增强高表达转玉米C4型PEPC水稻耐旱性的机制. 植物学报 56, 296- 314. | |
| [27] | Ling LL (2007). Photosynthetic Characteristic and Physiological Mechanism of C4 Photosynthetic Gene Transgenic Rice. PhD disseration. Chengdu: Sichuan University. pp. 9-10. (in Chinese) |
| 凌丽俐 (2007). 转C4光合酶基因水稻的光合特性及生理机制研究. 博士论文. 成都: 四川大学. pp. 9-10. | |
| [28] | Liu DG, Hu RB, Zhang J, Guo HB, Cheng H, Li LL, Borland AM, Qin H, Chen JG, Muchero W, Tuskan GA, Yang XH (2021). Overexpression of an Agave phosphoenolpyruvate carboxylase improves plant growth and stress tolerance. Cells 10, 582. |
| [29] | Liu XL, Li X, Dai CC, Zhou JY, Yan T, Zhang JF (2017a). Improved short-term drought response of transgenic rice over-expressing maize C4 phosphoenolpyruvate carboxylase via calcium signal cascade. J Plant Physiol 218, 206- 221. |
| [30] | Liu XL, Li X, Qian BY (2015). Photosynthetic and physiological regulation of C4 phosphoenolpyruvate carboxylase transgenic rice (Oryza sativa) by exogenous Ca2+ under polyethylene glycol stress. Chin Bull Bot 50, 206-216. (in Chinese) |
|
刘小龙, 李霞, 钱宝云 (2015). 外源Ca2+对PEG处理下转C4型PEPC基因水稻光合生理的调节. 植物学报 50, 206-216.
DOI |
|
| [31] | Liu XL, Li X, Zhang C, Dai CC, Zhou JY, Ren CG, Zhang JF (2017b). Phosphoenolpyruvate carboxylase regulation in C4-PEPC-expressing transgenic rice during early responses to drought stress. Physiol Plant 159, 178-200. |
| [32] | Liu YX, Maimaitijiang T, Zhang JH, Ma YL, Lan HY (2020). The developmental enhancement of a C4 system with non-typical C4 physiological characteristics in Salsola ferganica (Kranz anatomy), an annual desert halophyte. Front Plant Sci 11, 152. |
| [33] |
Magnin NC, Cooley BA, Reiskind JB, Bowes G (1997). Regulation and localization of key enzymes during the induction of Kranz-Less, C4-type photosynthesis in Hydrilla verticillata. Plant Physiol 115, 1681-1689.
PMID |
| [34] | Miyao M, Masumoto C, Miyazawa SI, Fukayama H (2011). Lessons from engineering a single-cell C4 photosynthetic pathway into rice. J Exp Bot 62, 3021-3029. |
| [35] |
Nomura M, Mai HT, Fujii M, Hata S, Izui K, Tajima S (2006). Phosphoenolpyruvate carboxylase plays a crucial role in limiting nitrogen fixation in Lotus japonicus nodules. Plant Cell Physiol 47, 613-621.
DOI PMID |
| [36] | Ogren WL (1984). Photorespiration: pathways, regulation, and modification. Ann Rev Plant Physiol 35, 415-442. |
| [37] | O’Leary B, Fedosejevs ET, Hill AT, Bettridge J, Park J, Rao SK, Leach CA, Plaxton WC (2011). Tissue-specific expression and post-translational modifications of plant- and bacterial-type phosphoenolpyruvate carboxylase isozymes of the castor oil plant, Ricinus communis L. J Exp Bot 62, 5485-5495. |
| [38] | Qi XL (2016). Study on Physiological Characteristics in Transgenic Wheat with Maize C4-PEPC Gene under High Temperature Stresses. PhD disseration. Zhengzhou: Henan Agricultural University. pp. 45-55. (in Chinese) |
| 齐学礼 (2016). 高温胁迫下转玉米PEPC基因小麦的生理特性研究. 博士论文. 郑州: 河南农业大学. pp. 45-55. | |
| [39] | Qi XL, Xu WG, Zhang JZ, Guo R, Zhao MZ, Hu L, Wang HW, Dong HB, Li Y (2017). Physiological characteristics and metabolomics of transgenic wheat containing the maize C4 phosphoenolpyruvate carboxylase (PEPC) gene under high temperature stress. Protoplasma 254, 1017- 1030. |
| [40] | Qian BY, Li X, Liu XL, Chen PB, Ren CG, Dai CC (2015). Enhanced drought tolerance in transgenic rice over-expressing of maize C4 phosphoenolpyruvate carboxylase gene via NO and Ca2+. J Plant Physiol 175, 9-20. |
| [41] |
Qin N, Xu WG, Hu L, Li Y, Wang HW, Qi XL, Fang YH, Hua X (2016). Drought tolerance and proteomics studies of transgenic wheat containing the maize C4 phosphoenolpyruvate carboxylase (PEPC) gene. Protoplasma 253, 1503-1512.
PMID |
| [42] |
Ruiz-Ballesta I, Baena G, Gandullo J, Wang LQ, She YM, Plaxton WC, Echevarría C (2016). New insights into the post-translational modification of multiple phosphoenolpyruvate carboxylase isoenzymes by phosphorylation and monoubiquitination during sorghum seed development and germination. J Exp Bot 67, 3523-3536.
DOI PMID |
| [43] | Shao RX, Li LL, Zheng HF, Zhang JY, Yang SJ, Ma Y, Xin LF, Su XY, Ran WL, Mao J, Zheng BY, Yang QH (2016). Effects of exogenous nitric oxide on photosynthesis of maize seedlings under drought stress. Sci Agric Sin 49, 251-259. (in Chinese) |
|
邵瑞鑫, 李蕾蕾, 郑会芳, 张寄阳, 杨慎娇, 马野, 信龙飞, 苏小雨, 冉午玲, 毛俊, 郑博元, 杨青华 (2016). 外源一氧化氮对干旱胁迫下玉米幼苗光合作用的影响. 中国农业科学 49, 251-259.
DOI |
|
| [44] | Sharpe RM, Offermann S (2014). One decade after the discovery of single-cell C4 species in terrestrial plants: what did we learn about the minimal requirements of C4 photosynthesis? Photosynth Res 119, 169-180. |
| [45] | Shi HM, Maimaiti R, Cao J, Lan HY (2022). Cloning, prokaryotic expression and response to abiotic stress of two types of PEPC genes from Salsola ferganica. Mol Plant Breeding [2024-06-24]. https://kns.cnki.net/kcms/detail//46.1068.S.20221201.1241.003.html. (in Chinese) |
| 石慧敏, 热孜亚·麦麦提, 曹婧, 兰海燕 (2022). 费尔干猪毛菜两种PEPC基因的克隆、原核表达及胁迫耐受性分析. 分子植物育种 [2024-06-24]. https://kns.cnki.net/kcms/detail//46.1068.S.20221201.1241.003.html. | |
| [46] | Shi JH, Yi KK, Liu Y, Xie L, Zhou ZJ, Chen Y, Hu ZH, Zheng T, Liu RH, Chen YL, Chen JQ (2015). Phosphoenolpyruvate carboxylase in Arabidopsis leaves plays a crucial role in carbon and nitrogen metabolism. Plant Physiol 167, 671-681. |
| [47] | Smith ME, Koteyeva NK, Voznesenskaya EV, Okita TW, Edwards GE (2009). Photosynthetic features of non-Kranz type C4 versus Kranz type C4 and C3 species in subfamily Suaedoideae (Chenopodiaceae). Funct Plant Biol 36, 770-782. |
| [48] | Song NX, Xie YF, Li X (2020). Effects of epigenetic mechanisms on C4phosphoenolpyruvate carboxylase transgenic rice (Oryza sativa) seed germination under drought stress. Chin Bull Bot 55, 677-692. (in Chinese) |
| 宋凝曦, 谢寅峰, 李霞 (2020). 干旱胁迫下表观遗传机制对转C4型PEPC基因水稻种子萌发的影响. 植物学报 55, 677-692. | |
| [49] | Suganuma N, Okada Y, Kanayama Y (1997). Isolation of a cDNA for nodule-enhanced phosphoenolpyruvate carboxylase from pea and its expression in effective and plant-determined ineffective pea nodules. J Exp Bot 48, 1165-1173. |
| [50] | Sun JY (2003). Localization, Function and Gene Regulation of Triose Phosphate/Phosphate Translocator (TPT) in wheat. PhD disseration. Beijing: China Agricultural University. pp. 1-5. (in Chinese) |
| 孙金月 (2003). 小麦磷酸丙糖转运器(TPT)的定位、功能及基因表达的调节. 博士论文. 北京: 中国农业大学. pp. 1-5. | |
| [51] | Suzuki S, Murai N, Kasaoka K, Hiyoshi T, Imaseki H, Burnell JN, Arai M (2006). Carbon metabolism in transgenic rice plants that express phosphoenolpyruvate carboxylase and/or phosphoenolpyruvate carboxykinase. Plant Sci 170, 1010-1019. |
| [52] | Tang YT, Li X, Lu W, Wei XD, Zhang QJ, Lv CG, Song NX (2018). Enhanced photorespiration in transgenic rice over-expressing maize C4 phosphoenolpyruvate carboxylase gene contributes to alleviating low nitrogen stress. Plant Physiol Biochem 130, 577-588. |
| [53] |
Taniguchi Y, Ohkawa H, Masumoto C, Fukuda T, Tamai T, Lee K, Sudoh S, Tsuchida H, Sasaki H, Fukayama H, Miyao M (2008). Overproduction of C4 photosynthetic enzymes in transgenic rice plants: an approach to introduce the C4-like photosynthetic pathway into rice. J Exp Bot 59, 1799-1809.
DOI PMID |
| [54] |
Voznesenskaya EV, Franceschi VR, Kiirats O, Artyusheva EG, Freitag H, Edwards GE (2002). Proof of C4 photosynthesis without Kranz anatomy in Bienertia cycloptera (Chenopodiaceae). Plant J 31, 649-662.
DOI PMID |
| [55] | Voznesenskaya EV, Franceschi VR, Kiirats O, Freitag H, Edwards GE (2001). Kranz anatomy is not essential for terrestrial C4 plant photosynthesis. Nature 414, 543-546. |
| [56] | Wang C, Li X, Cai QS (2008). Comparison of photosynthetic characteristics of PEPC transgenic rice and its hybrid rice lines under field and lab conditions. Jiangsu J Agric Sci 24, 232-236. (in Chinese) |
| 王超, 李霞, 蔡庆生 (2008). 不同测定环境条件下转PEPC基因水稻及杂交后代光合特性的比较. 江苏农业学报 24, 232-236. | |
| [57] | Wang FL, Liu RH, Wu GT, Lang CX, Chen JQ, Shi CH (2012). Specific downregulation of the bacterial-type PEPC gene by artificial microRNA improves salt tolerance in Arabidopsis. Plant Mol Biol Rep 30, 1080-1087. |
| [58] | Waseem M, Ahmad F (2019). The phosphoenolpyruvate carboxylase gene family identification and expression analysis under abiotic and phytohormone stresses in Solanum lycopersicum L. Gene 690, 11-20. |
| [59] | Wu M (2011). Research on Transgenic Brassica napus with C4-type PEPC gene. Master’s thesis. Hefei: Anhui Agricultural University. pp. 29-33. (in Chinese) |
| 吴梅 (2011). C4型PEPC基因导入油菜的研究. 硕士论文. 合肥: 安徽农业大学. pp. 29-33. | |
| [60] | Yang L (2015). Clone and Functional Analysis of Carbonic Anhydrase Gene of Tobacco. Master’s thesis. Ya’an: Sichuan Agricultural University. pp. 2-6. (in Chinese) |
| 杨朗 (2015). 烟草碳酸酐酶基因的克隆及其生物信息学分析. 硕士论文. 雅安: 四川农业大学. pp. 2-6. | |
| [61] | Yin W (2009). Preliminary Analysis of Transgenic Poplars with PEPC Key Gene in C4 Plant Photosynthesis. Master’s thesis. Nanjing: Nanjing Forestry University. pp. 22- 41. (in Chinese) |
| 尹吴 (2009). 转C4植物光合关键基因PEPC杨树的初步分析. 硕士论文. 南京: 南京林业大学. pp. 22-41. | |
| [62] | Zhang C, Li X, He YF, Zhang JF, Yan T, Liu XL (2017). Physiological investigation of C4-phosphoenolpyruvate- carboxylase-introduced rice line shows that sucrose metabolism is involved in the improved drought tolerance. Plant Physiol Biochem 115, 328-342. |
| [63] | Zhang Y, Man WQ, Nan XR, Li ZG (2015). Sorghum C4-specific pepc gene transformed into soybean can improve the photosynthetic characteristics of soybean. Mol Plant Breeding 13, 294-300. (in Chinese) |
| 张艳, 满为群, 南相日, 李柱刚 (2015). 高粱C4型pepc基因转入大豆可改善大豆光合特性. 分子植物育种 13, 294- 300. | |
| [64] | Zhou BY, Ding ZS, Zhao M (2011). Alleviation of drought stress inhibition on photosynthesis by overexpression of PEPC gene in rice. Acta Agron Sin 37, 112-118. (in Chinese) |
|
周宝元, 丁在松, 赵明 (2011). PEPC过表达可以减轻干旱胁迫对水稻光合的抑制作用. 作物学报 37, 112-118.
DOI |
|
| [65] | Zou CL, Guo ZQ, Zhao SS, Chen JS, Zhang CL, Han HR (2023). Genome-wide analysis of long non-coding RNAs in sugar beet (Beta vulgaris L.) under drought stress. Front Plant Sci 14, 1118011. |
| [1] | 王子阳, 刘升学, 杨志蕊, 秦峰. 玉米抗旱性的遗传解析[J]. 植物学报, 2024, 59(6): 883-902. |
| [2] | 王贺萍, 孙震, 刘雨辰, 苏彦龙, 杜锦瑜, 赵彦, 赵竑博, 王召明, 苑峰, 刘亚玲, 吴振映, 何峰, 付春祥. 蒙古冰草肉桂醇脱氢酶基因序列鉴定及功能分析[J]. 植物学报, 2024, 59(2): 204-216. |
| [3] | 李伟斌, 张红霞, 张玉书, 陈妮娜. 昼夜不对称增温对长白山阔叶红松林碳汇能力的影响[J]. 植物生态学报, 2023, 47(9): 1225-1233. |
| [4] | 蒋海港, 曾云鸿, 唐华欣, 刘伟, 李杰林, 何国华, 秦海燕, 王丽超, 姚银安. 三种藓类植物固碳耗水节律调节作用[J]. 植物生态学报, 2023, 47(7): 988-997. |
| [5] | 张蕾, 姜鹏飞, 王一鸣, 兰婷, 刘妍婧, 曾庆银. 苦杨×小叶杨杂交F1代苗期抗旱性比较研究[J]. 植物学报, 2023, 58(4): 519-534. |
| [6] | 孙永江, 王琪, 邵琪雯, 辛智鸣, 肖辉杰, 程瑾. 高温胁迫对植物光合作用的影响研究进展[J]. 植物学报, 2023, 58(3): 486-498. |
| [7] | 金佳怡, 罗怿婷, 杨惠敏, 芦涛, 叶涵斐, 谢继毅, 王珂欣, 陈芊羽, 方媛, 王跃星, 饶玉春. 水稻叶绿素含量QTL定位与候选基因表达分析[J]. 植物学报, 2023, 58(3): 394-403. |
| [8] | 刘海燕, 臧纱纱, 张春霞, 左进城, 阮祚禧, 吴红艳. 磷饥饿下硅藻光系统II光化学反应及其对高光强的响应[J]. 植物生态学报, 2023, 47(12): 1718-1727. |
| [9] | 孔照胜, 杨文强, 王柏臣, 林荣呈. 豆科饲草碳氮高效固定、转运和同化利用研究进展[J]. 植物学报, 2022, 57(6): 764-773. |
| [10] | 邹青青, 吴含玉, 刘东焕, 姜闯道. 植物光合作用的三维特性研究进展[J]. 植物学报, 2022, 57(2): 250-258. |
| [11] | 吴霖升, 张永光, 章钊颖, 张小康, 吴云飞. 日光诱导叶绿素荧光遥感及其在陆地生态系统监测中的应用[J]. 植物生态学报, 2022, 46(10): 1167-1199. |
| [12] | 罗丹丹, 王传宽, 金鹰. 木本植物水力系统对干旱胁迫的响应机制[J]. 植物生态学报, 2021, 45(9): 925-941. |
| [13] | 靳川, 李鑫豪, 蒋燕, 徐铭泽, 田赟, 刘鹏, 贾昕, 查天山. 黑沙蒿光合能量分配组分在生长季的相对变化与调控机制[J]. 植物生态学报, 2021, 45(8): 870-879. |
| [14] | 武洪敏, 双升普, 张金燕, 寸竹, 孟珍贵, 李龙根, 沙本才, 陈军文. 短期生长环境光强骤增导致典型阴生植物三七光系统受损的机制[J]. 植物生态学报, 2021, 45(4): 404-419. |
| [15] | 叶子飘, 于冯, 安婷, 王复标, 康华靖. 植物气孔导度对CO2响应模型的构建[J]. 植物生态学报, 2021, 45(4): 420-428. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||