

植物学报 ›› 2024, Vol. 59 ›› Issue (3): 397-413.DOI: 10.11983/CBB23130 cstr: 32102.14.CBB23130
刘笑1,2, 杜琬莹1,2, 张云秀2, 唐成名1,2, 李华伟2, 夏海勇2, 樊守金1, 孔令安1,2,*( )
)
收稿日期:2023-09-16
接受日期:2024-04-15
出版日期:2024-05-10
发布日期:2024-05-10
通讯作者:
孔令安
基金资助:
Xiao Liu1,2, Wanying Du1,2, Yunxiu Zhang2, Chengming Tang1,2, Huawei Li2, Haiyong Xia2, Shoujin Fan1, Ling’an Kong1,2,*( )
)
Received:2023-09-16
Accepted:2024-04-15
Online:2024-05-10
Published:2024-05-10
Contact:
Ling’an Kong
摘要: 该研究探讨了小麦(Triticum aestivum)根系NH4+毒性以及NO3-缓解其毒性机理。与7.5 mmol·L-1 NO3-处理(CK)相比, 7.5 mmol·L-1 NH4+处理(SA)抑制小麦根系生长, 添加1 mmol·L-1 NO3- (AN)后缓解了对根系生长的抑制。转录组分析表明, 与CK相比, SA处理下, 编码糖酵解途径酶、发酵关键酶、呼吸爆发性氧化酶同源物(Rbohs)、交替氧化酶(AOX)和双加氧酶相关基因显著上调表达; 编码TCA循环酶、ATP合酶和水通道蛋白(AQPs)相关基因显著下调表达。与SA相比, AN处理下, 糖酵解、发酵途径、Rbohs、AOX和双加氧酶相关基因的表达下调, 编码TCA循环酶、ATP合酶和AQPs的基因表达上调。蛋白质组分析表明, SA处理下, 糖酵解与发酵相关酶以及AOX相关基因表达上调, 而AQPs相关基因表达下调。AN处理下, 糖酵解与发酵相关酶以及AOX相关基因表达下调, AQPs相关基因表达上调。综上所述, 单独NH4+处理促进糖酵解和发酵途径, 抑制TCA循环, 能量生成减少, 最终抑制小麦根系生长, 这可能与NH4+处理引起根系缺O2胁迫有关。添加NO3-后抑制了糖酵解和发酵途径, 促进TCA循环和能量产生, 显著缓解了根系缺O2胁迫以及NH4+对根系生长的抑制。
刘笑, 杜琬莹, 张云秀, 唐成名, 李华伟, 夏海勇, 樊守金, 孔令安. NO3-缓解小麦根部NH4+毒性机理(长英文摘要). 植物学报, 2024, 59(3): 397-413.
Xiao Liu, Wanying Du, Yunxiu Zhang, Chengming Tang, Huawei Li, Haiyong Xia, Shoujin Fan, Ling’an Kong. Nitrate-dependent Alleviation of Root Ammonium Toxicity in Wheat (Triticum aestivum). Chinese Bulletin of Botany, 2024, 59(3): 397-413.
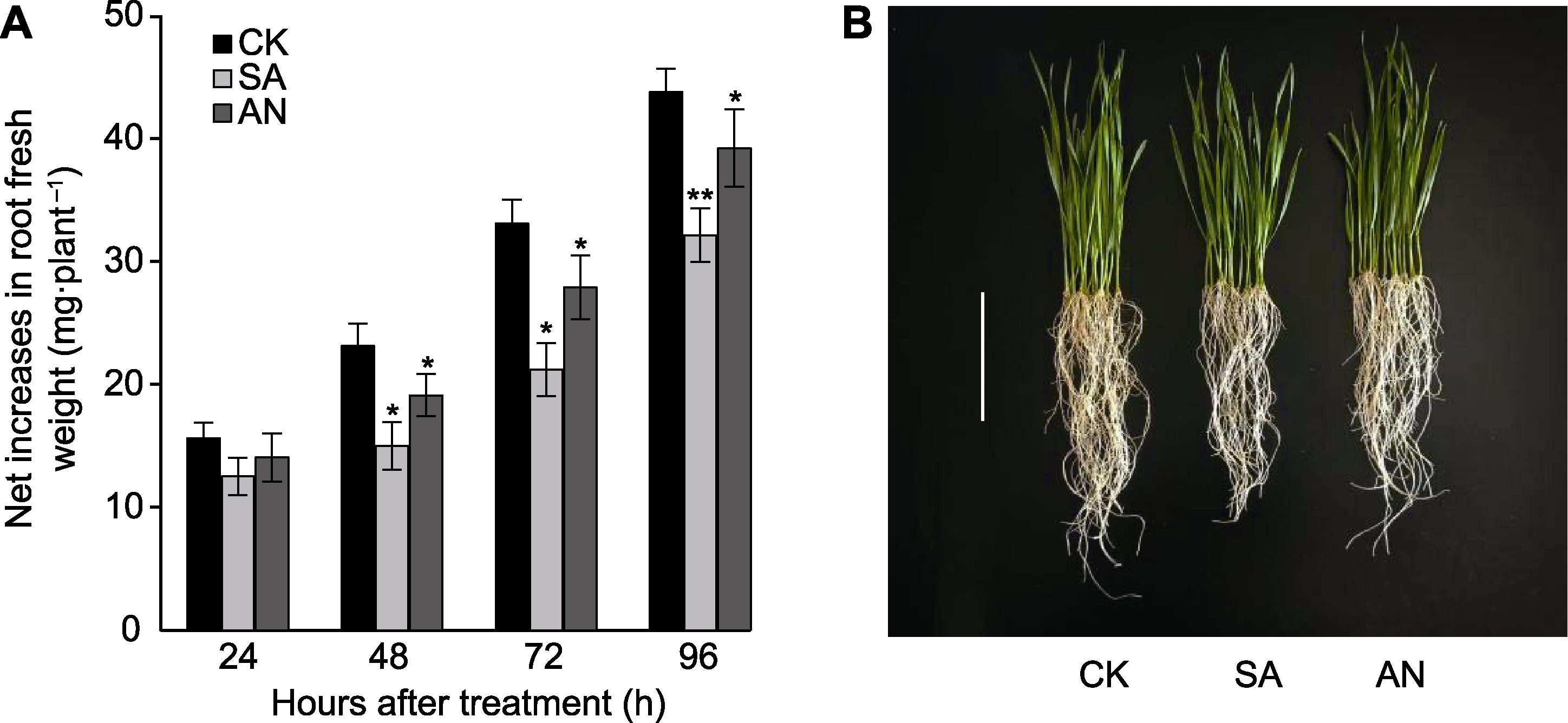
图1 不同态氮素处理对小麦幼苗根系生长的影响 (A) 不同态氮素处理24、48、72和96小时幼苗根系鲜重净增加量(结果为3次独立实验的平均值±标准差); (B) 不同态氮素处理48小时小麦幼苗根系表型(bar=10 cm)。CK: 7.5 mmol·L-1 NO3-处理; SA: 7.5 mmol·L-1 NH4+处理; AN: 7.5 mmol·L-1 NH4++1.0 mmol·L-1 NO3-处理。* P<0.05; ** P<0.01
Figure 1 Effects of different N treatments on the root growth of wheat seedlings (A) Net increases in root fresh weight of wheat seedlings at 24, 48, 72 and 96 h after different N treatments (data are means±SD of three independent experiments); (B) Phenotype of wheat seedling roots at 48 h after different N treatments (bar=10 cm). CK: 7.5 mmol·L-1 NO3- treatment; SA: 7.5 mmol·L-1 NH4+ treatment; AN: 7.5 mmol·L-1 NH4++1.0 mmol·L-1 NO3- treatment. * P<0.05; ** P<0.01
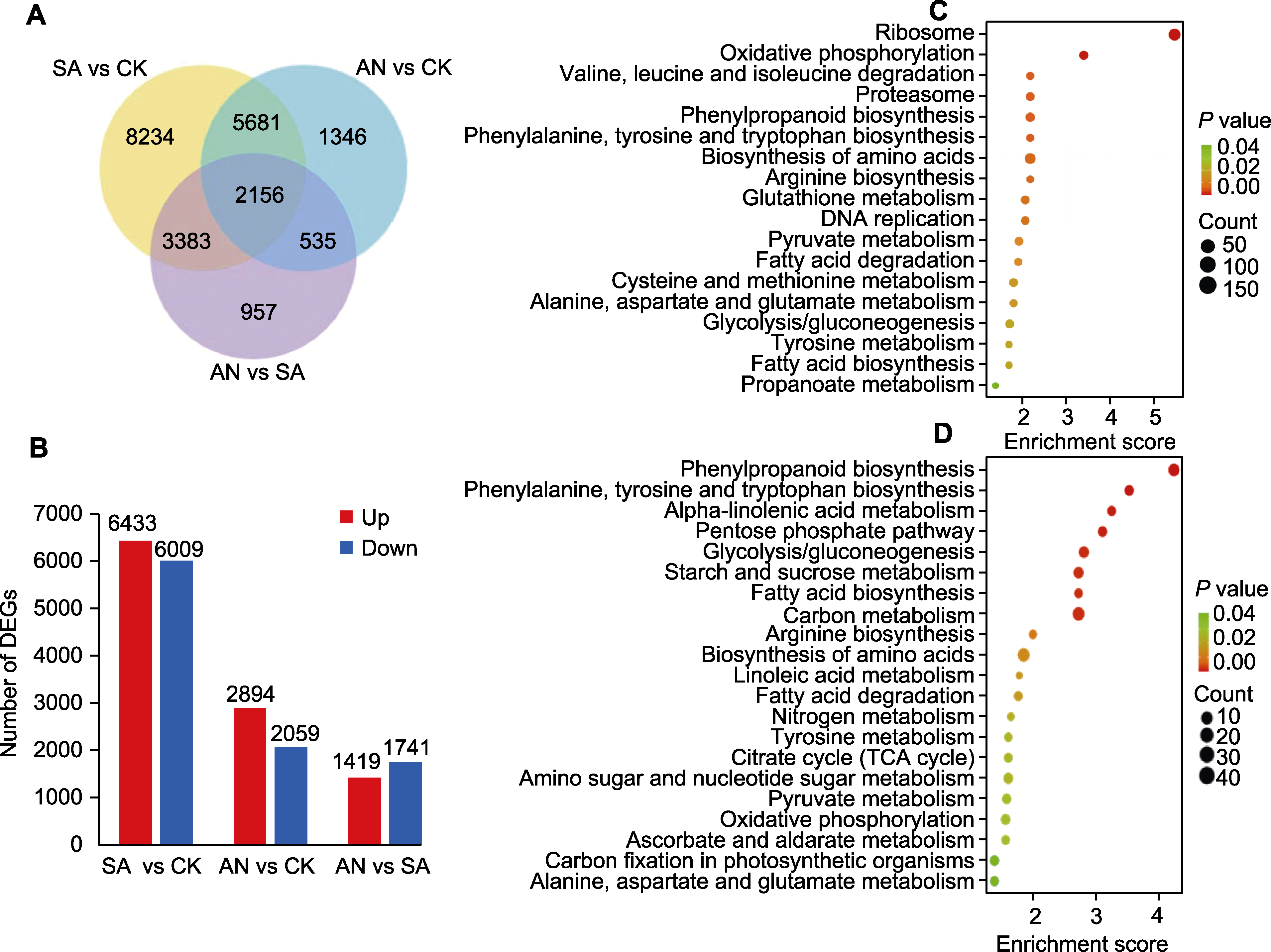
图2 不同态氮素处理间差异表达基因(DEGs)的成对比较 (A) 不同态氮素处理间DEGs的韦恩图; (B) 不同态氮素处理间DEGs的统计图; (C) SA与CK比较下DEGs的KEGG富集分析; (D) AN与SA比较下DEGs的KEGG富集分析。CK、SA和AN同图1。
Figure 2 Paired comparisons of differentially expressed genes (DEGs) among different N treatments (A) Venn diagram of DEGs under different N treatments; (B) Statistical map of DEGs under different N treatments; (C) KEGG enrichment analysis of DEGs from SA vs CK comparison; (D) KEGG enrichment analysis of DEGs from AN vs SA comparison. CK, SA, and AN are the same as shown in Figure 1.
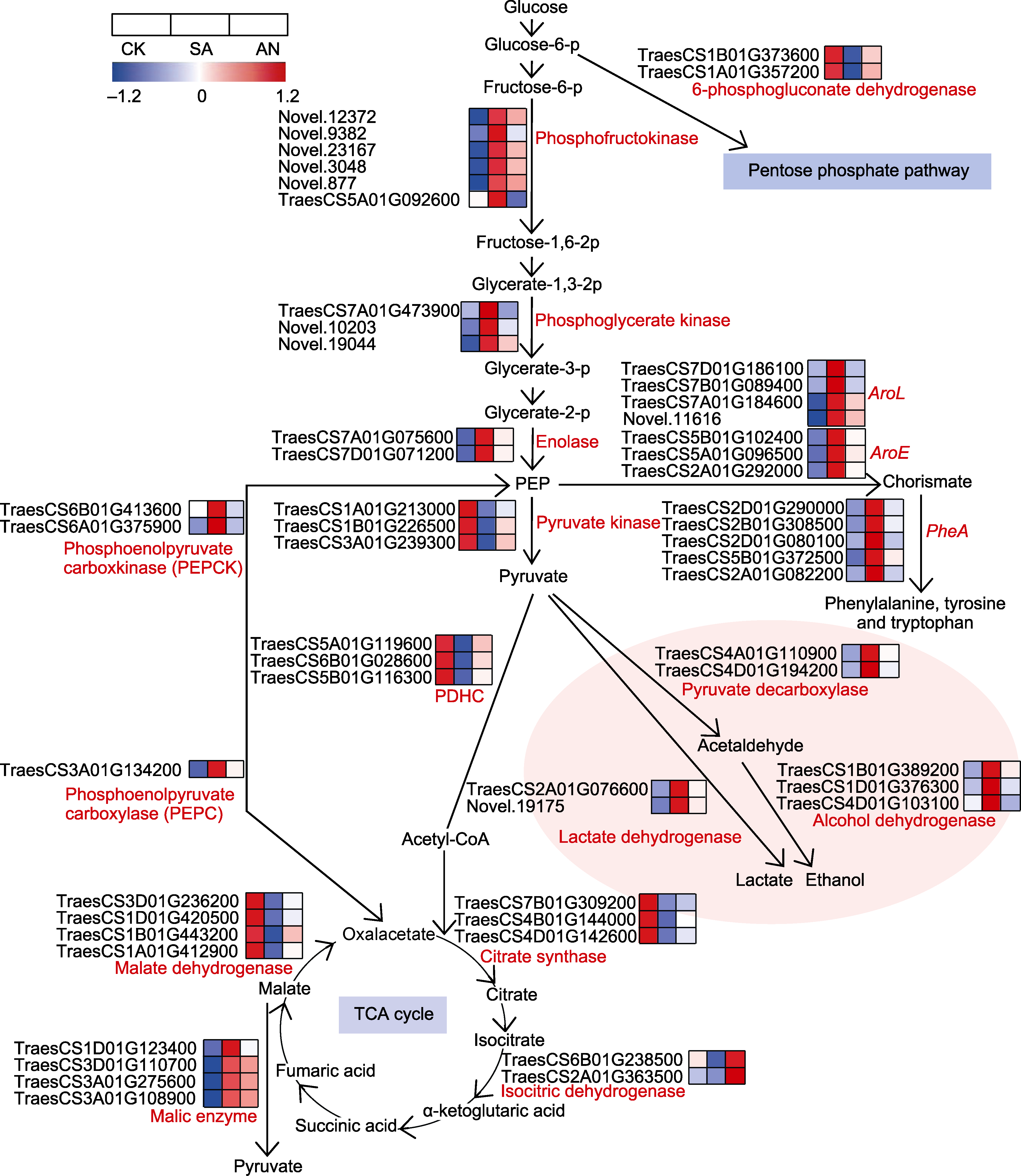
图3 不同态氮素处理下小麦根系与糖酵解、磷酸戊糖途径、丙酮酸代谢、TCA循环、发酵和莽草酸途径相关DEGs转录水平变化 热图中方格从左到右依次表示CK、SA和AN处理下该基因的表达水平; 彩色标尺为基因在3个不同处理下的相对表达量, 蓝色代表表达量较低, 红色代表表达量较高。CK、SA和AN同图1。
Figure 3 Changes in transcription level of DEGs involved in glycolysis, pentose phosphate pathway, pyruvate metabolism, TCA cycle, fermentation and shikimate pathway under different N treatments in the roots of wheat seedlings Squares from left to right indicate the expression level of this gene under CK, SA and AN treatments; the color scale indicates the relative gene expression, with blue color indicating lower expression and red color indicating higher expression. CK, SA, and AN are the same as shown in Figure 1.

图4 不同态氮素处理下小麦根系编码ATP合酶的差异表达基因(DEGs)转录水平变化 彩色标尺为基因在3个不同处理下的相对表达量, 蓝色代表表达量较低, 红色代表表达量较高。CK、SA和AN同图1。
Figure 4 Changes in transcription level of differentially expressed genes (DEGs) encoding ATP synthases under different N treatments in wheat roots The color scale indicates the relative gene expression, with blue color indicating lower expression and red color indicating higher expression. CK, SA, and AN are the same as shown in Figure 1.
| Gene ID | Gene relative expression level  | Gene description | Species | Reference |
|---|---|---|---|---|
| Respiratory burst oxidase homologs | ||||
| TraesCS5D01G105900 |  | Respiratory burst oxidase homolog protein B-like | Zea mays | Mira et al., |
| TraesCS1D01G284800 | Respiratory burst oxidase homolog protein B-like | Nicotiana tabacum | Zafari et al., | |
| TraesCS5A01G093600 | Respiratory burst oxidase homolog protein B-like | |||
| Alternative pathway | ||||
| TraesCS2A01G439400 | 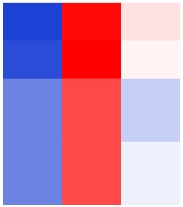 | Mitochondrial alternative oxidase | N. tabacum | Zafari et al., |
| TraesCS2B01G459300 | Mitochondrial alternative oxidase | |||
| TraesCS2D01G212500 | Internal alternative NADPH-ubiquinone oxidoreductase A1, mitochondrial-like | Z. mays | Igamberdiev and Hill, | |
| TraesCS3D01G314700 | PREDICTED: external alternative NADPH-ubiquinone oxidoreductase B3, mitochondrial-like | |||
| O2-binding and Fe transport | ||||
| TraesCS1D01G340600 | 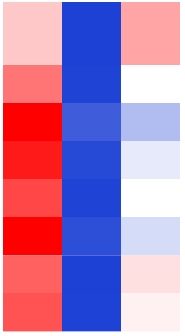 | Non-symbiotic hemoglobin-like isoform X1 | Oryza sativa | Narsai and Whelan, |
| TraesCS1B01G350800 | Non-symbiotic hemoglobin-like isoform X1 | |||
| TraesCS1A01G338400 | Non-symbiotic hemoglobin-like isoform X2 | |||
| TraesCS5A01G552000 | Nicotianamine synthase | Citrus junos | Xie et al., | |
| TraesCS6B01G425200 | Nicotianamine synthase | |||
| TraesCS6D01G148200 | Nicotianamine synthase | |||
| TraesCS2A01G049900 | Nicotianamine synthase | |||
| TraesCS6D01G382900 | Nicotianamine synthase-like 5 protein | |||
| Aquaporin | ||||
| TraesCS6A01G222100 |  | Probable aquaporin PIP2-2 | Glycine max | Matsuo et al., |
| TraesCS6D01G212900 | 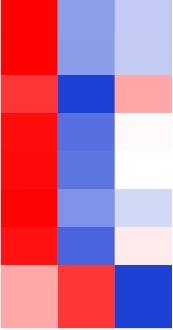 | Probable aquaporin PIP2-2 | Sorghum bicolor | Kadam et al., |
| TraesCS2A01G407700 | Aquaporin PIP1-2 | |||
| TraesCS2D01G404800 | Aquaporin PIP1-2 | |||
| TraesCS2B01G077700 | Probable aquaporin PIP2-6 | |||
| TraesCS2D01G063900 | Probable aquaporin PIP2-6 | |||
| TraesCS6A01G405600 | Aquaporin PIP1-5-like | |||
| TraesCS2A01G198500 | Aquaporin PIP2-1 | |||
| TraesCS5D01G347600 | Nodulin-26 like intrinsic protein | Arabidopsis thaliana | Beamer et al., | |
| Ethylene signaling | ||||
| TraesCS2A01G026500 | 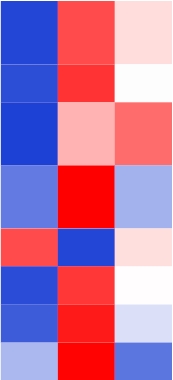 | 1-aminocyclopropane-1-carboxylate oxidase homolog 1-like | Cucumis sativus | Kęska et al., |
| TraesCS5A01G234200 | 1-aminocyclopropane-1-carboxylate oxidase 1-like | N. tabacum | Zafari et al., | |
| TraesCS7B01G110400 | 1-aminocyclopropane-1-carboxylate oxidase homolog 4-like | |||
| TraesCS2D01G028500 | 1-aminocyclopropane-1-carboxylate oxidase homolog 1-like | |||
| TraesCS4A01G221300 | 1-aminocyclopropane-1-carboxylate oxidase | |||
| TraesCS7B01G162100 | Ethylene-responsive transcription factor RAP2-6-like | A. thaliana | Hess et al., | |
| TraesCS6D01G281200 | Ethylene-responsive transcription factor RAP2-4-like | |||
| TraesCS1B01G243200 | Ethylene-responsive transcription factor RAP2-3-like | |||
| S-type anion channel | ||||
| TraesCS3A01G225100 |  | S-type anion channel SLAH3 | A. thaliana | Lehmann et al., |
| Allene oxide synthase (alpha-linolenic acid metabolism) | ||||
| TraesCS6D01G172200 |  | Allene oxide synthase 4-like | A. thaliana | Xie et al., |
| TraesCS5B01G408000 | Allene oxide synthase 1 |
表1 小麦根系中与缺O2胁迫相关的基因
Table 1 Genes related to hypoxic stress in the roots of wheat
| Gene ID | Gene relative expression level  | Gene description | Species | Reference |
|---|---|---|---|---|
| Respiratory burst oxidase homologs | ||||
| TraesCS5D01G105900 |  | Respiratory burst oxidase homolog protein B-like | Zea mays | Mira et al., |
| TraesCS1D01G284800 | Respiratory burst oxidase homolog protein B-like | Nicotiana tabacum | Zafari et al., | |
| TraesCS5A01G093600 | Respiratory burst oxidase homolog protein B-like | |||
| Alternative pathway | ||||
| TraesCS2A01G439400 |  | Mitochondrial alternative oxidase | N. tabacum | Zafari et al., |
| TraesCS2B01G459300 | Mitochondrial alternative oxidase | |||
| TraesCS2D01G212500 | Internal alternative NADPH-ubiquinone oxidoreductase A1, mitochondrial-like | Z. mays | Igamberdiev and Hill, | |
| TraesCS3D01G314700 | PREDICTED: external alternative NADPH-ubiquinone oxidoreductase B3, mitochondrial-like | |||
| O2-binding and Fe transport | ||||
| TraesCS1D01G340600 |  | Non-symbiotic hemoglobin-like isoform X1 | Oryza sativa | Narsai and Whelan, |
| TraesCS1B01G350800 | Non-symbiotic hemoglobin-like isoform X1 | |||
| TraesCS1A01G338400 | Non-symbiotic hemoglobin-like isoform X2 | |||
| TraesCS5A01G552000 | Nicotianamine synthase | Citrus junos | Xie et al., | |
| TraesCS6B01G425200 | Nicotianamine synthase | |||
| TraesCS6D01G148200 | Nicotianamine synthase | |||
| TraesCS2A01G049900 | Nicotianamine synthase | |||
| TraesCS6D01G382900 | Nicotianamine synthase-like 5 protein | |||
| Aquaporin | ||||
| TraesCS6A01G222100 |  | Probable aquaporin PIP2-2 | Glycine max | Matsuo et al., |
| TraesCS6D01G212900 |  | Probable aquaporin PIP2-2 | Sorghum bicolor | Kadam et al., |
| TraesCS2A01G407700 | Aquaporin PIP1-2 | |||
| TraesCS2D01G404800 | Aquaporin PIP1-2 | |||
| TraesCS2B01G077700 | Probable aquaporin PIP2-6 | |||
| TraesCS2D01G063900 | Probable aquaporin PIP2-6 | |||
| TraesCS6A01G405600 | Aquaporin PIP1-5-like | |||
| TraesCS2A01G198500 | Aquaporin PIP2-1 | |||
| TraesCS5D01G347600 | Nodulin-26 like intrinsic protein | Arabidopsis thaliana | Beamer et al., | |
| Ethylene signaling | ||||
| TraesCS2A01G026500 |  | 1-aminocyclopropane-1-carboxylate oxidase homolog 1-like | Cucumis sativus | Kęska et al., |
| TraesCS5A01G234200 | 1-aminocyclopropane-1-carboxylate oxidase 1-like | N. tabacum | Zafari et al., | |
| TraesCS7B01G110400 | 1-aminocyclopropane-1-carboxylate oxidase homolog 4-like | |||
| TraesCS2D01G028500 | 1-aminocyclopropane-1-carboxylate oxidase homolog 1-like | |||
| TraesCS4A01G221300 | 1-aminocyclopropane-1-carboxylate oxidase | |||
| TraesCS7B01G162100 | Ethylene-responsive transcription factor RAP2-6-like | A. thaliana | Hess et al., | |
| TraesCS6D01G281200 | Ethylene-responsive transcription factor RAP2-4-like | |||
| TraesCS1B01G243200 | Ethylene-responsive transcription factor RAP2-3-like | |||
| S-type anion channel | ||||
| TraesCS3A01G225100 |  | S-type anion channel SLAH3 | A. thaliana | Lehmann et al., |
| Allene oxide synthase (alpha-linolenic acid metabolism) | ||||
| TraesCS6D01G172200 |  | Allene oxide synthase 4-like | A. thaliana | Xie et al., |
| TraesCS5B01G408000 | Allene oxide synthase 1 |
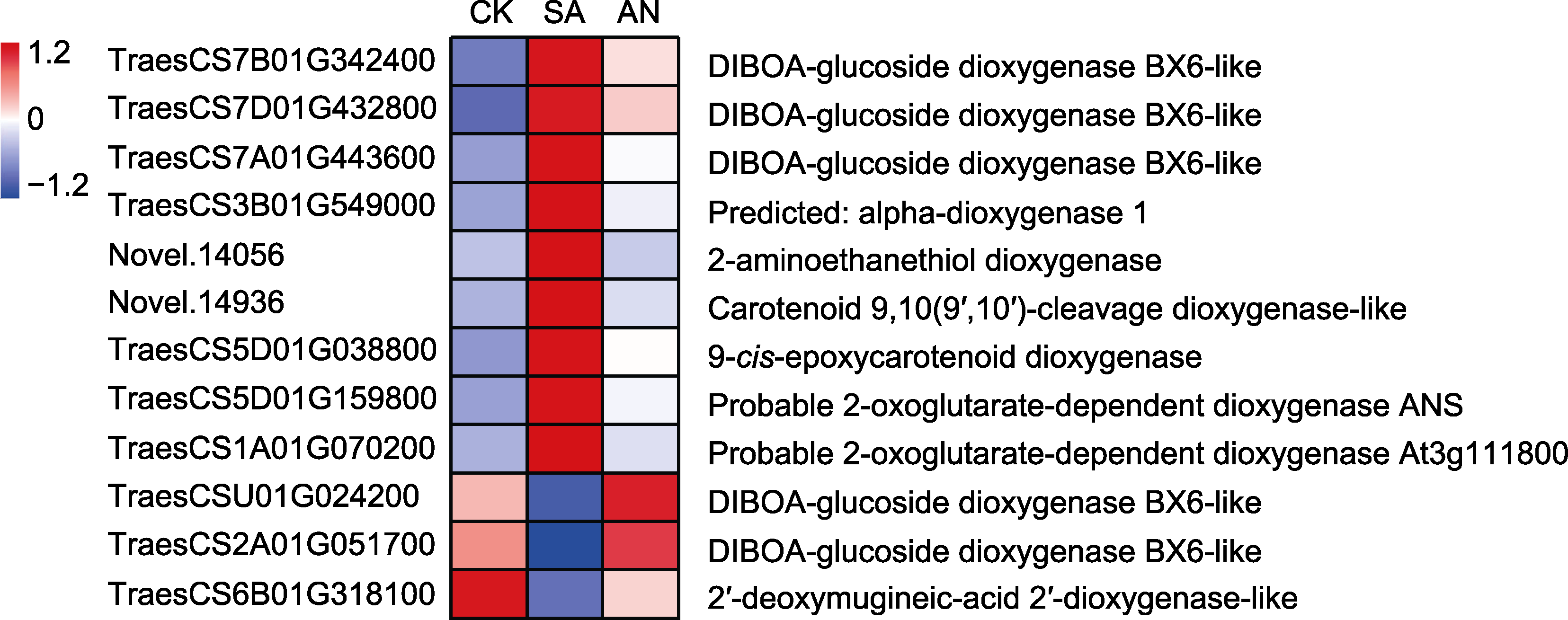
图5 不同态氮素处理下小麦根系编码双加氧酶的差异表达基因(DEGs)转录水平变化 彩色标尺为基因在3个不同处理下的相对表达量, 蓝色代表表达量较低, 红色代表表达量较高。CK、SA和AN同图1。
Figure 5 Changes in transcription levels of differentially expressed genes (DEGs) encoding kinds of dioxygenases under different N treatments in roots of wheat seedlings The color scale indicates the relative gene expression, with blue color indicating lower expression and red color indicating higher expression. CK, SA, and AN are the same as shown in Figure 1.
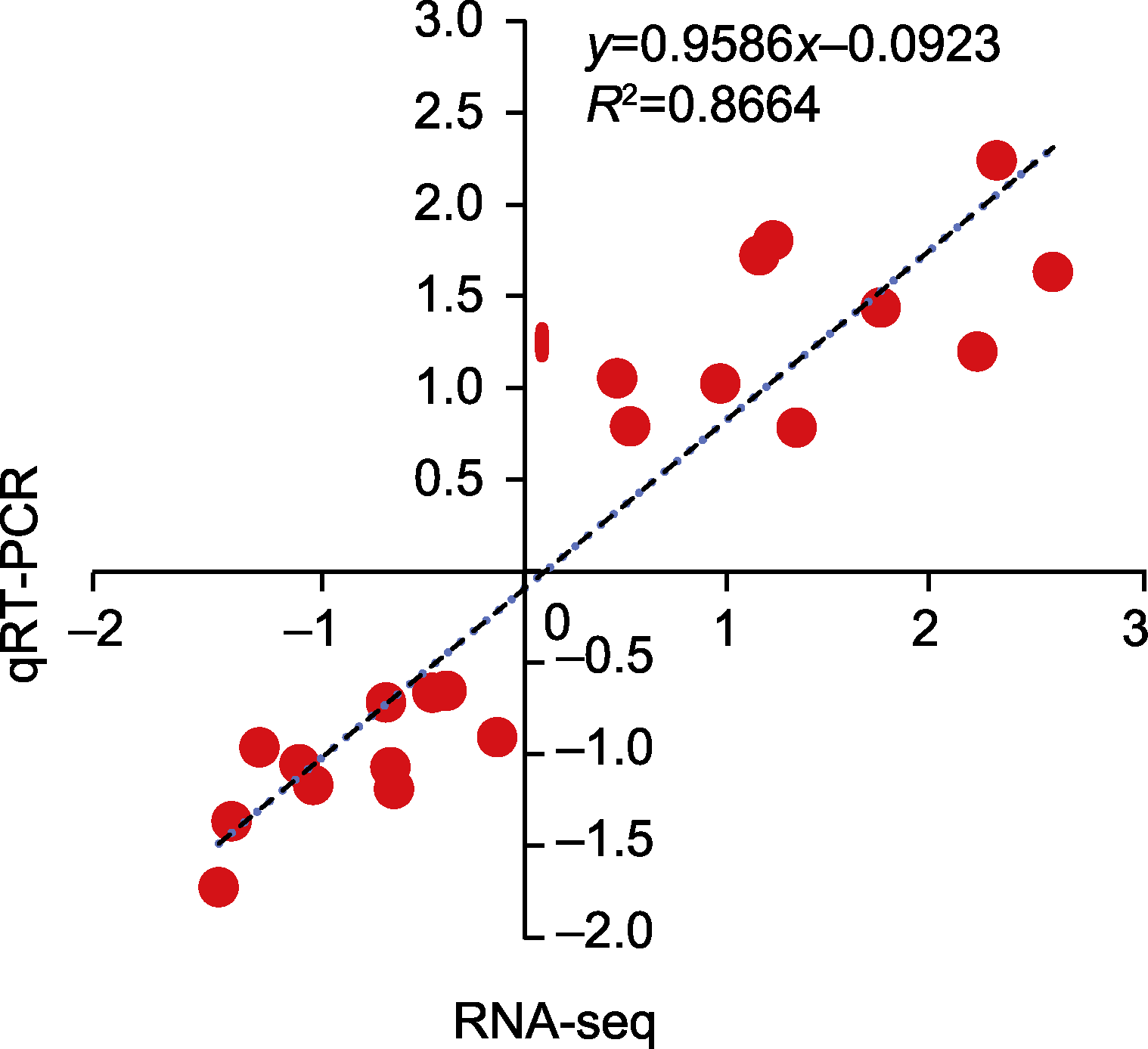
图6 RNA-seq数据的准确性验证 横坐标轴和纵坐标轴分别表示SA与CK和AN与SA比较下qRT- PCR的2-ΔΔCT值和RNA-seq的log2(FC)值。CK、SA和AN同图1。
Figure 6 RNA-seq data accuracy verification The X-axis and Y-axis represent the 2-ΔΔCT values for qRT- PCR analysis and the log2(FC) values for RNA-seq for comparisons of SA vs CK and AN vs SA, respectively. CK, SA, and AN are the same as shown in Figure 1.
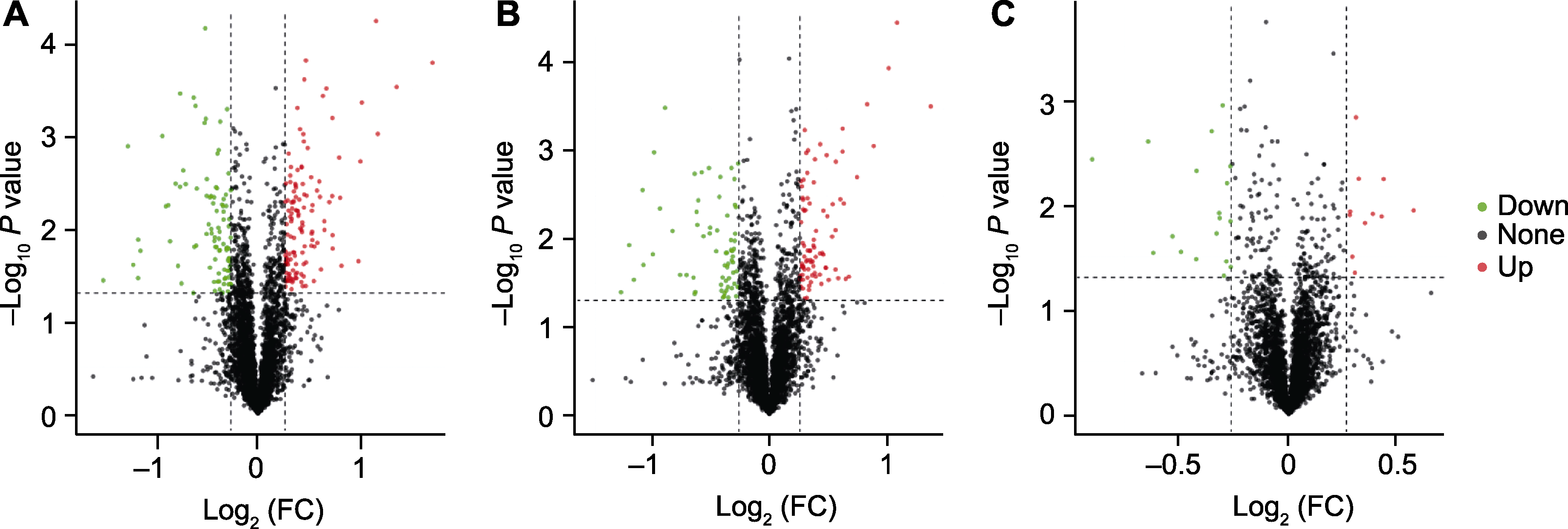
图7 不同态氮素处理下小麦根系差异表达蛋白(DEPs)火山图 (A) SA与CK比较; (B) AN与SA比较; (C) AN与CK比较。绿色表示蛋白表达下调, 红色表示蛋白表达上调, 黑色表示蛋白表达无显著变化。FC: 差异倍数。CK、SA和AN同图1。
Figure 7 Volcano plots of differentially expressed proteins (DEPs) under different N treatments in the roots of wheat seedlings (A) SA vs CK; (B) AN vs SA; (C) AN vs CK. Green dots indicate downregulated proteins, red dots indicate upregulated proteins and black dots indicate not significantly changed proteins. FC: Fold change. CK, SA, and AN are the same as shown in Figure 1.
| Protein ID | Protein expression | Protein description | ||
|---|---|---|---|---|
| Glycolysis | ||||
| CDM81680.1 | 6-phosphofructokinase 1 | |||
| SPT18714.1 | 6-phosphofructokinase 1 | |||
| AAO32641.1 | Phosphoglycerate kinase | |||
| CAA33303.1 | Phosphoglycerate kinase | |||
| CBN64599.1 | Phosphoglycerate kinase | |||
| CBG02671.1 | Pyruvate kinase | |||
| CBG02673.1 | Pyruvate kinase | |||
| CDM82952.1 | Pyruvate kinase | |||
| Pyruvate metabolism | ||||
| AIV42031.1 | Pyruvate dehydrogenase E2 component | |||
| CCA64976.1 | Pyruvate dehydrogenase E1 component beta subunit | |||
| SPT15511.1 | Pyruvate dehydrogenase E1 component alpha subunit | |||
| CAW61069.1 | Phosphoenolpyruvate carboxylase | |||
| CDM84220.1 | Phosphoenolpyruvate carboxylase | |||
| ABW77317.1 | Malic enzyme | |||
| SPT17588.1 | Malic enzyme | |||
| CDM81737.1 | Malic enzyme | |||
| Fermentation | ||||
| CBM36708.1 | Pyruvate decarboxylase | |||
| CBM36829.1 | Pyruvate decarboxylase | |||
| ABL74255.1 | Alcohol dehydrogenase class-P | |||
| ABL74259.1 | Alcohol dehydrogenase class-P | |||
| ABL74258.1 | Alcohol dehydrogenase class-P | |||
| SPT16036.1 | L-lactate dehydrogenase | |||
| Alternative pathway | ||||
| SPT19754.1 | Alternative oxidase | |||
| Aquaporin | ||||
| ABI96816.1 | Aquaporin TIP | |||
| BAP33940.1 | Aquaporin TIP | |||
| SPT19521.1 | Aquaporin TIP | |||
| AAD10494.1 | Aquaporin TIP | |||
| ACZ51371.1 | Aquaporin PIP | |||
| AEO13898.1 | Aquaporin PIP | |||
| Fe transport | ||||
| AND77086.1 | Nicotianamine synthase | |||
| AHG95976.1 | Nicotianamine synthase | |||
| AND77090.1 | Nicotianamine synthase | |||
| AND77092.1 | Nicotianamine synthase | |||
表2 不同态氮素处理下小麦根系能量代谢与低O2胁迫相关差异表达蛋白(DEPs)
Table 2 Differentially expressed proteins (DEPs) related to hypoxia and energy metabolism under different N treatments in wheat roots
| Protein ID | Protein expression | Protein description | ||
|---|---|---|---|---|
| Glycolysis | ||||
| CDM81680.1 | 6-phosphofructokinase 1 | |||
| SPT18714.1 | 6-phosphofructokinase 1 | |||
| AAO32641.1 | Phosphoglycerate kinase | |||
| CAA33303.1 | Phosphoglycerate kinase | |||
| CBN64599.1 | Phosphoglycerate kinase | |||
| CBG02671.1 | Pyruvate kinase | |||
| CBG02673.1 | Pyruvate kinase | |||
| CDM82952.1 | Pyruvate kinase | |||
| Pyruvate metabolism | ||||
| AIV42031.1 | Pyruvate dehydrogenase E2 component | |||
| CCA64976.1 | Pyruvate dehydrogenase E1 component beta subunit | |||
| SPT15511.1 | Pyruvate dehydrogenase E1 component alpha subunit | |||
| CAW61069.1 | Phosphoenolpyruvate carboxylase | |||
| CDM84220.1 | Phosphoenolpyruvate carboxylase | |||
| ABW77317.1 | Malic enzyme | |||
| SPT17588.1 | Malic enzyme | |||
| CDM81737.1 | Malic enzyme | |||
| Fermentation | ||||
| CBM36708.1 | Pyruvate decarboxylase | |||
| CBM36829.1 | Pyruvate decarboxylase | |||
| ABL74255.1 | Alcohol dehydrogenase class-P | |||
| ABL74259.1 | Alcohol dehydrogenase class-P | |||
| ABL74258.1 | Alcohol dehydrogenase class-P | |||
| SPT16036.1 | L-lactate dehydrogenase | |||
| Alternative pathway | ||||
| SPT19754.1 | Alternative oxidase | |||
| Aquaporin | ||||
| ABI96816.1 | Aquaporin TIP | |||
| BAP33940.1 | Aquaporin TIP | |||
| SPT19521.1 | Aquaporin TIP | |||
| AAD10494.1 | Aquaporin TIP | |||
| ACZ51371.1 | Aquaporin PIP | |||
| AEO13898.1 | Aquaporin PIP | |||
| Fe transport | ||||
| AND77086.1 | Nicotianamine synthase | |||
| AHG95976.1 | Nicotianamine synthase | |||
| AND77090.1 | Nicotianamine synthase | |||
| AND77092.1 | Nicotianamine synthase | |||
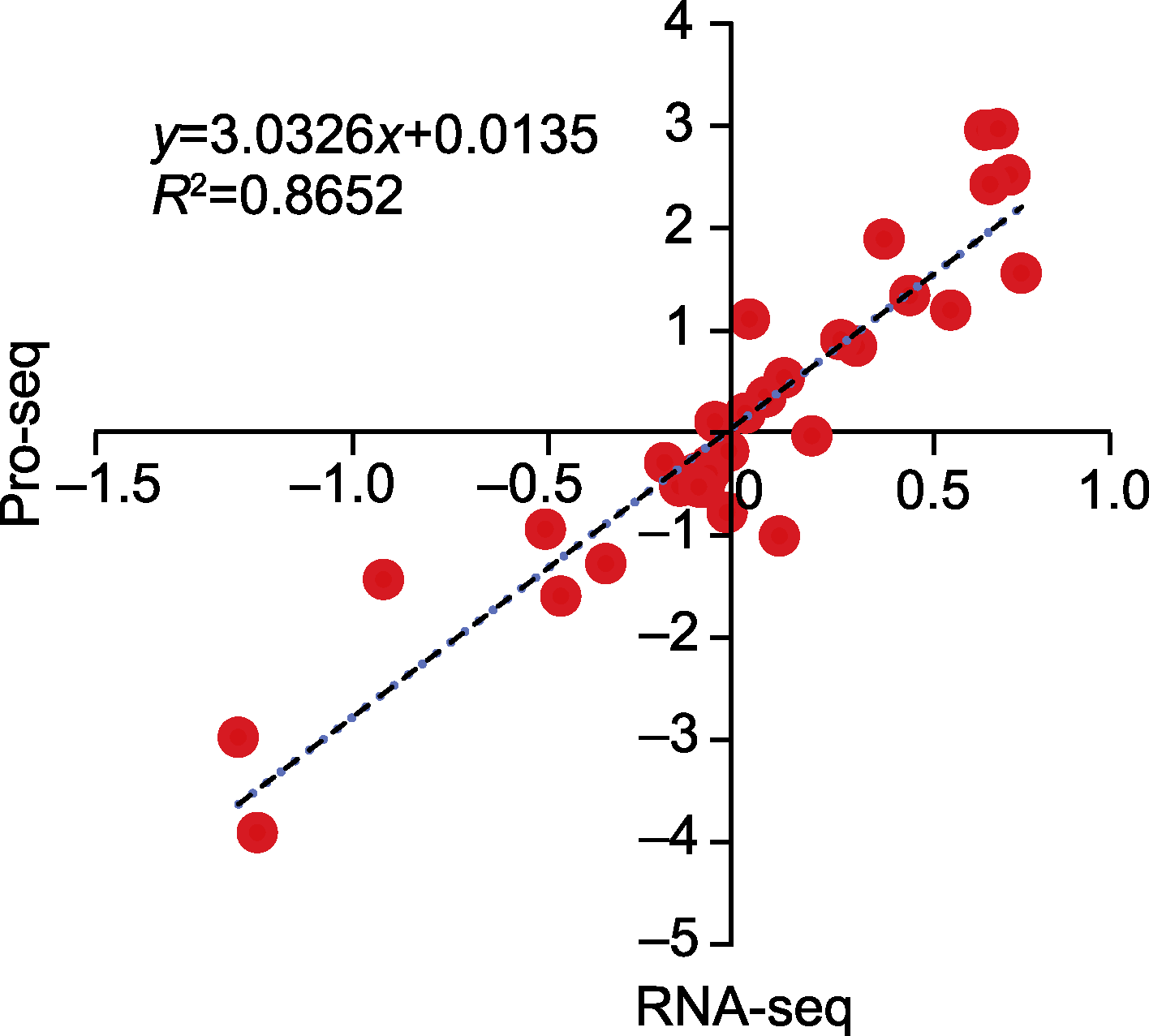
图8 蛋白质组和转录组测序数据相关性分析 横坐标轴和纵坐标轴分别表示SA与CK和AN与SA比较下基因和蛋白表达差异log2(FC)值。FC同图7。CK、SA和AN同图1。
Figure 8 Correlation analysis of proteomic and transcriptomic sequencing data The X-axis and Y-axis represent the log2(FC) values from paired comparison of SA vs CK and AN vs SA for gene and protein expressions, respectively. FC is the same as shown in Figure 7. CK, SA, and AN are the same as shown in Figure 1.
| [1] | Ariz I, Artola E, Asensio AC, Cruchaga S, Aparicio-Tejo PM, Moran JF (2011). High irradiance increases NH4+ tolerance in Pisum sativum: higher carbon and energy availability improve ion balance but not N assimilation. J Plant Physiol 168, 1009-1015. |
| [2] | Beamer ZG, Routray P, Choi WG, Spangler MK, Lokdarshi A, Roberts DM (2021). Aquaporin family lactic acid channel NIP2;1 promotes plant survival under low oxygen stress in Arabidopsis. Plant Physiol 187, 2262-2278. |
| [3] |
Bradford MM (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72, 248-254.
PMID |
| [4] | Britto DT, Kronzucker HJ (2002). NH4+ toxicity in higher plants: a critical review. J Plant Physiol 159, 567-584. |
| [5] | Britto DT, Kronzucker HJ (2005). Nitrogen acquisition, PEP carboxylase, and cellular pH homeostasis: new views on old paradigms. Plant Cell Environ 28, 1396-1409. |
| [6] | Damerval C, De Vienne D, Zivy M, Thiellement H (1986). Technical improvements in two-dimensional electrophoresis increase the level of genetic variation detected in wheat-seedling proteins. Electrophoresis 7, 52-54. |
| [7] | Di DW, Wu JJ, Ma MK, Li GJ, Wang M, Kronzucker HJ, Shi WM (2023). PIN5 is involved in regulating NH4+ efflux and primary root growth under high-ammonium stress via mediating intracellular auxin transport. Plant Soil doi:10. 1007/s11104-023-05869-z. |
| [8] | Diab H, Limami AM (2016). Reconfiguration of N metabolism upon hypoxia stress and recovery: roles of alanine aminotransferase (AlaAT) and glutamate dehydrogenase (GDH). Plants 5(2), 25. |
| [9] | Du WY, Zhang YX, Si JS, Zhang Y, Fan SJ, Xia HY, Kong LA (2021). Nitrate alleviates ammonium toxicity in wheat (Triticum aestivum L.) by regulating tricarboxylic acid cycle and reducing rhizospheric acidification and oxidative damage. Plant Signal Behav 16, 1991687. |
| [10] |
Esteban R, Ariz I, Cruz C, Moran JF (2016). Review: mechanisms of ammonium toxicity and the quest for tolerance. Plant Sci 248, 92-101.
DOI PMID |
| [11] | Feng XM, Gao X, Zang HD, Hu YG, Ren CZ, Hao ZP, Lü HQ, Zeng ZH (2023). Intercropping effect and nitrogen transfer characteristics of oat-mungbean intercrop. Chin Bull Bot 58, 122-131. (in Chinese) |
|
冯晓敏, 高翔, 臧华栋, 胡跃高, 任长忠, 郝志萍, 吕慧卿, 曾昭海 (2023). 燕麦-绿豆间作效应及氮素转移特性. 植物学报 58, 122-131.
DOI |
|
| [12] |
Hachiya T, Noguchi K (2011). Integrative response of plant mitochondrial electron transport chain to nitrogen source. Plant Cell Rep 30, 195-204.
DOI PMID |
| [13] | Hartman MD, Rojas BE, Iglesias AA, Figueroa CM (2023). The involvement of allosteric effectors and post-translational modifications in the control of plant central carbon metabolism. Plant J 114, 1037-1058. |
| [14] | Heckwolf M, Pater D, Hanson DT, Kaldenhoff R (2011). The Arabidopsis thaliana aquaporin AtPIP1;2 is a physiologically relevant CO2 transport facilitator. Plant J 67, 795-804. |
| [15] | Hess N, Klode M, Anders M, Sauter M (2011). The hypoxia responsive transcription factor genes ERF71/HRE2 and ERF73/HRE1of Arabidopsis are differentially regulated by ethylene. Physiol Plant 143, 41-49. |
| [16] | Hoffmann A, Milde S, Desel C, Hümpel A, Kaiser H, Hammes E, Piippo M, Soitamo A, Aro EM, Gerendás J, Sattelmacher B, Hansen UP (2007). N form-dependent growth retardation of Arabidopsis thaliana seedlings as revealed from physiological and microarray studies. J Plant Nutr Soil Sci 170, 87-97. |
| [17] | Huang HM, Gao YK, Tai YY, Liu C, Qu DJ, Tang RH, Wang YN (2023). Research advances in elucidating the function and molecular mechanism of the nitrate transporter 2 (NRT2) proteins in plants. Chin Bull Bot 58, 783-798. (in Chinese) |
|
黄慧梅, 高永康, 台玉莹, 刘超, 曲德杰, 汤锐恒, 王幼宁 (2023). 硝酸盐转运蛋白NRT2在植物中的功能及分子机制研究进展. 植物学报 58, 783-798.
DOI |
|
| [18] |
Iacopino S, Licausi F (2020). The contribution of plant dioxygenases to hypoxia signaling. Front Plant Sci 11, 1008.
DOI PMID |
| [19] |
Igamberdiev AU, Hill RD (2018). Elevation of cytosolic Ca2+ in response to energy deficiency in plants: the general mechanism of adaptation to low oxygen stress. Biochem J 475, 1411-1425.
DOI PMID |
| [20] | Kadam S, Abril A, Dhanapal AP, Koester RP, Vermerris W, Jose S, Fritschi FB (2017). Characterization and regulation of aquaporin genes of sorghum [Sorghum bicolor (L.) Moench] in response to waterlogging stress. Front Plant Sci 8, 862. |
| [21] | Kedar P, Colah R, Shimizu K (2007). Proteomic investigation on the pyk-F gene knockout Escherichia coli for aromatic amino acid production. Enzyme Microb Technol 41, 455-465. |
| [22] | Kęska K, Szcześniak MW, Makałowska I, Czernicka M (2021). Long-term waterlogging as factor contributing to hypoxia stress tolerance enhancement in cucumber: com- parative transcriptome analysis of waterlogging sensitive and tolerant accessions. Genes 12, 189. |
| [23] |
Kong LA, Zhang YX, Zhang B, Li HW, Wang ZS, Si JS, Fan SJ, Feng B (2022). Does energy cost constitute the primary cause of ammonium toxicity in plants? Planta 256, 62.
DOI PMID |
| [24] | Lehmann J, Jørgensen ME, Fratz S, Müller HM, Kusch J, Scherzer S, Navarro-Retamal C, Mayer D, Böhm J, Konrad KR, Terpitz U, Dreyer I, Mueller TD, Sauer M, Hedrich R, Geiger D, Maierhofer T (2021). Acidosis-induced activation of anion channel SLAH3 in the flooding-related stress response of Arabidopsis. Curr Biol 31, 3575-3585. |
| [25] | Matsuo N, Nanjo Y, Tougou M, Nakamura T, Nishizawa K, Komatsu S, Shimamura S (2012). Identification of putative aquaporin genes and their expression analysis under hypoxic conditions in soybean [Glycine max (L.) Merr.]. Plant Prod Sci 15, 278-283. |
| [26] |
Mira MM, Hill RD, Stasolla C (2016). Phytoglobins improve hypoxic root growth by alleviating apical meristem cell death. Plant Physiol 172, 2044-2056.
PMID |
| [27] |
Narsai R, Whelan J (2013). How unique is the low oxygen response? An analysis of the anaerobic response during germination and comparison with abiotic stress in rice and Arabidopsis. Front Plant Sci 4, 349.
DOI PMID |
| [28] |
O’Leary B, Plaxton WC (2020). Multifaceted functions of post-translational enzyme modifications in the control of plant glycolysis. Curr Opin Plant Biol 55, 28-37.
DOI PMID |
| [29] | Ou XH, Li SP, Liao PR, Cui XM, Zheng BL, Yang Y, Liu DH, Zheng Y (2019). The transcriptome variations of Panax notoginseng roots treated with different forms of nitrogen fertilizers. BMC Genomics 20, 965. |
| [30] | Patel MK, Pandey S, Burritt DJ, Tran LSP (2019). Plant responses to low-oxygen stress: interplay between ROS and NO signaling pathways. Environ Exp Bot 161, 134-142. |
| [31] | Pfaffl MW, Horgan GW, Dempfle L (2002). Relative expression software tool (REST©) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res 30, e36. |
| [32] | Prasad K, Reddy TSK, Sharma A (2022). Pollination, emasculation and hybridization methods in wheat: a review. Pharm Innov J SP-11, 1087-1091. |
| [33] | Ricoult C, Cliquet JB, Limami AM (2005). Stimulation of alanine amino transferase (AlaAT) gene expression and alanine accumulation in embryo axis of the model legume Medicago truncatula contribute to anoxia stress tolerance. Physiol Plant 123, 30-39. |
| [34] | Roosta HR, Schjoerring JK (2008). Effects of nitrate and potassium on ammonium toxicity in cucumber plants. J Plant Nutr 31, 1270-1283. |
| [35] | Vejan P, Khadiran T, Abdullah R, Ahmad N (2021). Controlled release fertilizer: a review on developments, applications and potential in agriculture. J Control Release 339, 321-334. |
| [36] | Verdoucq L, Maurel C (2018). Plant aquaporins. In: Advances in Botanical Research, Vol. 87. London: Academic Press. pp. 25-56. |
| [37] | Wang Y, Qiu T, Han Q, Kang XY (2018). Comparative proteomics of two Populus spp. (Section tacamahaca) allotriploid derived by different types of 2n female gamete and their parents. J Beijing For Univ 40(5), 1-9. (in Chinese) |
| 王溢, 邱彤, 韩强, 康向阳 (2018). 不同2n雌配子来源的青杨杂种三倍体与其亲本蛋白质组差异研究. 北京林业大学学报 40(5), 1-9. | |
| [38] |
Xiao CB, Fang Y, Wang SM, He K (2023). The alleviation of ammonium toxicity in plants. J Integr Plant Biol 65, 1362-1368.
DOI |
| [39] | Xie LJ, Zhou Y, Chen QF, Xiao S (2021a). New insights into the role of lipids in plant hypoxia responses. Prog Lipid Res 81, 101072. |
| [40] | Xie RJ, Zheng L, Jiao Y, Huang X (2021b). Understanding physiological and molecular mechanisms of citrus rootstock seedlings in response to root zone hypoxia by RNA-seq. Environ Exp Bot 192, 104647. |
| [41] | Xu XP, Fu XD, Liao H (2016). Advances in study of ammonium assimilation and its regulatory mechanism in plants. Chin Bull Bot 51, 152-166. (in Chinese) |
|
徐晓鹏, 傅向东, 廖红 (2016). 植物铵态氮同化及其调控机制的研究进展. 植物学报 51, 152-166.
DOI |
|
| [42] | Xu Y, Zhang K, Li SH, Zhou YQ, Ran SX, Xu R, Lin YZ, Shen L, Huang WQ, Zhong FL (2023). Carbon and nitrogen metabolism in tomato (Solanum lycopersicum L.) leaves response to nitrogen treatment. Plant Growth Regul 100, 747-756. |
| [43] | Yu HL, Du XQ, Zhang FX, Zhang F, Hu Y, Liu SC, Jiang XN, Wang GD, Liu D (2012). A mutation in the E2 subunit of the mitochondrial pyruvate dehydrogenase complex in Arabidopsis reduces plant organ size and enhances the accumulation of amino acids and intermediate products of the TCA cycle. Planta 236, 387-399. |
| [44] | Zafari S, Vanlerberghe GC, Igamberdiev AU (2022). The role of alternative oxidase in the interplay between nitric oxide, reactive oxygen species, and ethylene in tobacco (Nicotiana tabacum L.) plants incubated under normoxic and hypoxic conditions. Int J Mol Sci 23, 7153. |
| [45] | Zhang WJ, Zhang T, Zhang J, Lei WW, Zhao L, Wang S, Shi MY, Wei M (2023). Low nitrogen stress promotes root nitrogen uptake and assimilation in strawberry: contribution of hormone networks. Horticulturae 9, 249. |
| [46] | Zhang Y, Zhang YX, Lü XM, Du WY, Feng B, Li HW, Wang ZS, Xia HY, Fan SJ, Kong LA (2021). Study on physiological mechanism of NO3- alleviating NH4+ stress in wheat. Plant Physiol J 57, 480-492. (in Chinese) |
| 张燕, 张云秀, 吕雪梅, 杜琬莹, 冯波, 李华伟, 王宗帅, 夏海勇, 樊守金, 孔令安 (2021). NO3-缓解小麦NH4+胁迫的生理机制研究. 植物生理学报 57, 480-492. | |
| [47] | Zhu YN, Qi BF, Hao YW, Liu HC, Sun GW, Chen RY, Song SW (2021). Appropriate NH4+/NO3- ratio triggers plant growth and nutrient uptake of flowering Chinese cabbage by optimizing the pH value of nutrient solution. Front Plant Sci 12, 656144. |
| [48] |
Zwiazek JJ, Xu H, Tan XF, Navarro-Ródenas A, Morte A (2017). Significance of oxygen transport through aquaporins. Sci Rep 7, 40411.
DOI PMID |
| [1] | 张子睿, 周静, 胡艳萍, 梁爽, 马永鹏, 陈伟乐. 极度濒危植物巧家五针松的根内和根际真菌群落特征[J]. , 2025, 49(濒危植物的保护与恢复): 0-. |
| [2] | 王秀媛, 申磊, 刘婷婷, 尉雯雯, 张帅, 张伟. ‘塞外红’苹果-大豆复合系统根系时空分布与种间竞争策略[J]. 植物生态学报, 2025, 49(5): 748-759. |
| [3] | 杜英杰, 范爱连, 王雪, 闫晓俊, 陈廷廷, 贾林巧, 姜琦, 陈光水. 亚热带天然常绿阔叶林乔木树种与林下灌木树种根-叶功能性状协调性及差异[J]. 植物生态学报, 2025, 49(4): 585-595. |
| [4] | 田伟, 陈佳杰, 陈渊戈, 徐清, 周进. 浙江象山港蟹类(十足目: 短尾下目)物种多样性[J]. 生物多样性, 2025, 33(4): 24461-. |
| [5] | 宋威, 程才, 王嘉伟, 吴纪华. 土壤微生物对植物多样性–生态系统功能关系的调控作用[J]. 生物多样性, 2025, 33(4): 24579-. |
| [6] | 李梦琦, 苗灵凤, 李大东, 龙奕帆, 叶冰冰, 杨帆. 海南东寨港红树林植物细根功能性状对不同潮位沉积物养分变化的响应[J]. 植物生态学报, 2025, 49(4): 552-561. |
| [7] | 刘雨函, 曹启江, 张诗晗, 李益慧, 王菁, 谭晓萌, 刘筱儒, 王显玲. 拟南芥AtFTCD-L参与根系响应土壤紧实度的机制研究[J]. 植物学报, 2025, 60(4): 1-0. |
| [8] | 王娟, 张登山, 肖元明, 裴全帮, 王博, 樊博, 周国英. 长期围封后高寒草原植物根系分泌物特征与环境因子关系[J]. 植物生态学报, 2025, 49(4): 596-609. |
| [9] | 郭李琦, 闫晓蕾, 曹磊, 高景, 刘瑞强, 周旭辉. 树种菌根类型与根系性状对根际微生物网络复杂性的影响[J]. 植物生态学报, 2025, 49(4): 573-584. |
| [10] | 许庭旸, 刘雨辰, 王万鹏, 苏航, 苏昆龙, 吴振映, 吕明, 李福利, 王小山, 付春祥. 喷施不同植物生长调节剂对盐碱地小麦生长发育的影响[J]. 植物学报, 2025, 60(3): 354-362. |
| [11] | 曾文丹, 严华兵, 吴正丹, 尚小红, 曹升, 陆柳英, 肖亮, 施平丽, 程冬, 龙紫媛, 李婕宇. 发根农杆菌介导的野葛毛状根遗传转化体系[J]. 植物学报, 2025, 60(3): 425-434. |
| [12] | 郑琳敏, 熊小玲, 姜永孟, 王曼, 张锦秀, 曾志伟, 吕茂奎, 谢锦升. 武夷山不同海拔杉木凋落叶和细根分解规律以及驱动因素的差异[J]. 植物生态学报, 2025, 49(2): 244-255. |
| [13] | 李建国, 张怡, 张文君. 水稻根系铁膜形成及对磷吸收的影响[J]. 植物学报, 2025, 60(1): 132-143. |
| [14] | 牛云明, 贾国栋, 王欣, 刘子赫. 庐山不同海拔植物蒸腾水龄动态及用水策略[J]. 植物生态学报, 2024, 48(9): 1104-1117. |
| [15] | 何欣怡, 潘玉梅, 祝燕, 陈佳仪, 张思榕, 张乃莉. 暖温带森林外生菌根树种优势和植物多样性对土壤氮素周转的影响[J]. 生物多样性, 2024, 32(9): 24173-. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||