

植物学报 ›› 2018, Vol. 53 ›› Issue (5): 603-611.DOI: 10.11983/CBB17129 cstr: 32102.14.CBB17129
陈建权, 程晨, 张梦恬, 张向前, 张尧, 王爱英, 祝建波*( )
)
收稿日期:2017-07-08
接受日期:2017-10-25
出版日期:2018-09-01
发布日期:2018-11-29
通讯作者:
祝建波
作者简介:
作者简介: 路安民(图中左), 植物系统分类学家。20世纪60-70年代编著《中国植物志》等, 后从事植物系统发育和进化研究。“七五”以来主持了4项中科院、国家自然科学基金委重大和重点项目。1991年获国务院颁发的有突出贡献科学家荣誉证书。1987年8月-1990年12月担任中科院植物所所长。
基金资助:
Chen Jianquan, Cheng Chen, Zhang Mengtian, Zhang Xiangqian, Zhang Yao, Wang Aiying, Zhu Jianbo*( )
)
Received:2017-07-08
Accepted:2017-10-25
Online:2018-09-01
Published:2018-11-29
Contact:
Zhu Jianbo
About author:† These authors contributed equally to this paper
摘要: 通过转基因烟草(Nicotiana tabacum)验证天山雪莲(Saussurea involucrata) Δ9硬脂酰-ACP脱饱和酶基因SiSAD与拟南芥(Arabidopsis thaliana)中同源基因AtFAB2的抗寒性功能。利用农杆菌介导法将植物表达载体PSiSAD:AtFAB2和PSiSAD:SiSAD导入烟草, 然后将2种转基因和野生型烟草分别置于20°C、10°C、5°C、0°C及-2°C下处理2小时, 检测其相对电导率、丙二醛(MDA)含量、叶绿素荧光参数(Fv/Fm)及脂肪酸含量。将-2°C处理2小时后的植株置于25°C培养1周进行生长恢复实验。结果表明, 生长恢复实验中转SiSAD基因烟草的恢复效果显著优于转AtFAB2基因和野生型烟草。在0°C和-2°C处理2小时后, 转SiSAD、AtFAB2基因型和野生型烟草的相对电导率和丙二醛含量呈现显著递增趋势; 转SiSAD、AtFAB2基因型烟草的Fv/Fm显著高于野生型烟草, 其中, 转SiSAD基因烟草的Fv/Fm显著高于转AtFAB2基因烟草。转AtFAB2基因型和野生型烟草的油酸(C18:1)含量随着温度的降低逐渐升高后降低并在0°C时达到最高值; 而转SiSAD基因型烟草C18:1含量持续升高, 并在-2°C时达到最高值, 其含量分别是转AtFAB2基因型和野生型烟草的1.58倍和1.7倍。以上结果表明, 天山雪莲Δ9硬脂酰-ACP脱饱和酶基因SiSAD与拟南芥中同源基因AtFAB2均可以显著增强非低温驯化烟草的抗寒性, 但是SiSAD基因效果显著优于AtFAB2。
陈建权, 程晨, 张梦恬, 张向前, 张尧, 王爱英, 祝建波. 天山雪莲SiSAD基因与拟南芥AtFAB2基因转化 烟草的抗寒性分析. 植物学报, 2018, 53(5): 603-611.
Chen Jianquan, Cheng Chen, Zhang Mengtian, Zhang Xiangqian, Zhang Yao, Wang Aiying, Zhu Jianbo. Cold-tolerance Analysis of Tobacco Plants Transformed with Saussurea involucrata SiSAD and Arabidopsis thaliana AtFAB2 Gene. Chinese Bulletin of Botany, 2018, 53(5): 603-611.
| Primer name | Primer sequence (5'-3') |
|---|---|
| SAD F | GTTGGAGATATGATCCACGAGGAAGC |
| SikSAD R | TTCCAGTATATCGGCATAGTCCTT |
| AtFAB2 F | GCACATGCGTGACATGCTTC |
| AtFAB2 R | CTGATCGACGGTCAATTGGC |
| GAPDH F | GTTGCTAGAGTTGCACTTCAGAGAG |
| GAPDH R | TTCCTGAAGCCGAAAACAGC |
表1 RT-PCR引物
Table 2 Primers of RT-PCR
| Primer name | Primer sequence (5'-3') |
|---|---|
| SAD F | GTTGGAGATATGATCCACGAGGAAGC |
| SikSAD R | TTCCAGTATATCGGCATAGTCCTT |
| AtFAB2 F | GCACATGCGTGACATGCTTC |
| AtFAB2 R | CTGATCGACGGTCAATTGGC |
| GAPDH F | GTTGCTAGAGTTGCACTTCAGAGAG |
| GAPDH R | TTCCTGAAGCCGAAAACAGC |
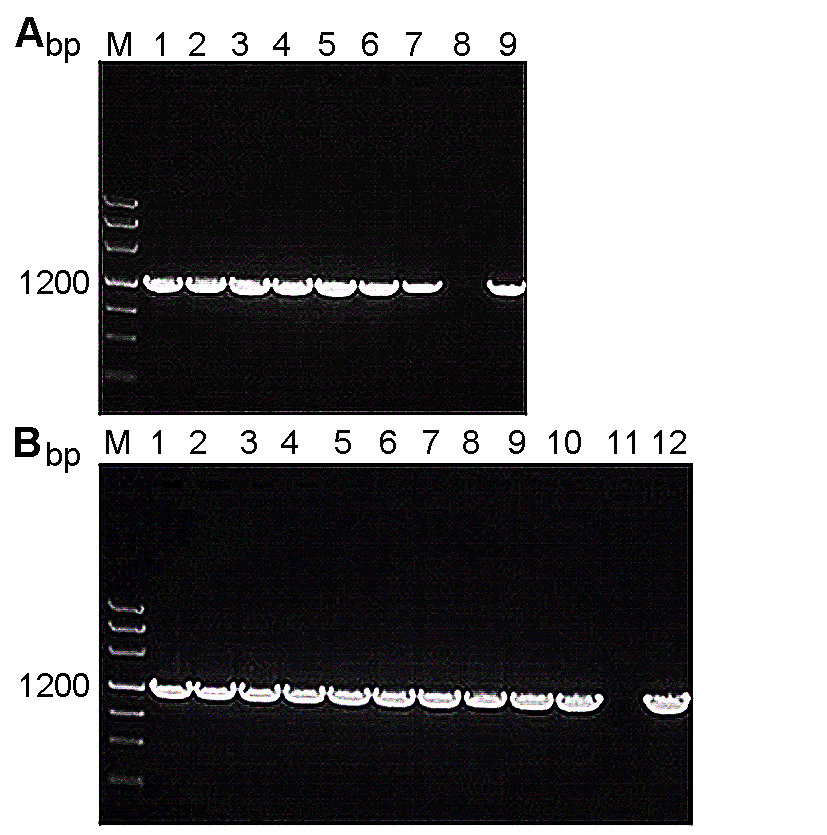
图2 转PSiSAD:SiSAD和PSiSAD:AtFAB2重组质粒烟草的PCR鉴定(A) 转PSiSAD:SiSAD重组质粒烟草基因PCR鉴定(M: Marker III DNA分子标量; 1-7: 不同转基因株系; 8: 野生型(阴性对照); 9: PSiSAD:SiSAD质粒(阳性对照); (B) 转PSiSAD:AtFAB2重组质粒烟草基因PCR鉴定(M: Marker III DNA分子标量; 1-10: 不同转基因株系; 11: 野生型(阴性对照); 12: PSiSAD:AtFAB2质粒(阳性对照))
Figure 2 PCR identification of tobacco transferred with PSiSAD:SiSAD and PSiSAD:AtFAB2 recombinant plasmid, respectively (A) PCR identification of tobacco transferred with PSiSAD: SiSAD recombinant plasmid (M: Marker III DNA marker; 1-7: Different transgenic lines; 8: Wild type (negative control); 9: PSiSAD:SiSAD plasmid (positive control)); (B) PCR identification of tobacco transferred with PSiSAD:AtFAB2 recombinant plasmid (M: Marker III DNA marker; 1-10: Different transgenic lines; 11: Wild type (negative control); 12: PSiSAD:SiSAD plasmid (positive control))
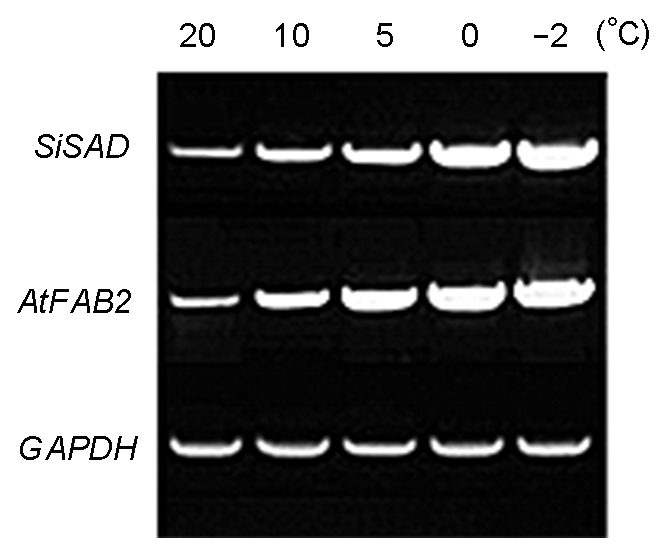
图3 转PSiSAD:SiSAD和PSiSAD:AtFAB2重组质粒烟草的RT- PCR鉴定
Figure 3 RT-PCR identification of tobacco transferred with PSiSAD:SiSAD and PSiSAD:AtFAB2 recombinant plasmid, respectively

图4 不同温度处理下野生型和转基因烟草表型(A)-(E) 野生型和转基因烟草在20°C、10°C、5°C、0°C及-2°C各处理2小时; (F) -2°C处理后, 25°C恢复培养1周。s-f: PSiSAD: AtFAB2; s-s: PSiSAD:SiSAD; WT: 野生型
Figure 4 Phenotype of wild-type and transgenic tobacco under different temperatures(A)-(E) Wild-type and transgenic tobacco plants grown at 20°C, 10°C, 5°C, 0°C, and -2°C for 2 hours, respectively; (F) After -2°C treatment recovering in 25°C for one week.s-f: PSiSAD:AtFAB2; s-s: PSiSAD:SiSAD; WT: Wild-type
| Temperature (°C) | Plant | Fatty acid (%) | |||||
|---|---|---|---|---|---|---|---|
| C16:0 | C18:0 | C18:1 | C18:2 | C18:3 | Total desaturation products | ||
| 20 | WT | 43.70±1.10 a | 24.77±0.66 a | 14.33±0.94 a | 3.27±0.43 a | 12.97±1.56 a | 16.57 |
| s-f | 42.27±1.11 a | 20.89±1.09 b | 15.20±0.38 b | 2.77±0.92 a | 7.37±1.24 b | 17.34 | |
| s-s | 46.53±0.90 a | 20.03±0.32 c | 17.23±1.60 c | 4.61±1.15 a | 7.07±0.93 b | 21.91 | |
| 10 | WT | 38.99±0.58 a | 19.83±0.65 a | 15.87±0.92 a | 2.53±0.30 a | 8.17±1.22 a | 17.57 |
| s-f | 42.97±0.62 a | 15.87±0.86 a | 17.43±0.48 a | 2.07±0.12 a | 8.19±1.12 a | 19.69 | |
| s-s | 40.76±0.79 a | 15.70±2.80 a | 19.40±1.23 c | 1.77±0.38 a | 6.00±1.11 a | 23.17 | |
| 5 | WT | 30.30±0.80 a | 16.47±0.66 a | 17.50±0.44 a | 3.70±0.12 a | 17.20±2.02 a | 19.4 |
| s-f | 25.27±0.66 b | 13.83±0.71 b | 18.70±1.29 b | 1.90±0.17 a | 10.20±0.86 b | 20.8 | |
| s-s | 46.43±0.41 c | 12.30±0.67 a | 20.70±0.57 b | 2.67±0.09 a | 6.60±0.68 c | 31.97 | |
| 0 | WT | 40.53±0.44 a | 16.23±0.32 a | 17.90±0.76 a | 2.20±0.17 a | 8.47±0.41 a | 22.67 |
| s-f | 47.63±0.47 b | 13.67±0.73 b | 19.17±1.45 b | 2.33±0.27 a | 11.97±1.44 a | 28.47 | |
| s-s | 44.33±0.07 b | 12.47±0.45 c | 23.50±1.33 a | 2.30±0.46 a | 11.87±2.07 a | 37.67 | |
| -2 | WT | 38.20±1.07 a | 15.57±0.76 a | 15.63±2.21 a | 2.87±0.20 a | 10.23±0.72 a | 26.73 |
| s-f | 40.20±0.23 b | 11.20±1.56 b | 16.54±0.49 a | 3.13±0.59 a | 16.30±1.22 b | 31.97 | |
| s-s | 53.60±0.47 c | 10.30±0.64 b | 30.47±0.89 b | 2.33±0.48 a | 9.83±2.24 a | 49.63 | |
| Recover treatment | WT | 36.30±1.04 a | 14.46±0.68 a | 14.77±2.11 a | 2.46±0.22 a | 10.02±0.68 a | 25.94 |
| s-f | 41.40±1.03 a | 20.05±1.03 b | 14.91±0.34 b | 2.58±0.85 a | 7.19±1.18 b | 16.87 | |
| s-s | 46.33±0.87 a | 20.01±0.30 c | 17.14±1.53 c | 4.57±1.12 a | 6.98±0.90 b | 21.23 | |
表2 不同温度下转基因烟草的脂肪酸含量测定
Table 2 The analysis of the content of desaturation products in transgenic tobacco under different temperatures
| Temperature (°C) | Plant | Fatty acid (%) | |||||
|---|---|---|---|---|---|---|---|
| C16:0 | C18:0 | C18:1 | C18:2 | C18:3 | Total desaturation products | ||
| 20 | WT | 43.70±1.10 a | 24.77±0.66 a | 14.33±0.94 a | 3.27±0.43 a | 12.97±1.56 a | 16.57 |
| s-f | 42.27±1.11 a | 20.89±1.09 b | 15.20±0.38 b | 2.77±0.92 a | 7.37±1.24 b | 17.34 | |
| s-s | 46.53±0.90 a | 20.03±0.32 c | 17.23±1.60 c | 4.61±1.15 a | 7.07±0.93 b | 21.91 | |
| 10 | WT | 38.99±0.58 a | 19.83±0.65 a | 15.87±0.92 a | 2.53±0.30 a | 8.17±1.22 a | 17.57 |
| s-f | 42.97±0.62 a | 15.87±0.86 a | 17.43±0.48 a | 2.07±0.12 a | 8.19±1.12 a | 19.69 | |
| s-s | 40.76±0.79 a | 15.70±2.80 a | 19.40±1.23 c | 1.77±0.38 a | 6.00±1.11 a | 23.17 | |
| 5 | WT | 30.30±0.80 a | 16.47±0.66 a | 17.50±0.44 a | 3.70±0.12 a | 17.20±2.02 a | 19.4 |
| s-f | 25.27±0.66 b | 13.83±0.71 b | 18.70±1.29 b | 1.90±0.17 a | 10.20±0.86 b | 20.8 | |
| s-s | 46.43±0.41 c | 12.30±0.67 a | 20.70±0.57 b | 2.67±0.09 a | 6.60±0.68 c | 31.97 | |
| 0 | WT | 40.53±0.44 a | 16.23±0.32 a | 17.90±0.76 a | 2.20±0.17 a | 8.47±0.41 a | 22.67 |
| s-f | 47.63±0.47 b | 13.67±0.73 b | 19.17±1.45 b | 2.33±0.27 a | 11.97±1.44 a | 28.47 | |
| s-s | 44.33±0.07 b | 12.47±0.45 c | 23.50±1.33 a | 2.30±0.46 a | 11.87±2.07 a | 37.67 | |
| -2 | WT | 38.20±1.07 a | 15.57±0.76 a | 15.63±2.21 a | 2.87±0.20 a | 10.23±0.72 a | 26.73 |
| s-f | 40.20±0.23 b | 11.20±1.56 b | 16.54±0.49 a | 3.13±0.59 a | 16.30±1.22 b | 31.97 | |
| s-s | 53.60±0.47 c | 10.30±0.64 b | 30.47±0.89 b | 2.33±0.48 a | 9.83±2.24 a | 49.63 | |
| Recover treatment | WT | 36.30±1.04 a | 14.46±0.68 a | 14.77±2.11 a | 2.46±0.22 a | 10.02±0.68 a | 25.94 |
| s-f | 41.40±1.03 a | 20.05±1.03 b | 14.91±0.34 b | 2.58±0.85 a | 7.19±1.18 b | 16.87 | |
| s-s | 46.33±0.87 a | 20.01±0.30 c | 17.14±1.53 c | 4.57±1.12 a | 6.98±0.90 b | 21.23 | |
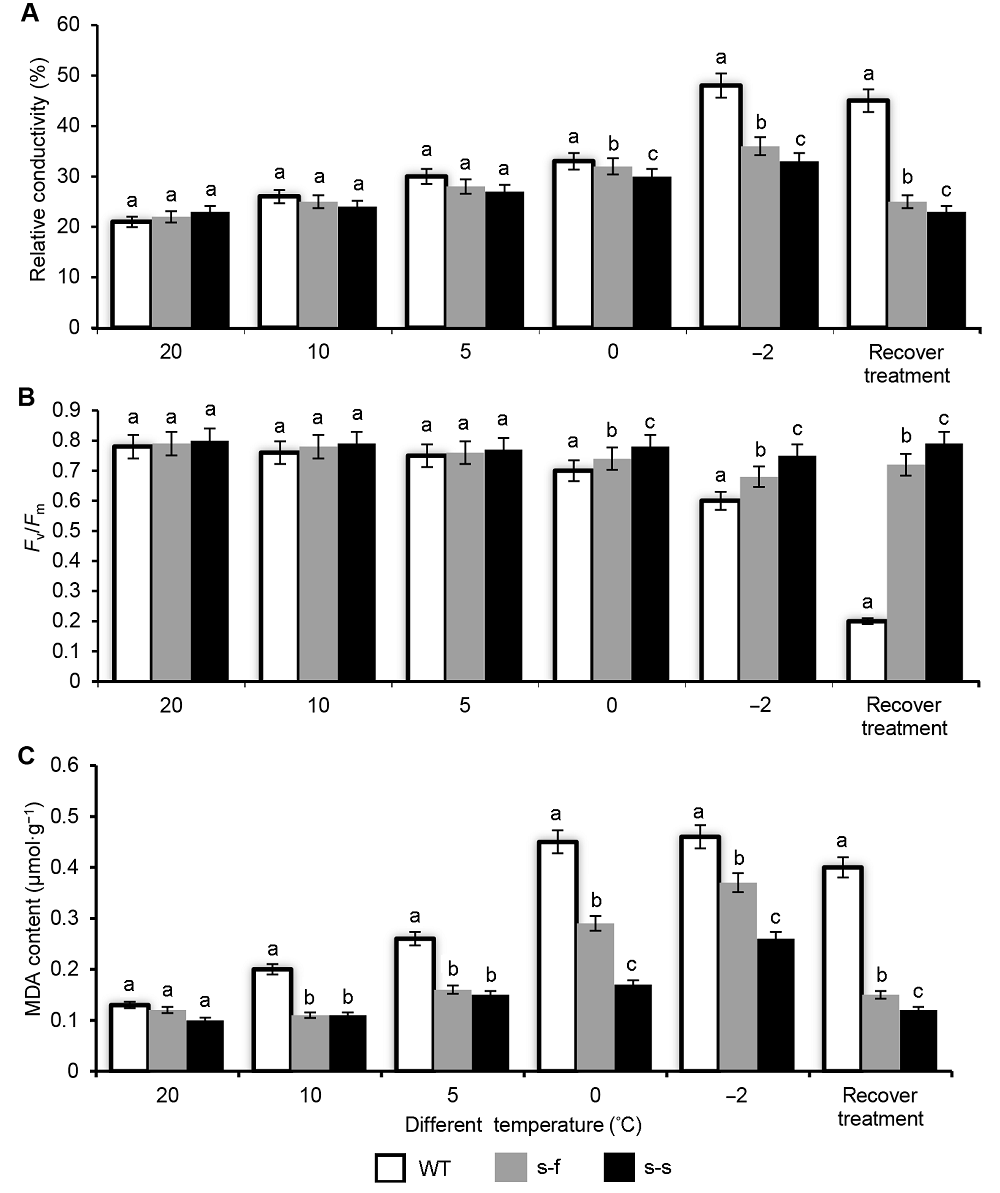
图5 不同温度处理下野生型和转基因烟草的生理指标(A) 相对电导率; (B) 叶绿素荧光参数(Fv/Fm); (C) 丙二醛(MDA)含量。处理条件: 野生型和转基因烟草在20°C、10°C、5°C、0°C和-2°C各处理2小时, 恢复处理是在-2°C处理2小时后25°C恢复培养1周。不同小写字母代表差异显著(P<0.05)。s-f: PSiSAD:AtFAB2; s-s: PSiSAD:SiSAD; WT: 野生型
Figure 5 The physiological analysis of wild-type and transgenic tobacco plant after different processing temperature (A) Relative conductivity; (B) Maximum efficiency of photosystem II photochemistry (Fv/Fm); (C) Malondialdehyde (MDA) content; Wild-type and transgenic tobacco plants grown at 20°C, 10°C, 5°C, 0°C, and -2°C; recovery treatment: after -2°C treatment for 2 hours recovery in 25°C for 1 week. Different lowercase letters indicate significant differences at P<0.05. s-f: PSiSAD:AtFAB2; s-s: PSiSAD:SiSAD; WT: Wild-type
| 1 | 陈爱葵, 韩瑞宏, 李东洋, 凌连莲, 罗惠霞, 唐上剑 (2010). 植物叶片相对电导率测定方法比较研究. 广东教育学院学报 30(5), 88-91. |
| 2 | 陈东亮 (2010). 根癌农杆菌介导的千年桐SAD基因对产油酵母的遗传转化. 硕士论文. 北京: 中国林业科学研究院. pp. 38-50. |
| 3 | 陈发菊, 杨映根, 赵德修, 桂耀林, 郭仲琛 (1999). 我国雪莲植物的种类、生境分布及化学成分的研究进展. 植物学通报 16, 561-566. |
| 4 | 陈思羽, 刘鹏, 朱末, 夏冬冬, 李亮, 徐克章, 陈展宇, 张治安 (2016). 大豆植株不同冠层种子活力及其萌发中抗氧化酶活性. 植物学报 51, 24-30. |
| 5 | 程晨, 郭新勇, 王爱英, 祝建波 (2011). 转新疆雪莲去饱和酶基因sikSAD重组酵母低温和酒精耐受性分析. 微生物学通报 38, 1647-1656. |
| 6 | 范妙华, 李纪元, 范正琪, 田敏, 倪穗 (2008). 千年桐SAD基因克隆与分析及其丝状真菌表达载体构建. 西北植物学报 28, 18-22. |
| 7 | 桂仁意, 刘亚迪, 郭小勤, 季海宝, 贾月, 余明增, 方伟 (2010). 不同剂量137Cs-γ辐射对毛竹幼苗叶片叶绿素荧光参数的影响. 植物学报 45, 66-72. |
| 8 | 郭新勇, 程晨, 王爱英, 张煜星, 王重, 喻娜, 祝建波 (2012). 天山雪莲冷调节蛋白基因siCOR转化烟草植株的抗旱性分析. 植物学报 47, 111-119. |
| 9 | 贾艳丽, 吴磊, 卢长明 (2014). 甘蓝型油菜Δ9硬脂酰ACP脱氢酶(SAD)基因的克隆与表达分析. 中国油料作物学报 36, 135-141. |
| 10 | 李金璐, 王硕, 于婧, 王玲, 周世良 (2013). 一种改良的植物DNA提取方法. 植物学报 48, 72-78. |
| 11 | 罗华元, 董石飞, 倪明, 张峻松 (2010). 烟叶中多元酸和高级脂肪酸的分析. 安徽农业科学 38, 16212-16214. |
| 12 | 罗通 (2006). 麻疯树的抗冷性和SAD基因的克隆及表达研究. 博士论文. 成都: 四川大学. pp. 27-35. |
| 13 | 罗秀芹, 欧文军, 李开绵, 陈松笔 (2014). 抗寒蛋白硬脂酰-ACP脱饱和酶的结构与功能预测. 福建农林大学学报(自然科学版) 43, 484-489. |
| 14 | 庞磊, 周小生, 李叶云, 江昌俊 (2011). 应用叶绿素荧光法鉴定茶树品种抗寒性的研究. 茶叶科学 31, 521-524. |
| 15 | 张党权, 谭晓风, 陈鸿鹏, 曾艳玲, 蒋瑶, 李魏, 胡芳名 (2008). 油茶SAD基因的全长cDNA克隆及生物信息学分析. 林业科学 44, 155-159. |
| 16 | 甄伟, 陈溪, 孙思洋, 胡鸢雷, 林忠平 (2000). 冷诱导基因的转录因子CBF1转化油菜和烟草及抗寒性鉴定. 自然科学进展 10, 1104-1108. |
| 17 | 祝建波, 刘海亮, 王重, 周鹏 (2006). 天山雪莲叶片全长cDNA文库的构建. 西北农业学报 15(6), 170-173. |
| 18 | Aroca R, Amodeo G, Fernández-Illescas S, Herman EM, Chaumont F, Chrispeels MJ (2005). The role of aqu- aporins and membrane damage in chilling and hydrogen peroxide induced changes in the hydraulic conductance of maize roots.Plant Physiol 137, 341-353. |
| 19 | Barkan L, Vijayan P, Carlsson AS, Mekhedov S, Browse J (2006). A suppressor of fab1 challenges hypotheses on the role of thylakoid unsaturation in photosynthetic function. Plant Physiol 141, 1012-1020. |
| 20 | Byfield GE, Xue H, Upchurch RG (2006). Two genes from soybean encoding soluble Δ9 stearoyl-acp desaturas- es.Crop Sci 46, 840-846. |
| 21 | Craig W, Lenzi P, Scotti N, De Palma M, Saggese P, Carbone V, McGrath CN, Magee AM, Medgyesy P, Kavan- agh TA, Dix PJ, Grillo S, Cardi T (2008). Transplastomic tobacco plants expressing a fatty acid desaturase gene exhibit altered fatty acid profiles and improved cold tolerance.Transgenic Res 17, 769-782. |
| 22 | Du ZY, Bramlage WJ (1992). Modified thiobarbituric acid assay for measuring lipid oxidation in sugar-rich plant tissue extracts. J Agric Food Chem 40, 1566-1570. |
| 23 | James DW Jr, Dooner HK (1990). Isolation of EMS-induced mutants in Arabidopsis altered in seed fatty acid composition.Theor Appl Genet 80, 241-245. |
| 24 | Jung S, Tate PL, Horn R, Kochert G, Moore K, Abbott AG (2003). The phylogenetic relationship of possible progenitors of the cultivated peanut. J Hered 94, 334-340. |
| 25 | Kachroo A, Shanklin J, Whittle E, Lapchyk L, Hildebrand D, Kachroo P (2007). The Arabidopsis stearoyl-acyl carrier protein-desaturase family and the contribution of leaf isoforms to oleic acid synthesis.Plant Mol Biol 63, 257-271. |
| 26 | Krause GH, Weis E (1991). Chlorophyll fluorescence and photosynthesis: the basics.Ann Rev Plant Physiol Plant Mol Biol 42, 313-349. |
| 27 | Lightner J, James DW Jr, Dooner HK, Browse J (1994a). Altered body morphology is caused by increased stearate levels in a mutant of Arabidopsis.Plant J 6, 401-412. |
| 28 | Lightner J, Wu JR, Browse J (1994b). A mutant of Arabidopsis with increased levels of stearic acid. Plant Physiol 106, 1443-1451. |
| 29 | Lutts S, Kinet JM, Bouharmont J (1996). Nacl-induced senescence in leaves of rice (Oryza sativa L.) cultivars differing in salinity resistance. Ann Bot 78, 389-398. |
| 30 | Murata N, Wada H (1995). Acyl-lipid desaturases and their importance in the tolerance and acclimatization to cold of cyanobacteria. Biochem J 308, 1-8. |
| 31 | Shilman F, Brand Y, Brand A, Hedvat I, Hovav R (2011). Identification and molecular characterization of homeologousΔ9-stearoyl acyl carrier protein desaturase 3 genes from the allotetraploid peanut(Arachis hypogaea). Plant Mol Biol Rep 29, 232-241. |
| 32 | Tasseva G, de Virville JD, Cantrel C, Moreau F, Zacho- wski A (2004). Changes in the endoplasmic reticulum lipid properties in response to low temperature in Brassica napus. Plant Physiol Biochem 42, 811-822. |
| 33 | Thompson GA, Scherer DE, Foxall-Van Aken S, Kenny JW, Young HL, Shintani DK, Kridl JC, Knauf VC (1991). Primary structures of the precursor and mature forms of stearoyl-acyl carrier protein desaturase from safflower embryos and requirement of ferredoxin for enzyme activity.Proc Natl Acad Sci USA 88, 2578-2582. |
| 34 | Uemura M, Steponkus PL (1997). Effect of cold acclimation on the lipid composition of the inner and outer membrane of the chloroplast envelope isolated from rye leaves.Plant Physiol 114, 1493-1500. |
| 35 | Whittle E, Cahoon EB, Subrahmanyam S, Shanklin J (2005). A multifunctional acyl-acyl carrier protein desaturase from Hedera helix L.(English ivy) can synthesize 16- and 18-carbon monoene and diene products. J Biol Chem 280, 28169-28176. |
| 36 | Yukawa Y, Takaiwa F, Shoji K, Masuda K, Yamada K (1996). Structure and expression of two seed-specific cDNA clones encoding stearoyl-acyl carrier protein desaturase from sesame,Sesamum indicum L. Plant Cell Phy- siol 37, 201-205. |
| 37 | Zhang P, Burton JW, Upchurch RG, Whittle E, Shanklin J, Dewey RE (2008). Mutations in a Δ9-stearoyl-acpdes- aturase gene are associated with enhanced stearic acid levels in soybean seeds.Crop Sci 48, 2305-2313. |
| [1] | 闫小红 胡文海. 亚热带地区3种常绿阔叶植物冬季光保护机制的差异[J]. 植物生态学报, 2025, 49(预发表): 0-0. |
| [2] | 欧阳子龙, 贾湘璐, 石景忠, 滕维超, 刘秀. 生长调节剂对低温胁迫及复温下红海榄幼苗光合特性的影响[J]. 植物生态学报, 2025, 49(4): 638-652. |
| [3] | 樊蓓, 任敏, 王延峰, 党峰峰, 陈国梁, 程国亭, 杨金雨, 孙会茹. 番茄SlWRKY45转录因子在响应低温和干旱胁迫中的功能(长英文摘要)[J]. 植物学报, 2025, 60(2): 186-203. |
| [4] | 高敏, 缑倩倩, 王国华, 郭文婷, 张宇, 张妍. 低温胁迫对不同母树年龄柠条锦鸡儿种子萌发幼苗生理和生长的影响[J]. 植物生态学报, 2024, 48(2): 201-214. |
| [5] | 师生波, 周党卫, 李天才, 德科加, 杲秀珍, 马家麟, 孙涛, 王方琳. 青藏高原高山嵩草光合功能对模拟夜间低温的响应[J]. 植物生态学报, 2023, 47(3): 361-373. |
| [6] | 师生波, 师瑞, 周党卫, 张雯. 低温对高山嵩草叶片光化学和非光化学能量耗散特征的影响[J]. 植物生态学报, 2023, 47(10): 1441-1452. |
| [7] | 陈奕竹, 郎伟光, 陈效逑. 中国北方树木秋季物候的过程模拟及其区域分异归因[J]. 植物生态学报, 2022, 46(7): 753-765. |
| [8] | 于海英, 杨莉琳, 付素静, 张志敏, 姚琦馥. 暖温带森林木本植物展叶始期对低温和热量累积变化的响应[J]. 植物生态学报, 2022, 46(12): 1573-1584. |
| [9] | 吴丹丹, 陈永坤, 杨宇, 孔春艳, 龚明. 小桐子半胱氨酸蛋白酶家族和相应miRNAs的鉴定及其对低温锻炼的响应[J]. 植物学报, 2021, 56(5): 544-558. |
| [10] | 张雅文, 梁山, 徐国云, 郭无瑕, 邓书林. 烟草CONSTANS-like基因家族的鉴定与分析[J]. 植物学报, 2021, 56(1): 33-43. |
| [11] | 杨小青,黄晓琴,韩晓阳,刘腾飞,岳晓伟,伊冉. 外源物质对茶树耐寒及蔗糖代谢关键基因表达的影响[J]. 植物学报, 2020, 55(1): 21-30. |
| [12] | 刘栋峰,唐永严,雒胜韬,罗伟,李志涛,种康,徐云远. 利用低温水浴鉴定水稻苗期耐寒性[J]. 植物学报, 2019, 54(4): 509-514. |
| [13] | 段志坤, 秦晓惠, 朱晓红, 宋纯鹏. 解析植物冷信号转导途径: 植物如何感知低温[J]. 植物学报, 2018, 53(2): 149-153. |
| [14] | 刘静妍, 施怡婷, 杨淑华. CBF: 平衡植物低温应答与生长发育的关键[J]. 植物学报, 2017, 52(6): 689-698. |
| [15] | 马骊, 孙万仓, 袁金海, 刘自刚, 武军艳, 方彦, 许耀照, 蒲媛媛, 白静, 董小云, 何辉立. 白菜型冬油菜β-1,3-葡聚糖酶基因在低温胁迫下的表达[J]. 植物学报, 2017, 52(5): 568-578. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||