

植物学报 ›› 2020, Vol. 55 ›› Issue (6): 677-692.DOI: 10.11983/CBB20048 cstr: 32102.14.CBB20048
宋凝曦1,2, 谢寅峰2, 李霞1,2,3,*
收稿日期:2020-03-21
接受日期:2020-08-26
出版日期:2020-11-01
发布日期:2020-11-11
通讯作者:
李霞
作者简介:*E-mail: jspplx@jaas.ac.cn基金资助:Ningxi Song1,2, Yingfeng Xie2, Xia Li1,2,3,*
Received:2020-03-21
Accepted:2020-08-26
Online:2020-11-01
Published:2020-11-11
Contact:
Xia Li
摘要: 为探究干旱胁迫下表观遗传机制对高表达玉米(Zea mays) C4型PEPC转基因水稻(Oryza sativa)种子萌发的影响, 以转C4型PEPC水稻(PC)和野生型水稻Kitaake (WT)为试材, 采用10% (m/v)聚乙二醇6000 (PEG6000)模拟干旱条件, 通过单独和联合施用PEG6000、DNA甲基化抑制剂5-氮杂胞苷(5azaC)和可变剪接抑制剂大环内酯类(PB)进行种子发芽实验, 测定种子活力、萌发过程中可溶性糖和可溶性蛋白含量、α-淀粉酶活性以及PEPC、糖信号相关基因和部分剪接因子基因的表达。结果表明, 0.25 µmol·L-1PB处理对2种供试水稻在干旱条件下种子萌发均表现出显著抑制作用, 使干旱条件下种子萌发过程中可溶性总糖、蔗糖、葡萄糖和果糖含量以及可溶性蛋白含量均有所下降, PB也抑制糖信号-蔗糖非发酵1 (SNF1)相关蛋白激酶(SnRKs)家族和剪接因子丝氨酸/精氨酸富集蛋白家族(SR proteins)相关基因的表达以及α-淀粉酶的活性, 但对PC的抑制作用小于WT。5 µmol·L-15azaC处理对干旱条件下种子萌发的效果与可变剪接抑制剂相反。5 µmol·L -1 5azaC联合PEG6000干旱处理部分减缓了干旱对水稻种子发芽率的抑制作用, 使供试材料发芽率升高, 表明DNA甲基化和可变剪接机制参与了水稻芽期干旱耐性, 其中对PC的作用更大。
宋凝曦, 谢寅峰, 李霞. 干旱胁迫下表观遗传机制对转C4型PEPC基因水稻种子萌发的影响. 植物学报, 2020, 55(6): 677-692.
Ningxi Song, Yingfeng Xie, Xia Li. Effects of Epigenetic Mechanisms on C4 Phosphoenolpyruvate Carboxylase Transgenic Rice (Oryza sativa) Seed Germination Under Drought Stress. Chinese Bulletin of Botany, 2020, 55(6): 677-692.
| Gene | Forward primer (5'-3') | Reverse primer (5'-3') |
|---|---|---|
| Actin | CCCTCAAACATCGGTATGGA | TTGATCTTCATGCTGCTTGG |
| OsK1a | AACCAGAGGTAACAGGCAGG | AACCAGAGGTAACAGGCAGG |
| OsK24 | CGTGTTGGCTTCAGTGAAT | CCTTCTCTATCTAAGGGCCG |
| OsK35 | TTGTGTTGGCTTCAGTGAAA | CCTTCGCTGTCTAAGGACTG |
| C4-PEPC | CCCACTATCCTTCGCAAGAC | CTAGCCAGTGTTCTGCATGCCGG |
| Osppc2a | CTGGTTGAGATGGTTTTCGC | GGTGTGAATTCAGGCACTTC |
| SAPK8 | ATAGATGATAATGTCCAGCG | GTTCCTACAGTGGATTTTGG |
| SAPK9 | CACAGCAACGCCGTCTCC | CACACTTCCACCGCTACCAA |
| SAPK10 | TGCTGATGTGTGGTCGTGTG | TGCTGGTATGGTCGCCTCT |
| SR40 | CAATCTGGGGACTGCTTTC | TCCTGCTTGGGCTTTTACT |
| SR33 | ATATTGCCTGCTACCCGAAAG | CAGAGCAGCACCCAGTTTATTAC |
| OsAmy1A | TTTCGGTCCTCATCGTCCTCC | TCCACGACTCCCAGTTGAATC |
| OsAmy1C | TGGTATCGATCAGAAACCGGC | GTCCGACCTTCGTGATGACC |
| OsAmy3C | AAGCATTCCACCACAATGAGC | AGGAAGTTGTACCACCCACC |
| OsAmy3E | TCACCCTGTGTTGTGTCGTT | AAAGTTGTACCACCCGCCTT |
表1 引物序列
Table 1 Primers used in this study
| Gene | Forward primer (5'-3') | Reverse primer (5'-3') |
|---|---|---|
| Actin | CCCTCAAACATCGGTATGGA | TTGATCTTCATGCTGCTTGG |
| OsK1a | AACCAGAGGTAACAGGCAGG | AACCAGAGGTAACAGGCAGG |
| OsK24 | CGTGTTGGCTTCAGTGAAT | CCTTCTCTATCTAAGGGCCG |
| OsK35 | TTGTGTTGGCTTCAGTGAAA | CCTTCGCTGTCTAAGGACTG |
| C4-PEPC | CCCACTATCCTTCGCAAGAC | CTAGCCAGTGTTCTGCATGCCGG |
| Osppc2a | CTGGTTGAGATGGTTTTCGC | GGTGTGAATTCAGGCACTTC |
| SAPK8 | ATAGATGATAATGTCCAGCG | GTTCCTACAGTGGATTTTGG |
| SAPK9 | CACAGCAACGCCGTCTCC | CACACTTCCACCGCTACCAA |
| SAPK10 | TGCTGATGTGTGGTCGTGTG | TGCTGGTATGGTCGCCTCT |
| SR40 | CAATCTGGGGACTGCTTTC | TCCTGCTTGGGCTTTTACT |
| SR33 | ATATTGCCTGCTACCCGAAAG | CAGAGCAGCACCCAGTTTATTAC |
| OsAmy1A | TTTCGGTCCTCATCGTCCTCC | TCCACGACTCCCAGTTGAATC |
| OsAmy1C | TGGTATCGATCAGAAACCGGC | GTCCGACCTTCGTGATGACC |
| OsAmy3C | AAGCATTCCACCACAATGAGC | AGGAAGTTGTACCACCCACC |
| OsAmy3E | TCACCCTGTGTTGTGTCGTT | AAAGTTGTACCACCCGCCTT |
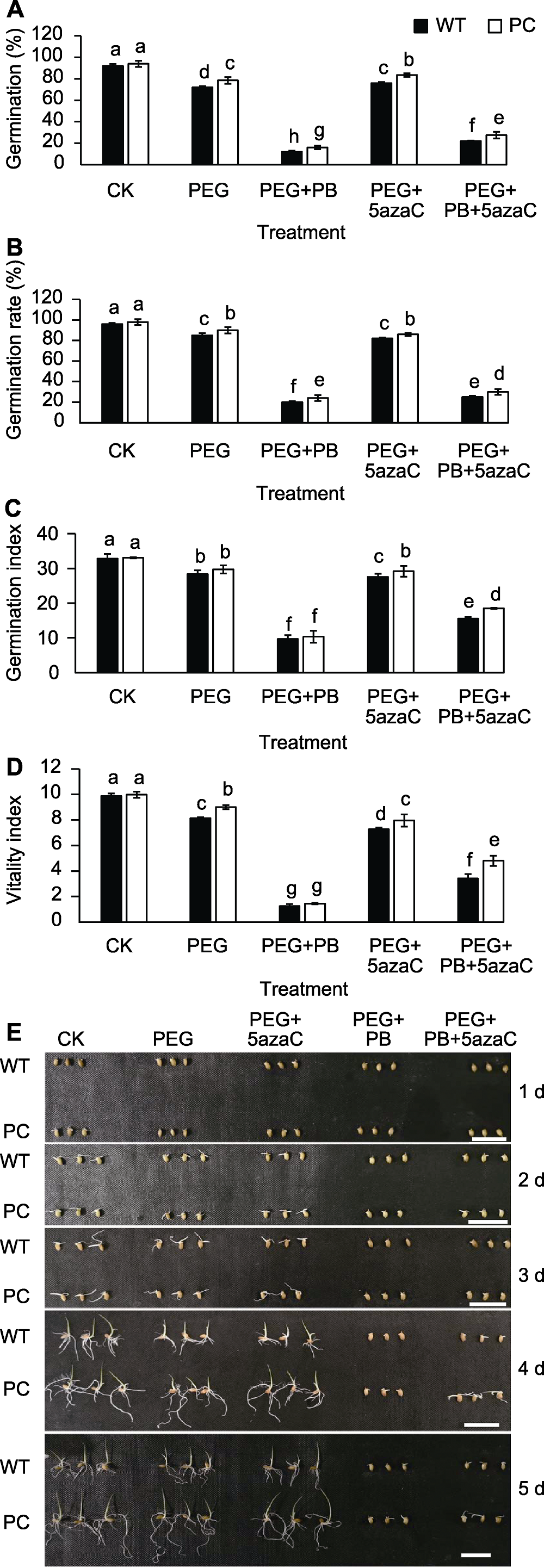
图1 不同处理对转基因水稻(PC)和野生型水稻(WT)种子活力的影响 (A) 发芽势; (B) 发芽率; (C) 发芽指数; (D) 活力指数; (E) 种子发芽图片。不同小写字母表示差异显著(P<0.05)。Bars=0.5 cm
Figure 1 Effects of different treatments on seed vigor of transgenic rice (PC) and wild type rice (WT) (A) Germination; (B) Germination rate; (C) Germination index; (D) Vigor index; (E) Images of germinating seeds at different time. Different lowercase letters indicate significant differences (P<0.05). Bars=0.5 cm
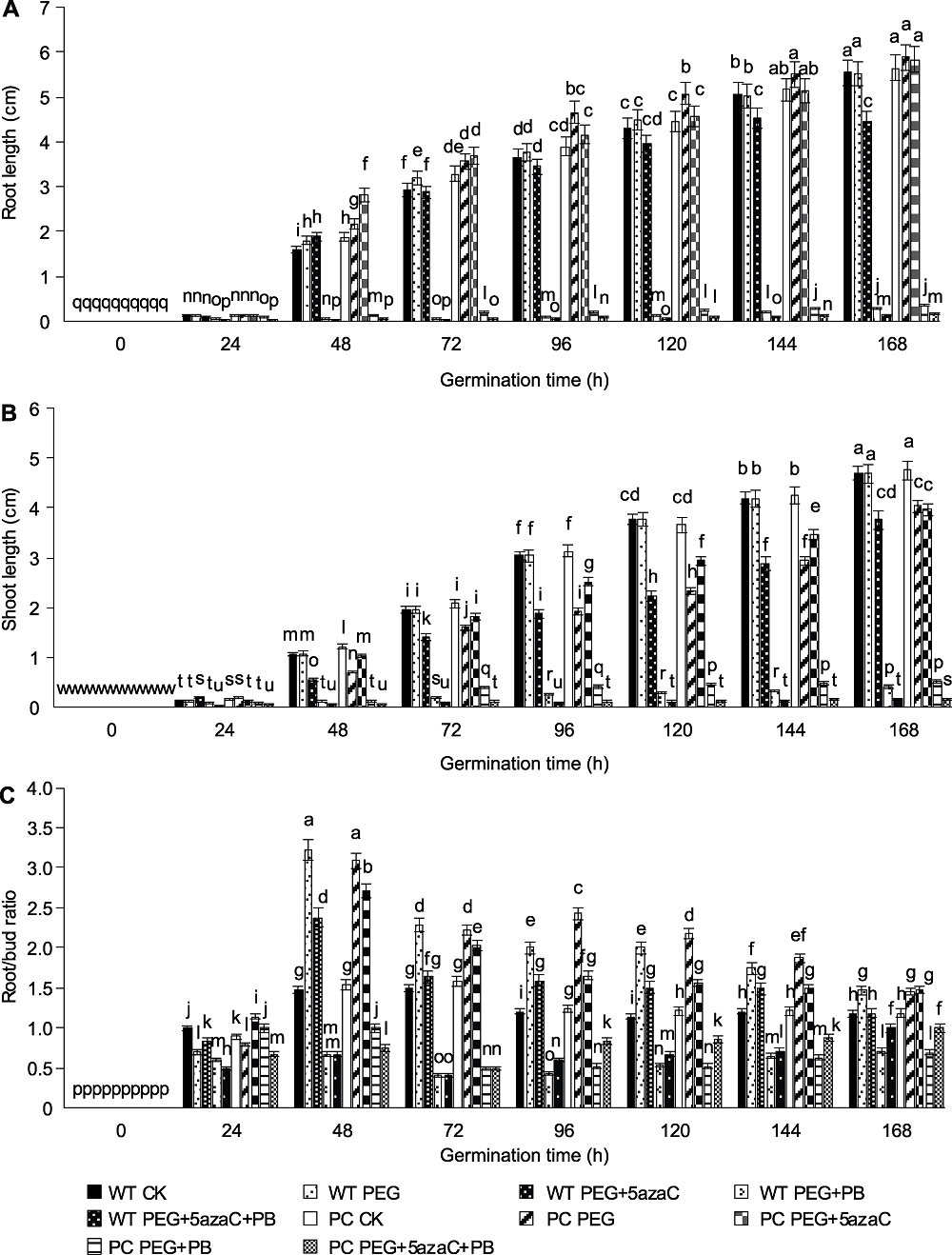
图2 不同处理下转基因水稻(PC)和野生型水稻(WT)萌发种子根长(A)、芽长(B)和根芽比(C)的变化 不同小写字母表示差异显著(P<0.05)。
Figure 2 Effects of different treatments on root length (A), shoot length (B) and root/bud ratio (C) of transgenic rice (PC) and wild type rice (WT) germinating seeds Different lowercase letters indicate significant differences (P<0.05).
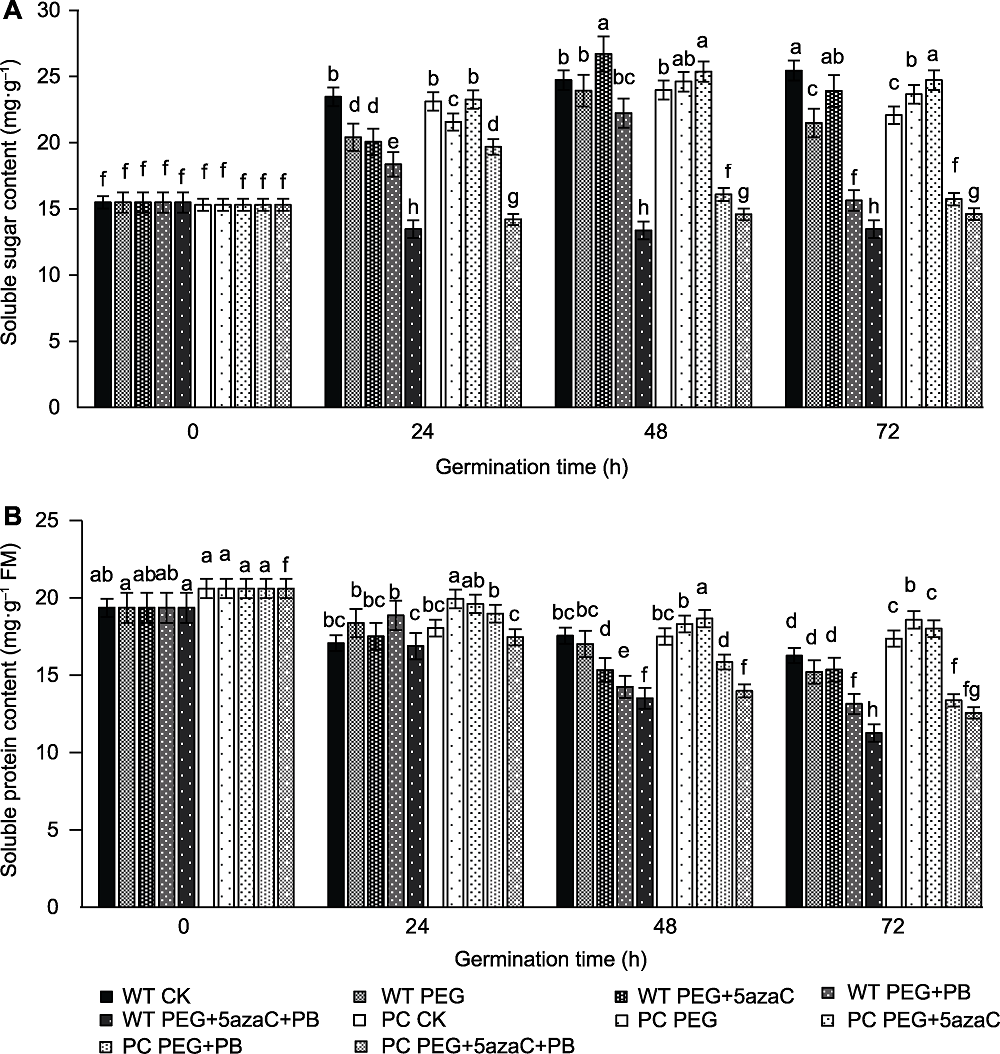
图3 不同处理对转基因水稻(PC)和野生型水稻(WT)种子萌发过程中可溶性总糖含量(A)和可溶性蛋白含量(B)的影响 不同小写字母表示差异显著(P<0.05)。
Figure 3 Effects of different treatments on total soluble sugar content (A) and soluble protein content (B) of transgenic rice (PC) and wild type rice (WT) germinating seeds Different lowercase letters indicate significant differences (P<0.05).
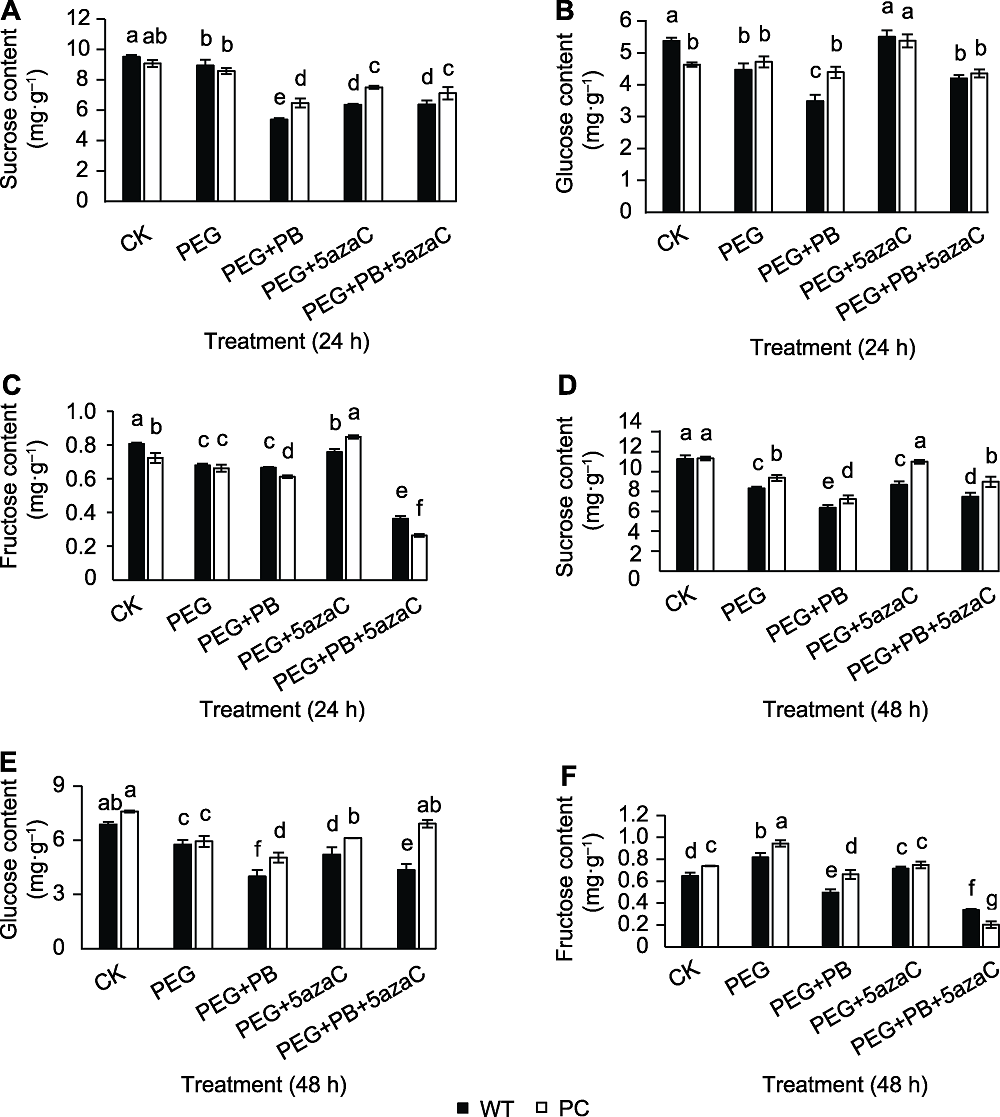
图4 不同处理对转基因水稻(PC)和野生型水稻(WT)种子萌发过程中糖组分含量的影响 (A), (D) 分别为发芽24和48小时的蔗糖含量; (B), (E) 分别为发芽24和48小时的葡萄糖含量; (C), (F) 分别为发芽24和48小时的果糖含量。不同小写字母表示差异显著(P<0.05)。
Figure 4 Effects of different treatments on sugar content of transgenic rice (PC) and wild type rice (WT) germinating seeds (A), (D) Sucrose content at 24 h and 48 h after germination, respectively; (B), (E) Glucose content at 24 h and 48 h after germination, respectively; (C), (F) Fructose content at 24 h and 48 h after germination, respectively. Different lowercase letters indicate significant differences (P<0.05).
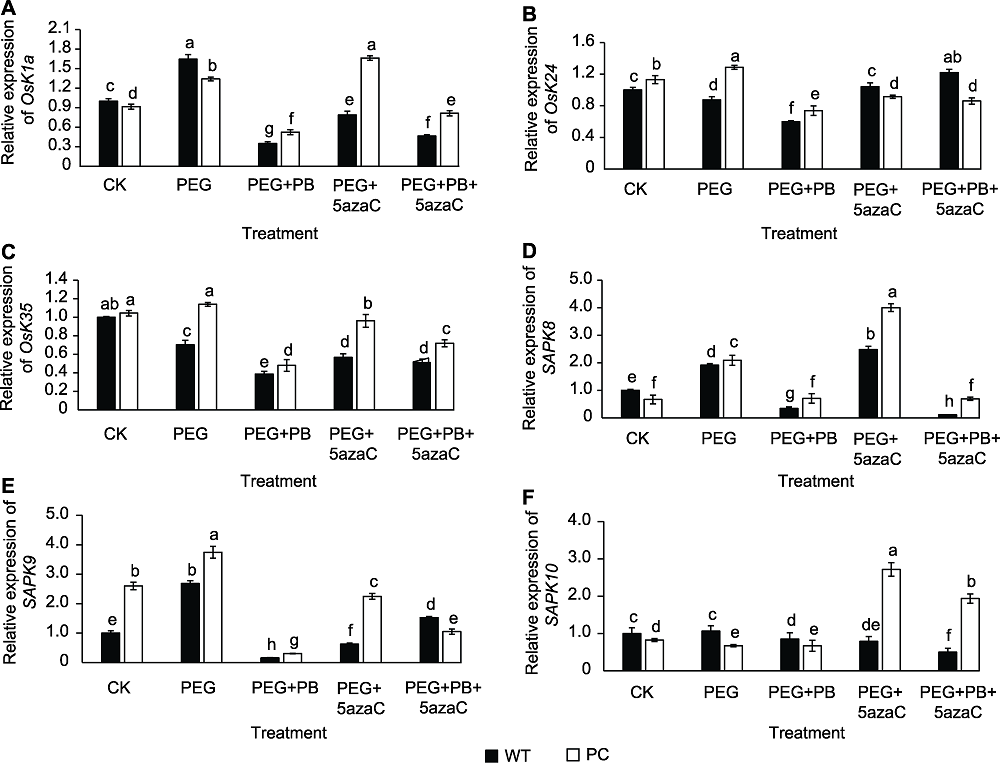
图5 不同处理对转基因水稻(PC)和野生型水稻(WT)种子萌发过程中SnRK相关基因表达的影响 不同小写字母表示差异显著(P<0.05)。
Figure 5 Effects of different treatments on the expression of SnRK related genes in transgenic rice (PC) and wild type rice (WT) germinating seeds Different lowercase letters indicate significant differences (P<0.05).
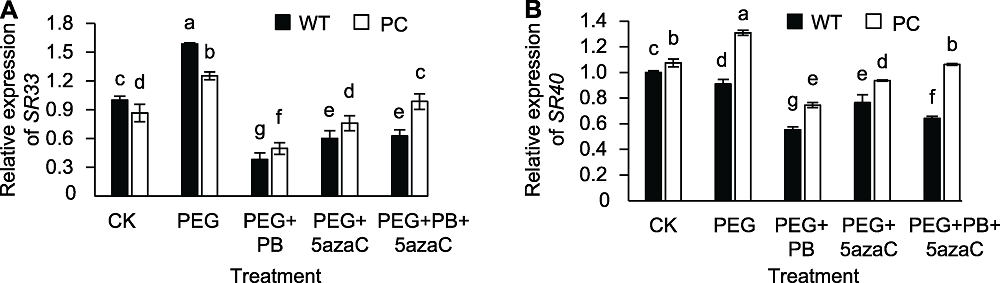
图6 不同处理对转基因水稻(PC)和野生型水稻(WT)种子萌发过程中剪接因子相关基因表达的影响 不同小写字母表示差异显著(P<0.05)。
Figure 6 Effects of different treatments on expression of splicing factor related genes in transgenic rice (PC) and wild type rice (WT) germinating seeds Different lowercase letters indicate significant differences (P<0.05).
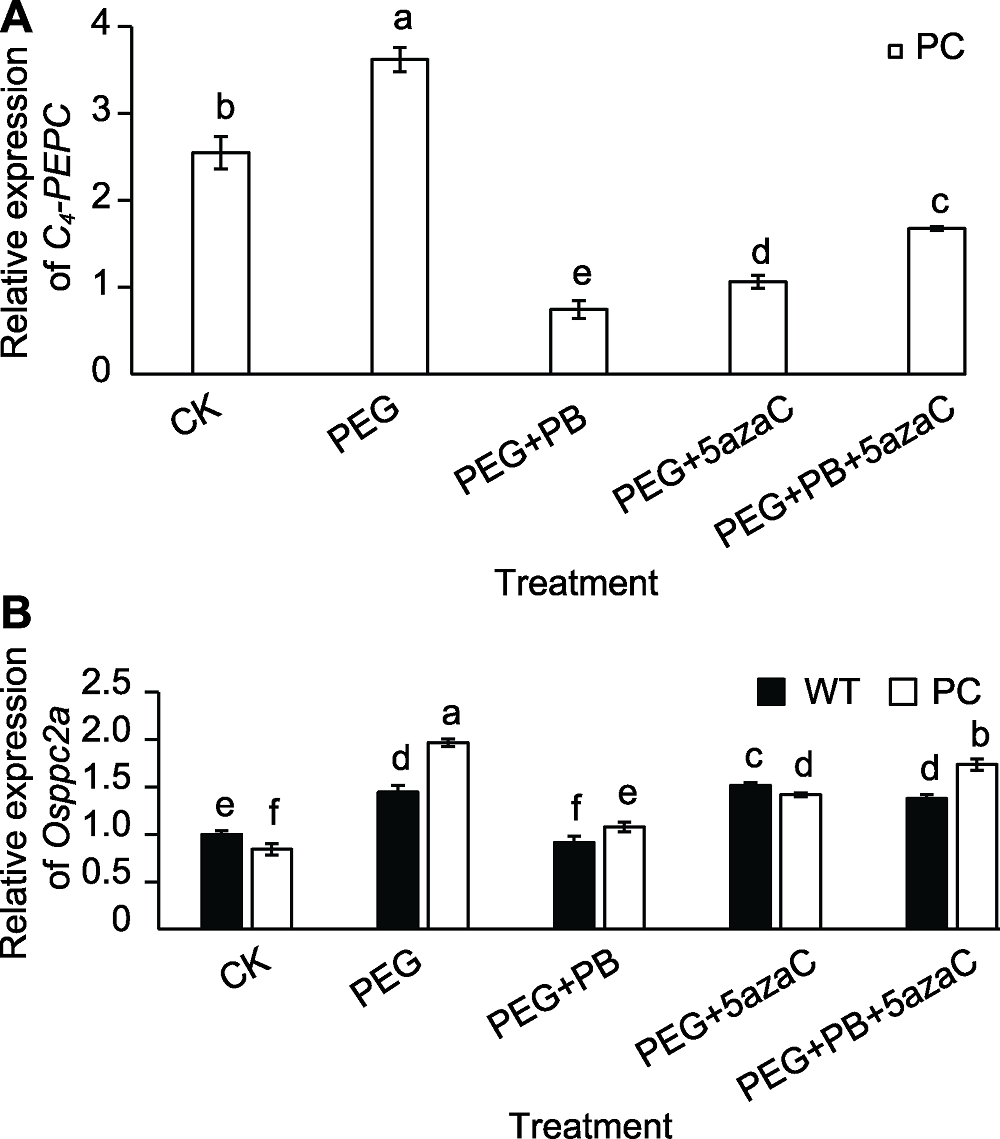
图7 不同处理对转基因水稻(PC)和野生型水稻(WT)种子萌发过程中PEPC相关基因表达的影响 不同小写字母表示差异显著(P<0.05)。
Figure 7 Effects of different treatments on expression of PEPC-related genes in transgenic rice (PC) and wild type rice (WT) germinating seeds Different lowercase letters indicate significant differences (P<0.05).
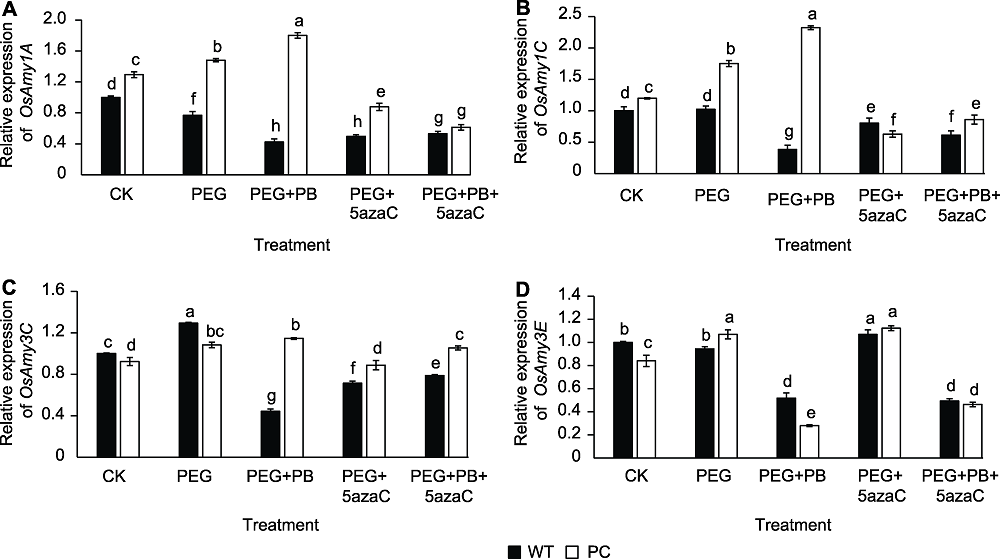
图8 不同处理对转基因水稻(PC)和野生型水稻(WT)种子萌发过程中α-淀粉酶相关基因表达的影响 不同小写字母表示差异显著(P<0.05)。
Figure 8 Effects of different treatments on expression of α-amylase related genes in transgenic rice (PC) and type wild rice (WT) germinating seeds Different lowercase letters indicate significant differences (P<0.05).
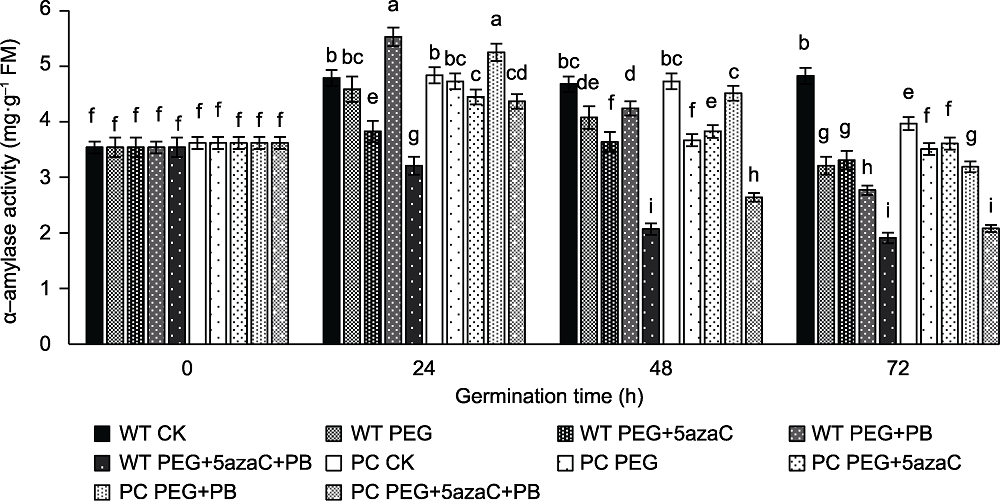
图9 不同处理对转基因水稻(PC)和野生型水稻(WT)种子萌发过程中α-淀粉酶活性的影响 不同小写字母表示差异显著(P<0.05)。
Figure 9 Effects of different treatments on α-amylase activity in transgenic rice (PC) and wild type rice (WT) germinating seeds Different lowercase letters indicate significant differences (P<0.05).
| [1] | 陈蕾太, 孙爱清, 杨敏, 陈路路, 马雪丽, 李美玲, 尹燕枰 (2016). 基于小麦种子发芽逆境抗逆指数的种子活力评价. 应用生态学报 27, 2968-2974. |
| [2] |
杜康兮, 沈文辉, 董爱武 (2018). 表观遗传调控植物响应非生物胁迫的研究进展. 植物学报 53, 581-593.
DOI URL |
| [3] | 焦德茂, 匡廷云, 李霞, 戈巧英, 黄雪清, 郝乃斌, 白克智 (2003). 转PEPC基因水稻具有初级CO2浓缩机制的生理特点. 中国科学(C辑) 33, 33-39. |
| [4] | 焦德茂, 李霞, 黄雪清, 迟伟, 匡廷云, 古森本 (2001). 转PEPC基因水稻的光合CO2同化和叶绿素荧光特性. 科学通报 46, 414-418. |
| [5] | 李合生 (2000). 植物生理生化实验原理和技术 北京: 高等教育出版社. pp. 123-124. |
| [6] | 李美玲, 孙爱清, 杨敏, 张杰道, 王振林, 陈蕾太, 陈路路, 马雪丽, 尹燕枰 (2017). 小麦干热风抗性鉴定及热胁迫相关基因TaHSPs的表达分析. 麦类作物学报 37, 162-174. |
| [7] |
刘小龙, 李霞, 钱宝云 (2015). 外源Ca2+对PEG处理下转C4型PEPC基因水稻光合生理的调节 . 植物学报 50, 206-216.
DOI URL |
| [8] | 曲瑞莲, 吴春霞, 冯献忠 (2014). mRNA选择性剪切在植物发育中的作用. 植物生理学报 50, 717-724. |
| [9] | 宋凝曦, 张晓敬, 陆佳岚, 李霞, 谢寅峰 (2020). 可变剪接在植物响应胁迫中的作用. 植物生理学报 56, 1201-1211. |
| [10] |
张金飞, 李霞, 何亚飞, 谢寅峰 (2018). 外源葡萄糖增强高表达转玉米C4型PEPC水稻耐旱性的生理机制. 作物学报 44, 82-94.
DOI URL |
| [11] |
张金飞, 李霞, 谢寅峰 (2017). 植物SnRKs家族在胁迫信号通路中的调节作用. 植物学报 52, 346-357.
DOI URL |
| [12] |
Ambavaram MMR, Basu S, Krishnan A, Ramegowda V, Batlang U, Rahman L, Baisakh N, Pereira A (2014). Coordinated regulation of photosynthesis in rice increases yield and tolerance to environmental stress. Nat Commun 5, 5302.
DOI URL PMID |
| [13] |
Bradford MM (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72, 248-254.
URL PMID |
| [14] |
Brummell DA, Chen RKY, Harris JC, Zhang HB, Hamiaux C, Kralicek AV, McKenzie MJ (2011). Induction of vacuolar invertase inhibitor mRNA in potato tubers contributes to cold-induced sweetening resistance and includes spliced hybrid mRNA variants. J Exp Bot 62, 3519-3534.
DOI URL PMID |
| [15] |
Cao Y, Ma LG (2019). To splice or to transcribe: SKIP- mediated environmental fitness and development in plants. Front Plant Sci 10, 1222.
DOI URL PMID |
| [16] | Chen PB, Li X, Huo K, Wei XD, Dai CC, Lv CG (2014). Promotion of photosynthesis in transgenic rice over-expressing of maize C4 phosphoenolpyruvate carboxylase gene by nitric oxide donors. J Plant Physiol 6, 458-466. |
| [17] |
Cruz TMD, Carvalho RF, Richardson DN, Duque P (2014). Abscisic acid (ABA) regulation of Arabidopsis SR protein gene expression. Int J Mol Sci 15, 17541-17564.
DOI URL PMID |
| [18] | Damaris RN, Lin ZY, Yang PF, He DL (2019). The rice α-amylase, conserved regulator of seed maturation and germination. Int J Mol Sci 20, 450. |
| [19] |
Dinesh SP, Baker BJ (2000). Alternatively spliced N resistance gene transcripts: their possible role in tobacco mosaic virus resistance. Proc Nat Acad Sci USA 97, 1908-1913.
DOI URL PMID |
| [20] | Dinkova TD, Márquez-Velázquez NA, Aguilar R, Lázaro- Mixteco PE, de Jiménez ES (2011). Tight translational control by the initiation factors eIF4E and eIF(iso)4E is required for maize seed germination. Seed Sci Res 21, 85-93. |
| [21] |
Dong CL, He F, Berkowitz O, Liu JX, Cao PF, Tang M, Shi HC, Wang WJ, Li QL, Shen ZG, Whelan J, Zheng LQ (2018). Alternative splicing plays a critical role in maintaining mineral nutrient homeostasis in rice (Oryza sativa). Plant Cell 30, 2267-2285.
DOI URL PMID |
| [22] |
Egawa C, Kobayashi F, Ishibashi M, Nakamura T, Nakamura C, Takumi S (2006). Differential regulation of transcript accumulation and alternative splicing of a DREB2 homolog under abiotic stress conditions in common wheat. Genes Genet Syst 81, 77-91.
DOI URL PMID |
| [23] |
Filichkin SA, Cumbie JS, Dharmawardhana P, Jaiswal P, Chang JH, Palusa SG, Reddy ASN, Megraw M, Mockler TC (2015). Environmental stresses modulate abundance and timing of alternatively spliced circadian transcripts in Arabidopsis. Mol Plant 8, 207-227.
DOI URL PMID |
| [24] |
Foolad MR, Subbiah P, Zhang LP (2007). Common QTL affect the rate of tomato seed germination under different stress and nonstress conditions. Int J Plant Genomics 2007, 97386.
URL PMID |
| [25] | Gassmann W (2008). Alternative splicing in plant defense. In: Reddy ASN, Golovkin M, eds. Nuclear Pre-mRNA Processing in Plants. Berlin:Springer.pp. 219-233. |
| [26] |
Golisz A, Sikorski PJ, Kruszka K, Kufel J (2013). Arabidopsis thaliana LSM proteins function in mRNA splicing and degradation. Nucleic Acids Res 41, 6232-6249.
DOI URL PMID |
| [27] |
Hakata M, Kuroda M, Miyashita T, Yamaguchi T, Kojima M, Sakakibara H, Mitsui T, Yamakawa H (2012). Suppression of α-amylase genes improves quality of rice grain ripened under high temperature. Plant Biotechnol J 10, 1110-1117.
DOI URL PMID |
| [28] | He YF, Xie YF, Li X, Yang J (2020). Drought tolerance of transgenic rice overexpressing maize C4-PEPC gene related to increased anthocyanin synthesis regulated by sucrose and calcium. Biologia Plantarum 64, 136-149. |
| [29] | Hu QJ, Fu YY, Guan YJ, Lin C, Cao DD, Hu WM, Sheteiwy M, Hu J (2016). Inhibitory effect of chemical combinations on seed germination and pre-harvest sprouting in hybrid rice. Plant Growth Regul 80, 281-289. |
| [30] |
Ku MSB, Agarie S, Nomura M, Fukayama H, Tsuchida H, Ono K, Hirose S, Toki S, Miyao M, Matsuoka M (1999). High-level expression of maize phosphoenolpyruvate carboxylase in transgenic rice plants. Nat Biotechnol 17, 76-80.
DOI URL PMID |
| [31] |
Lata C, Gupta S, Prasad M (2013). Foxtail millet: a model crop for genetic and genomic studies in bioenergy grasses. Crit Rev Biotechnol 33, 328-343.
DOI URL PMID |
| [32] | Lebouteiller B, Gousset-Dupont A, Pierre JN, Bleton J, Tchapla A, Maucourt M, Moing A, Rolin D, Vidal J (2007). Physiological impacts of modulating phosphoenolpyruvate carboxylase levels in leaves and seeds of Arabidopsis thaliana. Plant Sci 172, 265-272. |
| [33] |
Lev Maor G, Yearim A, Ast G (2015). The alternative role of DNA methylation in splicing regulation. Trends Genet 31, 274-280.
DOI URL PMID |
| [34] | Li X, Wang C (2013). Physiological and metabolic enzymes activity changes in transgenic rice plants with increased phosphoenolpyruvate carboxylase activity during the flowering stage. Acta Physiol Plant 35, 1503-1512. |
| [35] | Li X, Wang C, Ren CG (2011). Effects of 1-butanol, neomycin and calcium on the photosynthetic characteristics of pepc transgenic rice. Afr J Biotechnol 10, 17466-17476. |
| [36] |
Ling Y, Alshareef S, Butt H, Lozano-Juste J, Li LX, Galal AA, Moustafa A, Momin AA, Tashkandi M, Richardson DN, Fujii H, Arold S, Rodriguez PL, Duque P, Mahfouz MM (2017). Pre-mRNA splicing repression triggers abiotic stress signaling in plants. Plant J 89, 291-309.
DOI URL PMID |
| [37] |
Liu JJ, Sun N, Liu M, Liu JC, Du BJ, Wang XJ, Qi XT (2013). An autoregulatory loop controlling Arabidopsis HsfA2 expression: role of heat shock-induced alternative splicing. Plant Physiol 162, 512-521.
URL PMID |
| [38] |
Liu XL, Li X, Zhang C, Dai CC, Zhou JY, Ren CG, Zhang JF (2017). Phosphoenolpyruvate carboxylase regulation in C4-PEPC-expressing transgenic rice during early responses to drought stress. Physiol Plant 159, 178-200.
URL PMID |
| [39] |
Livak KJ, Schmittgen TD (2001). Analysis of relative gene expression data using reatime quantitative PCR and the 2-ΔΔCt method . Methods 25, 402-408.
DOI URL PMID |
| [40] |
Marquez Y, Brown JWS, Simpson C, Barta A, Kalyna M (2012). Transcriptome survey reveals increased comp- lexity of the alternative splicing landscape in Arabidopsis. Genome Res 22, 1184-1195.
DOI URL PMID |
| [41] |
Marsh JT, Sullivan S, Hartwell J, Nimmo HG (2003). Structure and expression of phospho enolpyruvate carboxylase kinase genes in solanaceae. A novel gene exhibits alternative splicing. Plant Physiol 133, 2021-2028.
URL PMID |
| [42] | Miransari M, Smith DL (2014). Plant hormones and seed germination. Environ Exp Bot 99, 110-121. |
| [43] |
Muthamilarasan M, Khandelwal R, Yadav CB, Bonthala VS, Khan Y, Prasad M (2014). Identification and molecular characterization of MYB transcription factor superfamily in C4 model plant foxtail millet ( Setaria italica L.). PLoS One 9, e109920.
DOI URL PMID |
| [44] |
Nakata M, Fukamatsu Y, Miyashita T, Hakata M, Kimura R, Nakata Y, Kuroda M, Yamaguchi T, Yamakawa H (2017). High temperature-induced expression of rice α-amylases in developing endosperm produces chalky grains. Front Plant Sci 8, 2089.
DOI URL PMID |
| [45] |
Nanjo Y, Asatsuma S, Itoh K, Hori H, Mitsui T (2004). Proteomic identification of α-amylase isoforms encoded by RAmy3B/3C from germinating rice seeds. Biosci Biotechnol Biochem 68, 112-118.
DOI URL PMID |
| [46] |
O’Leary B, Park J, Plaxton WC (2011). The remarkable diversity of plant PEPC (phosphoenolpyruvate carboxylase): recent insights into the physiological functions and post-translational controls of non-photosynthetic PEPCs. Biochem J 436, 15-34.
URL PMID |
| [47] | Prasad PVV, Pisipati SR, Momčilović I, Ristic Z (2011). Independent and combined effects of high temperature and drought stress during grain filling on plant yield and chloroplast EF-Tu expression in spring wheat. J Agron Crop Sci 197, 430-441. |
| [48] |
Qian BY, Li X, Liu XL, Wang M (2015). Improved oxidative tolerance in suspension-cultured cells of C4-pepc trans- genic rice by H2O2 and Ca2+ under PEG-6000. J Integr Plant Biol 57, 534-549.
DOI URL PMID |
| [49] |
Reddy ASN, Shad Ali G (2011). Plant serine/arginine-rich proteins: roles in precursor messenger RNA splicing, plant development, and stress responses. Wiley Interdiscip Rev RNA 2, 875-889.
DOI URL PMID |
| [50] |
Ren CG, Li X, Liu XL, Wei XD, Dai CC (2014). Hydrogen peroxide regulated photosynthesis in C4- pepc transgenic rice. Plant Physiol Biochem 74, 218-229.
DOI URL PMID |
| [51] |
Somani BL, Khanade J, Sinha R (1987). A modified anthrone-sulfuric acid method for the determination of fructose in the presence of certain proteins. Anal Biochem 167, 327-330.
DOI URL PMID |
| [52] |
Staiger D, Brown JWS (2013). Alternative splicing at the intersection of biological timing, development, and stress responses. Plant Cell 25, 3640-3656.
DOI URL PMID |
| [53] |
Syed NH, Kalyna M, Marquez Y, Barta A, Brown JWS (2012). Alternative splicing in plants-coming of age. Trends Plant Sci 17, 616-623.
DOI URL PMID |
| [54] |
Tang YT, Li X, Lu W, Wei XD, Zhang QJ, Lv CG, Song NX (2018). Transgenic rice over-expressing maize C4 phosphoenolpyruvate carboxylase gene contributes to alleviating low nitrogen stress. Plant Physiol Biochem 130, 577-588.
DOI URL PMID |
| [55] |
Wang BB, Brendel V (2006). Genomewide comparative analysis of alternative splicing in plants. Proc Natl Acad Sci USA 103, 7175-7180.
DOI URL PMID |
| [56] |
Yan HH, Kikuchi S, Neumann P, Zhang WL, Wu YF, Chen F, Jiang JM (2010). Genome-wide mapping of cytosine methylation revealed dynamic DNA methylation patterns associated with genes and centromeres in rice. Plant J 63, 353-365.
DOI URL PMID |
| [57] |
Zhang C, Li X, He YF, Zhang JF, Yan T, Liu XL (2017). Physiological investigation of C4-phosphoenolpyruvate- carboxylase-introduced rice line shows that sucrose metabolism is involved in the improved drought tolerance. Plant Physiol Biochem 115, 328-342.
URL PMID |
| [58] |
Zhang P, Deng H, Xiao FM, Liu YS (2013). Alterations of alternative splicing patterns of Ser/Arg-rich (SR) genes in response to hormones and stresses treatments in different ecotypes of rice (Oryza sativa). J Integr Agric 12, 737-748.
DOI URL |
| [59] |
Zhang XC, Gassmann W (2007). Alternative splicing and mRNA levels of the disease resistance gene RPS4 are induced during defense responses. Plant Physiol 145, 1577-1587.
DOI URL PMID |
| [1] | 周鑫宇, 刘会良, 高贝, 卢妤婷, 陶玲庆, 文晓虎, 张岚, 张元明. 新疆特有濒危植物雪白睡莲繁殖生物学研究[J]. , 2025, 49(濒危植物的保护与恢复): 0-. |
| [2] | 叶灿, 姚林波, 金莹, 高蓉, 谭琪, 李旭映, 张艳军, 陈析丰, 马伯军, 章薇, 张可伟. 水稻水杨酸代谢突变体高通量筛选方法的建立与应用[J]. 植物学报, 2025, 60(4): 1-0. |
| [3] | 赵凌, 管菊, 梁文化, 张勇, 路凯, 赵春芳, 李余生, 张亚东. 基于高密度Bin图谱的水稻苗期耐热性QTL定位[J]. 植物学报, 2025, 60(3): 342-353. |
| [4] | 熊良林, 梁国鲁, 郭启高, 景丹龙. 基因可变剪接调控植物响应非生物胁迫研究进展[J]. 植物学报, 2025, 60(3): 435-448. |
| [5] | 李新宇, 谷月, 徐非非, 包劲松. 水稻胚乳淀粉合成相关蛋白的翻译后修饰研究进展[J]. 植物学报, 2025, 60(2): 256-270. |
| [6] | 范惠玲, 路妍, 金文海, 王慧, 彭小星, 武学霞, 刘玉皎. 基于根系表型性状的蚕豆耐盐碱性鉴定与综合评价(长英文摘要)[J]. 植物学报, 2025, 60(2): 204-217. |
| [7] | 李建国, 张怡, 张文君. 水稻根系铁膜形成及对磷吸收的影响[J]. 植物学报, 2025, 60(1): 132-143. |
| [8] | 孙龙, 李文博, 娄虎, 于澄, 韩宇, 胡同欣. 火干扰对兴安落叶松种子萌发的影响[J]. 植物生态学报, 2024, 48(6): 770-779. |
| [9] | 姚瑞枫, 谢道昕. 水稻独脚金内酯信号感知的激活和终止[J]. 植物学报, 2024, 59(6): 873-877. |
| [10] | 罗燕, 刘奇源, 吕元兵, 吴越, 田耀宇, 安田, 李振华. 拟南芥光敏色素突变体种子萌发的光温敏感性[J]. 植物学报, 2024, 59(5): 752-762. |
| [11] | 连锦瑾, 唐璐瑶, 张伊诺, 郑佳兴, 朱超宇, 叶语涵, 王跃星, 商文楠, 傅正浩, 徐昕璇, 吴日成, 路梅, 王长春, 饶玉春. 水稻抗氧化性状遗传位点挖掘及候选基因分析[J]. 植物学报, 2024, 59(5): 738-751. |
| [12] | 袁涵, 钟爱文, 刘送平, 彭焱松, 徐磊. 水毛花种子萌发特性的差异及休眠解除方法[J]. 植物生态学报, 2024, 48(5): 638-650. |
| [13] | 黄佳慧, 杨惠敏, 陈欣雨, 朱超宇, 江亚楠, 胡程翔, 连锦瑾, 芦涛, 路梅, 张维林, 饶玉春. 水稻突变体pe-1对弱光胁迫的响应机制[J]. 植物学报, 2024, 59(4): 574-584. |
| [14] | 周俭民. 收放自如的明星战车[J]. 植物学报, 2024, 59(3): 343-346. |
| [15] | 朱晓博, 董张, 祝梦瑾, 胡晋, 林程, 陈敏, 关亚静. 重要的种子储存物质长寿命mRNA[J]. 植物学报, 2024, 59(3): 355-372. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||