

Chinese Bulletin of Botany ›› 2020, Vol. 55 ›› Issue (1): 49-61.DOI: 10.11983/CBB19047 cstr: 32102.14.CBB19047
• EXPERIMENTAL COMMUNICATIONS • Previous Articles Next Articles
Dongdong Cao1,3,Shanyu Chen1,Yebo Qin2,Huaping Wu3,Guanhai Ruan1,Yutao Huang1,*( )
)
Received:2019-03-14
Accepted:2019-07-26
Online:2020-01-01
Published:2019-12-20
Contact:
Yutao Huang
Dongdong Cao,Shanyu Chen,Yebo Qin,Huaping Wu,Guanhai Ruan,Yutao Huang. Regulatory Mechanism of Salicylic Acid on Seed Germination Under Salt Stress in Kale[J]. Chinese Bulletin of Botany, 2020, 55(1): 49-61.
| Gene name | No. of accession | Primer sequence (5'-3') |
|---|---|---|
| 18srRNA | NC_016118.1 | F: GGAAGGACTTGTACGGTAACATTG R: TGGACCTGCCTCATCATACTCA |
| BoPAL1 | XM_013781025.1 | F: TCGATCTTCCACAAGATTGGT R: TCCACTTCGTCAGGAAGCA |
| BoPAL2 | XM_013749252.1 | F: AATCAGCTGAGCAACATAACCA R: GACGTTTTGCGAGACGAGA |
| BoCAT1 | XM_013742034.1 | F: ATCCTCGTGGTTTTGCTGTC R: TGCCAACAAGATCAAAGTTCC |
| BoCAT2 | XM_013774413.1 | F: ATGGAAGGCTCAGGTGTCAA R: CGTAGTGAGCTTTTCCGGATT |
| BoSOD1 | XM_013783393.1 | F: ACATCATTGTTGGAGATGATGG R: GAGGGATCTGGCAGTCAGTG |
| BoSOD3 | XM_013750980.1 | F: TGGTGATCCTGATGACCTTG R: CCTGCGTTTCCTGTTGATTT |
| BoPOD2 | XM_013729485.1 | F: GGTGGTTTCTTGCGCTGA R: CCATGGTCCGTTGATCACTA |
| BoPOD3 | XM_013728218.1 | F: CGTCAGGAAACACGGAGAA R: CCTCAATGAAGCCAAATCCT |
Table 1 Primers used for qRT-PCR
| Gene name | No. of accession | Primer sequence (5'-3') |
|---|---|---|
| 18srRNA | NC_016118.1 | F: GGAAGGACTTGTACGGTAACATTG R: TGGACCTGCCTCATCATACTCA |
| BoPAL1 | XM_013781025.1 | F: TCGATCTTCCACAAGATTGGT R: TCCACTTCGTCAGGAAGCA |
| BoPAL2 | XM_013749252.1 | F: AATCAGCTGAGCAACATAACCA R: GACGTTTTGCGAGACGAGA |
| BoCAT1 | XM_013742034.1 | F: ATCCTCGTGGTTTTGCTGTC R: TGCCAACAAGATCAAAGTTCC |
| BoCAT2 | XM_013774413.1 | F: ATGGAAGGCTCAGGTGTCAA R: CGTAGTGAGCTTTTCCGGATT |
| BoSOD1 | XM_013783393.1 | F: ACATCATTGTTGGAGATGATGG R: GAGGGATCTGGCAGTCAGTG |
| BoSOD3 | XM_013750980.1 | F: TGGTGATCCTGATGACCTTG R: CCTGCGTTTCCTGTTGATTT |
| BoPOD2 | XM_013729485.1 | F: GGTGGTTTCTTGCGCTGA R: CCATGGTCCGTTGATCACTA |
| BoPOD3 | XM_013728218.1 | F: CGTCAGGAAACACGGAGAA R: CCTCAATGAAGCCAAATCCT |
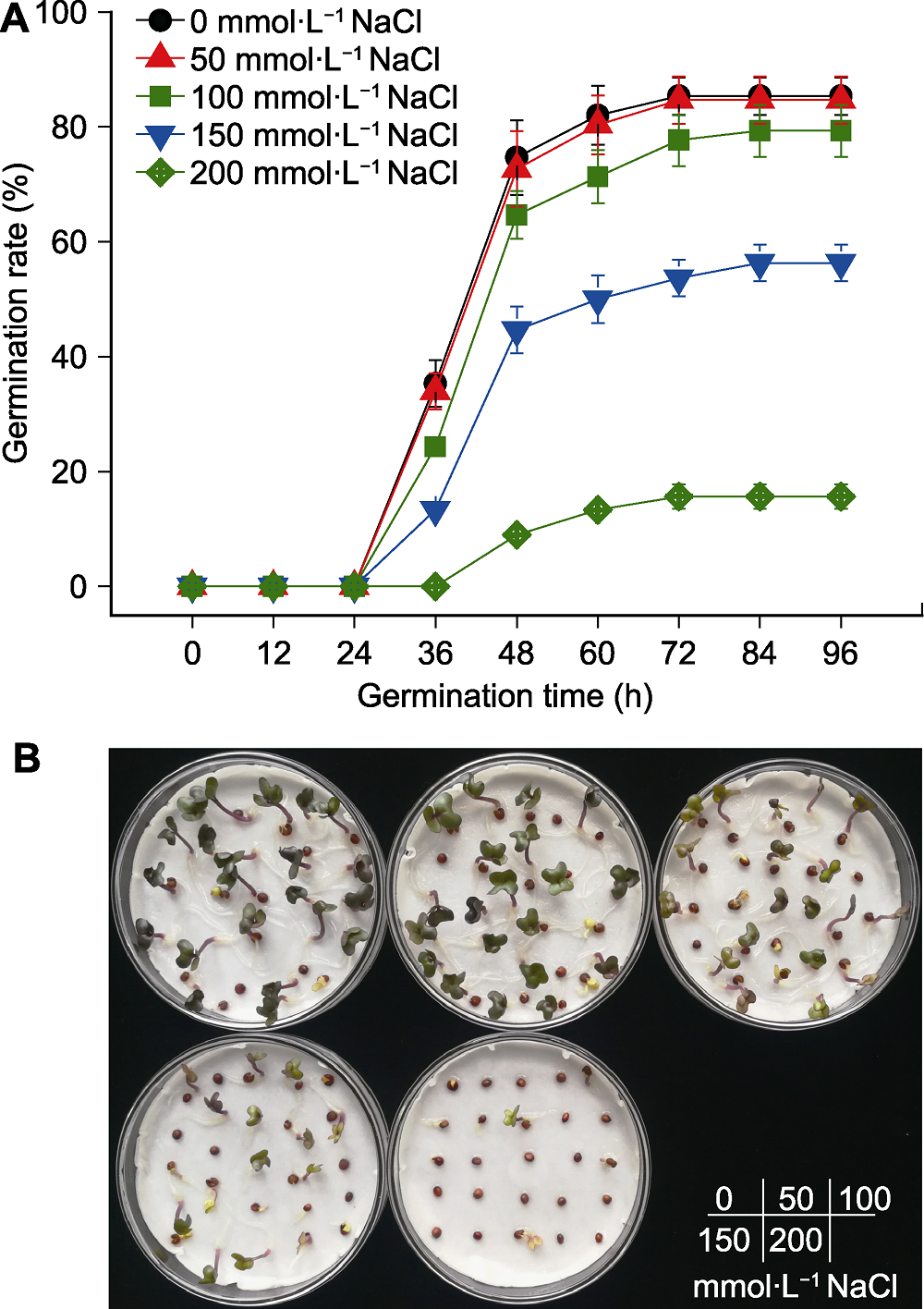
Figure 1 Effects of salt stress on seed germination of kale (A) Time course of seed germination under salt stress; (B) Seed germination after 96 hours of NaCl treatments with different concentration
| Treatments | Water uptake rate (%) | |||||
|---|---|---|---|---|---|---|
| 0-4 h | 4-8 h | 8-16 h | 16-24 h | 24-36 h | 36-48 h | |
| Control-1 | 47.18±3.52 a | 42.56±2.19 a | 19.14±0.81 a | 18.33±2.04 a | 18.21±1.33 a | 27.26±2.67 a |
| Control-2 | 37.11±4.31 b | 31.67±3.15 c | 14.62±1.09 b | 12.62±0.09 c | 10.21±1.02 b | 17.64±0.08 b |
| SA | 42.27±2.89 a | 37.75±3.09 b | 14.31±1.59 b | 14.24±0.15 b | 16.77±1.44 a | 25.81±1.77 a |
| AIP | 32.12±2.71 c | 30.41±4.93 c | 13.41±1.07 c | 12.78±0.21 c | 8.21±0.06 c | 7.55±1.23 c |
Table 2 Effects of salicylic acid (SA) and SA synthesis inhibitor (2-aminoindano-2-phosphonic acid, AIP) on water uptake rate during seed germination of kale under salt stress
| Treatments | Water uptake rate (%) | |||||
|---|---|---|---|---|---|---|
| 0-4 h | 4-8 h | 8-16 h | 16-24 h | 24-36 h | 36-48 h | |
| Control-1 | 47.18±3.52 a | 42.56±2.19 a | 19.14±0.81 a | 18.33±2.04 a | 18.21±1.33 a | 27.26±2.67 a |
| Control-2 | 37.11±4.31 b | 31.67±3.15 c | 14.62±1.09 b | 12.62±0.09 c | 10.21±1.02 b | 17.64±0.08 b |
| SA | 42.27±2.89 a | 37.75±3.09 b | 14.31±1.59 b | 14.24±0.15 b | 16.77±1.44 a | 25.81±1.77 a |
| AIP | 32.12±2.71 c | 30.41±4.93 c | 13.41±1.07 c | 12.78±0.21 c | 8.21±0.06 c | 7.55±1.23 c |
| Treatments | Germination energy (%) | Germination percentage (%) | Germination index | Vigor index | Seedling length (cm) | Seedling dry weight (g) |
|---|---|---|---|---|---|---|
| Control-1 | 85.333±3.270 a | 85.333±3.272 a | 15.742±0.742 a | 1.610±0.892 a | 8.241±0.711 a | 0.083±0.010 a |
| Control-2 | 53.667±3.208 c | 56.333±4.044 c | 8.231±0.810 c | 0.908±0.071 c | 7.822±0.635 b | 0.076±0.008 b |
| SA | 62.333±4.615 b | 68.667±4.730 b | 10.558±0.556 b | 1.167±0.064 b | 7.837±0.706 b | 0.079±0.009 b |
| AIP | 41.000±3.331 d | 46.667±2.516 d | 7.267±0.597 d | 0.762±0.079 d | 7.156±0.523 c | 0.064±0.004 c |
Table 3 Effects of salicylic acid (SA) and SA synthesis inhibitor (2-aminoindano-2-phosphonic acid, AIP) on seed germination of kale under salt stress
| Treatments | Germination energy (%) | Germination percentage (%) | Germination index | Vigor index | Seedling length (cm) | Seedling dry weight (g) |
|---|---|---|---|---|---|---|
| Control-1 | 85.333±3.270 a | 85.333±3.272 a | 15.742±0.742 a | 1.610±0.892 a | 8.241±0.711 a | 0.083±0.010 a |
| Control-2 | 53.667±3.208 c | 56.333±4.044 c | 8.231±0.810 c | 0.908±0.071 c | 7.822±0.635 b | 0.076±0.008 b |
| SA | 62.333±4.615 b | 68.667±4.730 b | 10.558±0.556 b | 1.167±0.064 b | 7.837±0.706 b | 0.079±0.009 b |
| AIP | 41.000±3.331 d | 46.667±2.516 d | 7.267±0.597 d | 0.762±0.079 d | 7.156±0.523 c | 0.064±0.004 c |
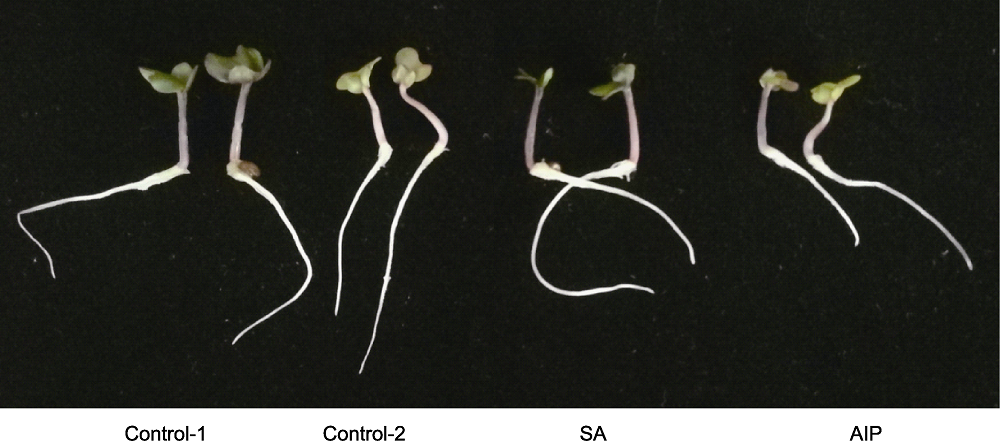
Figure 2 Effects of salicylic acid (SA) and SA synthesis inhibitor (2-aminoindano-2-phosphonic acid, AIP) on kale seedling growth at 5 days post-germination under salt stress Control-1, Control-2, SA and AIP are the same as Table 2.
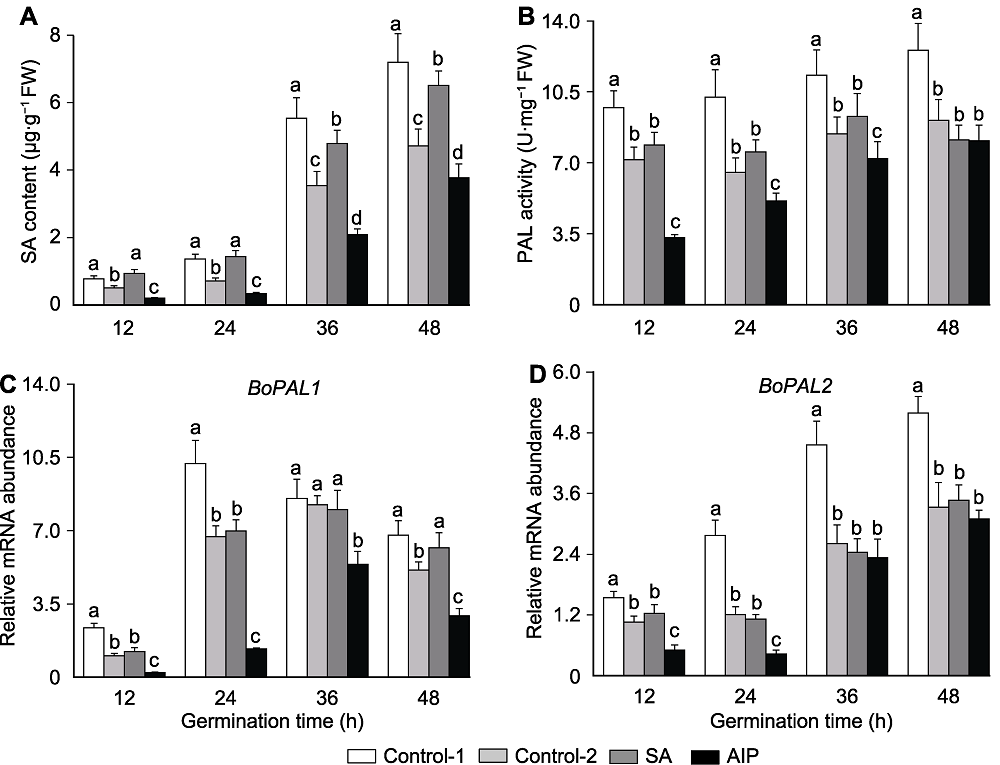
Figure 3 Effects of salicylic acid (SA) and SA synthesis inhibitor (2-aminoindano-2-phosphonic acid, AIP) on SA content (A), PAL activity (B) and BoPAL1-2 expression (C, D) in kale seeds during germination under salt stress PAL: Phenylalanine ammonia-lyase. Control-1, Control-2, SA and AIP are the same as Table 2. Different lowercase letters above the bars indicate significant differences (P<0.05) among treatments at the same sown time.
| Germination time (h) | Treatments | Ion content (mg·g-1 FW) | |||
|---|---|---|---|---|---|
| Na+ | K+ | Ca2+ | Mg2+ | ||
| 12 | Control-1 | 2.553±0.219 c | 1.471±0.134 a | 0.221±0.018 a | 0.431±0.026 a |
| Control-2 | 3.711±0.431 a | 1.227±0.203 b | 0.192±0.012 b | 0.313±0.033 c | |
| SA | 3.292±0.331 b | 1.435±0.053 a | 0.183±0.009 b | 0.372±0.042 b | |
| AIP | 3.809±0.504 a | 1.170±0.077 c | 0.211±0.018 a | 0.211±0.018 d | |
| 24 | Control-1 | 3.951±0.412 c | 1.682±0.117 a | 0.148±0.017 a | 0.477±0.033 a |
| Control-2 | 5.587±0.581 a | 1.427±0.109 b | 0.123±0.017 b | 0.385±0.049 b | |
| SA | 5.108±0.432 b | 1.623±0.152 a | 0.112±0.006 b | 0.462±0.053 a | |
| AIP | 5.717±0.367 a | 1.301±0.083 c | 0.118±0.008 b | 0.321±0.019 c | |
| 36 | Control-1 | 3.762±0.319 c | 1.677±0.092 a | 0.176±0.074 a | 0.597±0.055 a |
| Control-2 | 6.193±0.419 a | 1.486±0.106 c | 0.093±0.012 b | 0.511±0.022 b | |
| SA | 5.654±0.384 b | 1.583±0.201 b | 0.101±0.004 b | 0.590±0.044 a | |
| AIP | 6.202±0.609 a | 1.472±0.013 c | 0.095±0.011 b | 0.367±0.048 c | |
| 48 | Control-1 | 4.188±0.381 b | 1.769±0.208 a | 0.132±0.008 a | 0.646±0.054 a |
| Control-2 | 5.772±0.720 a | 1.311±0.011 c | 0.073±0.003 c | 0.491±0.060 c | |
| SA | 5.790±0.364 a | 1.422±0.015 b | 0.072±0.009 c | 0.582±0.015 b | |
| AIP | 5.831±0.519 a | 1.226±0.064 d | 0.094±0.014 b | 0.401±0.008 d | |
Table 4 Effects of salicylic acid (SA) and SA synthesis inhibitor (2-aminoindano-2-phosphonic acid, AIP) on ions contents during seed germination of kale under salt stress
| Germination time (h) | Treatments | Ion content (mg·g-1 FW) | |||
|---|---|---|---|---|---|
| Na+ | K+ | Ca2+ | Mg2+ | ||
| 12 | Control-1 | 2.553±0.219 c | 1.471±0.134 a | 0.221±0.018 a | 0.431±0.026 a |
| Control-2 | 3.711±0.431 a | 1.227±0.203 b | 0.192±0.012 b | 0.313±0.033 c | |
| SA | 3.292±0.331 b | 1.435±0.053 a | 0.183±0.009 b | 0.372±0.042 b | |
| AIP | 3.809±0.504 a | 1.170±0.077 c | 0.211±0.018 a | 0.211±0.018 d | |
| 24 | Control-1 | 3.951±0.412 c | 1.682±0.117 a | 0.148±0.017 a | 0.477±0.033 a |
| Control-2 | 5.587±0.581 a | 1.427±0.109 b | 0.123±0.017 b | 0.385±0.049 b | |
| SA | 5.108±0.432 b | 1.623±0.152 a | 0.112±0.006 b | 0.462±0.053 a | |
| AIP | 5.717±0.367 a | 1.301±0.083 c | 0.118±0.008 b | 0.321±0.019 c | |
| 36 | Control-1 | 3.762±0.319 c | 1.677±0.092 a | 0.176±0.074 a | 0.597±0.055 a |
| Control-2 | 6.193±0.419 a | 1.486±0.106 c | 0.093±0.012 b | 0.511±0.022 b | |
| SA | 5.654±0.384 b | 1.583±0.201 b | 0.101±0.004 b | 0.590±0.044 a | |
| AIP | 6.202±0.609 a | 1.472±0.013 c | 0.095±0.011 b | 0.367±0.048 c | |
| 48 | Control-1 | 4.188±0.381 b | 1.769±0.208 a | 0.132±0.008 a | 0.646±0.054 a |
| Control-2 | 5.772±0.720 a | 1.311±0.011 c | 0.073±0.003 c | 0.491±0.060 c | |
| SA | 5.790±0.364 a | 1.422±0.015 b | 0.072±0.009 c | 0.582±0.015 b | |
| AIP | 5.831±0.519 a | 1.226±0.064 d | 0.094±0.014 b | 0.401±0.008 d | |
| Germination time (h) | Treatments | Osmotic adjustment substance | ||
|---|---|---|---|---|
| Soluble sugar (mg·g-1 FW) | Soluble protein (mg·g-1 FW) | Free proline (μg·g-1 FW) | ||
| 12 | Control-1 | 13.56±0.81 d | 3.57±0.40 d | 108.19±1.23 c |
| Control-2 | 23.19±1.73 b | 8.11±0.71 b | 143.77±1.35 b | |
| SA | 29.28±2.00 a | 12.17±1.03 a | 156.53±1.17 a | |
| AIP | 19.13±1.23 c | 6.59±0.33 c | 157.11±0.90 a | |
| 24 | Control-1 | 17.11±0.92 c | 6.17±0.55 c | 115.64±0.87 c |
| Control-2 | 26.35±1.74 b | 9.27±0.49 b | 167.59±2.11 a | |
| SA | 33.27±4.02 a | 13.56±0.84 a | 142.13±1.33 b | |
| AIP | 25.77±3.19 b | 6.59±0.30 c | 122.64±2.16 c | |
| 36 | Control-1 | 23.85±1.39 d | 6.12±0.41 c | 117.26±1.55 c |
| Control-2 | 37.50±2.47 b | 11.73±1.23 a | 141.33±1.46 b | |
| SA | 43.59±4.33 a | 12.19±1.19 a | 172.57±1.15 a | |
| AIP | 30.66±2.20 c | 9.13±0.52 b | 109.33±0.82 c | |
| 48 | Control-1 | 22.74±3.10 c | 5.80±0.37 b | 133.52±0.81 c |
| Control-2 | 34.19±2.79 a | 8.62±0.79 a | 167.29±1.31 b | |
| SA | 36.82±2.12 a | 8.93±0.64 a | 128.19±1.55 c | |
| AIP | 26.70±2.34 b | 6.18±0.56 b | 186.53±2.08 a | |
Table 5 Effects of salicylic acid (SA) and SA synthesis inhibitor (2-aminoindano-2-phosphonic acid, AIP) on osmotic adjustment substances during seed germination of kale under salt stress
| Germination time (h) | Treatments | Osmotic adjustment substance | ||
|---|---|---|---|---|
| Soluble sugar (mg·g-1 FW) | Soluble protein (mg·g-1 FW) | Free proline (μg·g-1 FW) | ||
| 12 | Control-1 | 13.56±0.81 d | 3.57±0.40 d | 108.19±1.23 c |
| Control-2 | 23.19±1.73 b | 8.11±0.71 b | 143.77±1.35 b | |
| SA | 29.28±2.00 a | 12.17±1.03 a | 156.53±1.17 a | |
| AIP | 19.13±1.23 c | 6.59±0.33 c | 157.11±0.90 a | |
| 24 | Control-1 | 17.11±0.92 c | 6.17±0.55 c | 115.64±0.87 c |
| Control-2 | 26.35±1.74 b | 9.27±0.49 b | 167.59±2.11 a | |
| SA | 33.27±4.02 a | 13.56±0.84 a | 142.13±1.33 b | |
| AIP | 25.77±3.19 b | 6.59±0.30 c | 122.64±2.16 c | |
| 36 | Control-1 | 23.85±1.39 d | 6.12±0.41 c | 117.26±1.55 c |
| Control-2 | 37.50±2.47 b | 11.73±1.23 a | 141.33±1.46 b | |
| SA | 43.59±4.33 a | 12.19±1.19 a | 172.57±1.15 a | |
| AIP | 30.66±2.20 c | 9.13±0.52 b | 109.33±0.82 c | |
| 48 | Control-1 | 22.74±3.10 c | 5.80±0.37 b | 133.52±0.81 c |
| Control-2 | 34.19±2.79 a | 8.62±0.79 a | 167.29±1.31 b | |
| SA | 36.82±2.12 a | 8.93±0.64 a | 128.19±1.55 c | |
| AIP | 26.70±2.34 b | 6.18±0.56 b | 186.53±2.08 a | |
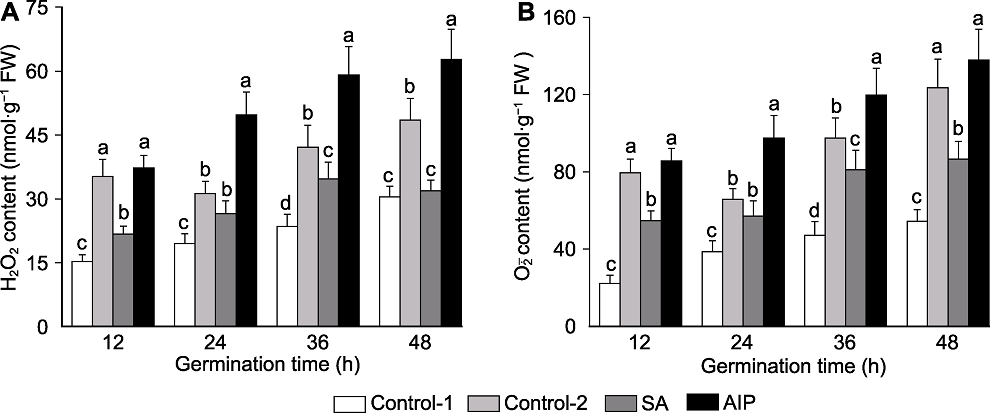
Figure 4 Effects of salicylic acid (SA) and SA synthesis inhibitor (2-aminoindano-2-phosphonic acid, AIP) on H2O2 (A) and O2-. (B) contents of kale seed during germination under salt stress Control-1, Control-2, SA and AIP are the same as Table 2. Different lowercase letters above the bars indicate significant differences (P<0.05) among treatments at the same sown time.
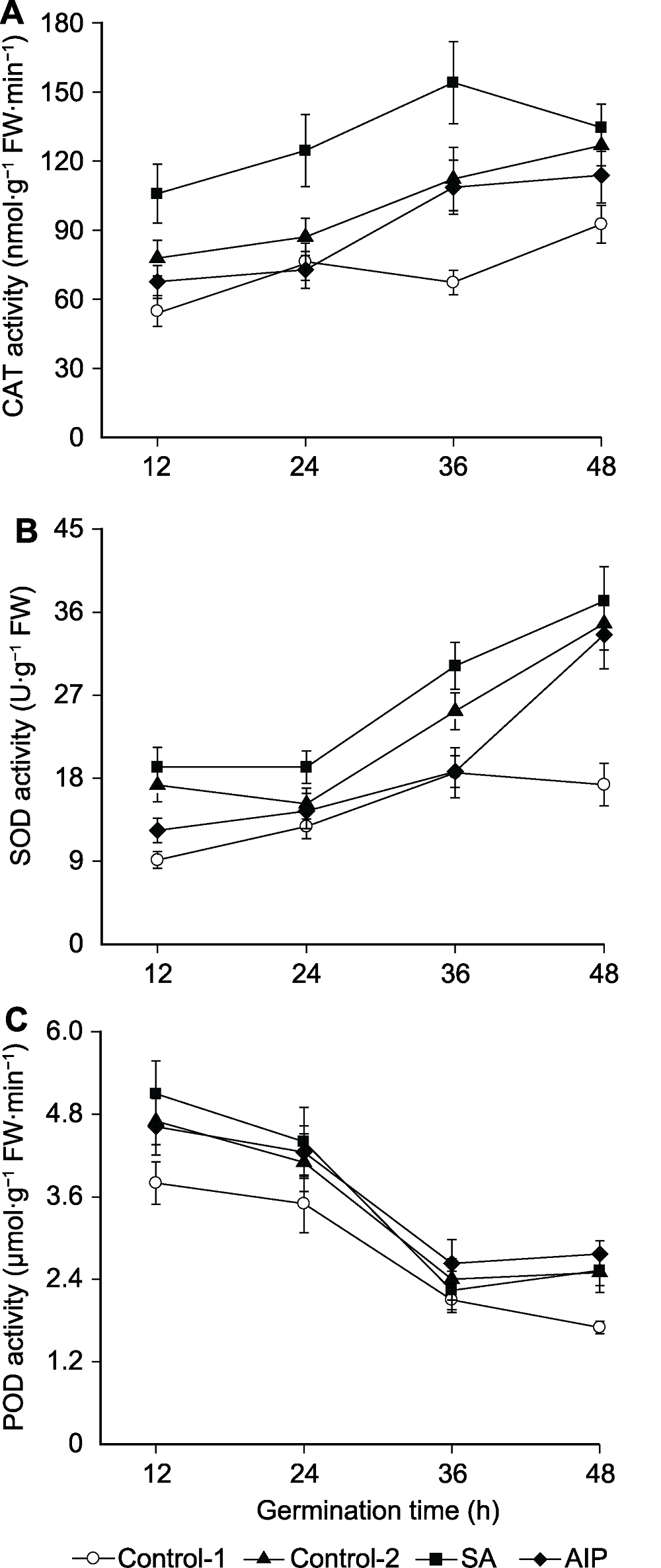
Figure 5 Effects of salicylic acid (SA) and SA synthesis inhibitor (2-aminoindano-2-phosphonic acid, AIP) on the activities of catalase (CAT) (A), superoxide dismutase (SOD) (B), and peroxidase (POD) (C) in kale seeds during germination under salt stress Control-1, Control-2, SA and AIP are the same as Table 2.
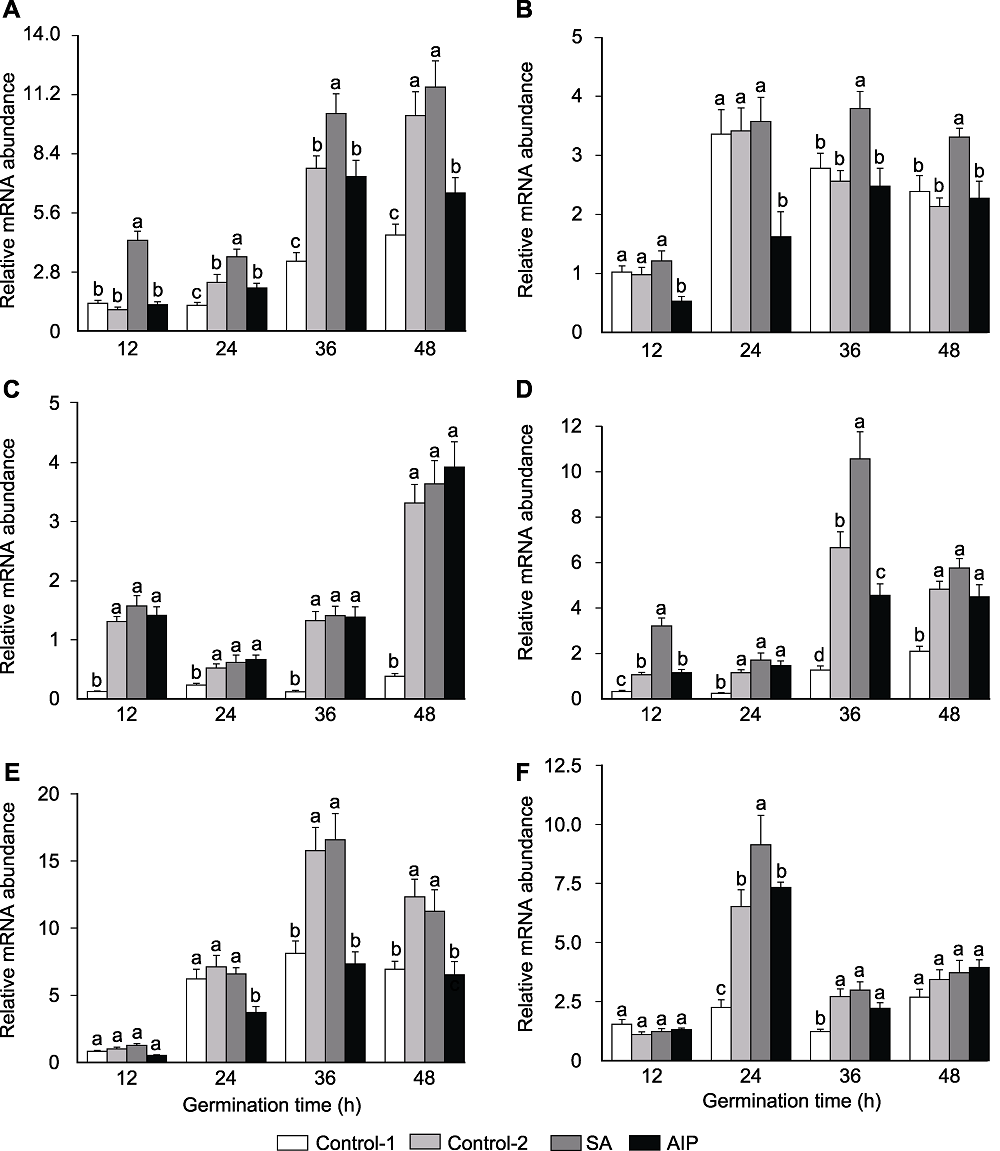
Figure 6 Effects of salicylic acid (SA) and SA synthesis inhibitor (2-aminoindano-2-phosphonic acid, AIP) on gene expression of BoCAT1 (A), BoCAT2 (B), BoSOD1 (C), BoSOD3 (D), BoPOD2 (E), and BoPOD3 (F) during seed germination of kale under salt stress Control-1, Control-2, SA and AIP are the same as Table 2. Different lowercase letters above the bars indicate significant differences (P<0.05) among treatments at the same sown time.
| [1] | 蔡晓锋, 胡体旭, 叶杰, 张余洋, 李汉霞, 叶志彪 (2015). 植物盐胁迫抗性的分子机制研究进展. 华中农业大学学报 34(3), 134-141. |
| [2] | 郭君洁, 贵红霞, 武欣, 李鸿雁, 李大红 (2018). 盐胁迫下过表达BoRACK1基因对羽衣甘蓝种子萌发的影响. 植物生理学报 54, 1365-1371. |
| [3] | 胡晋 (2014). 种子学. 北京: 中国农业出版社. pp. 82. |
| [4] | 李伟, 郭君洁, 李鸿雁 (2017). H2O2对盐胁迫下羽衣甘蓝幼苗生长的影响. 江苏农业科学 45(22), 149-152. |
| [5] | 沙汉景, 胡文成, 贾琰, 王新鹏, 田雪飞, 于美芳, 赵宏伟 (2017). 外源水杨酸、脯氨酸和γ-氨基丁酸对盐胁迫下水稻产量的影响. 作物学报 43, 1677-1688. |
| [6] | 王洋 (2014). 水杨酸对不同耐寒型玉米种子和幼苗抗寒性的调控作用研究. 博士论文. 杭州: 浙江大学. pp. 64-72. |
| [7] | 张万萍, 杨民, 江燕, 彭家国 (2008). H2O2浸种对羽衣甘蓝种子萌发及幼苗的影响. 种子 27(12), 98-100. |
| [8] | 赵秀枢, 李名扬, 张文玲, 刘凡 (2009). 观赏羽衣甘蓝高频再生体系的建立. 基因组学与应用生物学 28, 141-148. |
| [9] | 朱广龙, 宋成钰, 于林林, 陈许兵, 智文芳, 刘家玮, 焦秀荣, 周桂生 (2018). 外源生长调节物质对甜高粱种子萌发过程中盐分胁迫的缓解效应及其生理机制. 作物学报 44, 1713-1724. |
| [10] | Ci LJ, Yang XH (2010). Desertification and Its Control in China. Beijing: Higher Education Press. pp. 17-26. |
| [11] | Fariduddin Q, Khan TA, Yusuf M, Aafaqee ST, Khalil RRAE (2018). Ameliorative role of salicylic acid and spermidine in the presence of excess salt in Lycopersicon esculentum. Photosynthetica 56, 750-762. |
| [12] | Farooq M, Gogoi N, Hussain M, Barthakur S, Paul S, Bharadwaj N, Migdadi HM, Alghamdi SS, Siddique KHM (2017). Effects, tolerance mechanisms and management of salt stress in grain legumes. Plant Physiol Biochem 118, 199-217. |
| [13] | Hao L, Zhao Y, Jin DD, Zhang L, Bi XH, Chen HX, Xu Q, Ma CY, Li GZ (2012). Salicylic acid-altering Arabidopsis mutants response to salt stress. Plant Soil 354, 81-95. |
| [14] | Hayat Q, Hayat S, Irfan M, Ahmad A (2010). Effect of exogenous salicylic acid under changing environment: a review. Environ Exp Bot 68, 14-25. |
| [15] | Jayakannan M, Bose J, Babourina O, Shabala S, Massart A, Poschenrieder C, Rengel Z (2015). The NPR1-dependent salicylic acid signaling pathway is pivotal for enhanced salt and oxidative stress tolerance in Arabidopsis. J Exp Bot 66, 1865-1875. |
| [16] | Ke QB, Ye J, Wang BM, Ren JH, Yin LN, Deng XP, Wang SW (2018). Melatonin mitigates salt stress in wheat seedlings by modulating polyamine metabolism. Front Plant Sci 9, 914. |
| [17] | Liu J, Gao H, Wang X, Zheng Q, Wang Y, Wang X, Wang Q (2014). Effects of 24-epibrassinolide on plant growth, osmotic regulation and ion homeostasis of salt-stressed canola. Plant Biol 16, 440-450. |
| [18] | Ma XH, Zheng J, Zhang XL, Hu QD, Qian RJ (2017). Salicylic acid alleviates the adverse effects of salt stress on Dianthus superbus(Caryophyllaceae) by activating photosynthesis, protecting morphological structure, and enhancing the antioxidant system. Front Plant Sci 8, 600. |
| [19] | Maathuis FJM, Ahmad I, Patishtan J (2014). Regulation of Na+ fluxes in plants. Front Plant Sci 5, 467. |
| [20] | Nimir NEA, Lu SY, Zhou GS, Guo WS, Ma BL, Wang YH (2015). Comparative effects of gibberellic acid, kinetin and salicylic acid on emergence, seedling growth and the antioxidant defence system of sweet sorghum (Sorghum bicolor) under salinity and temperature stresses. Crop Pasture Sci 66, 145-157. |
| [21] | Nimir NEA, Zhou G, Guo W, Ma B, Lu S, Wang Y (2017). Effect of foliar application of GA3, kinetin, and salicylic acid on ions content, membrane permeability, and photosynthesis under salt stress of sweet sorghum [Sorghum bicolor(L.) Moench]. Can J Plant Sci 97, 525-535. |
| [22] | Rajjou L, Belghazi M, Huguet R, Robin C, Moreau A, Job C, Job D (2006). Proteomic investigation of the effect of salicylic acid on Arabidopsis seed germination and establishment of early defense mechanisms. Plant Physiol 141, 910-923. |
| [23] | Rajjou L, Duval M, Gallardo K, Catusse J, Bally J, Job C, Job D (2012). Seed germination and vigor. Annu Rev Plant Biol 63, 507-533. |
| [24] | Sharma P, Dubey RS (2007). Involvement of oxidative stress and role of antioxidative defense system in growing rice seedlings exposed to toxic concentrations of aluminum. Plant Cell Rep 26, 2027-2038. |
| [25] | Shu K, Qi Y, Chen F, Meng YJ, Luo XF, Shuai HW, Zhou WG, Ding J, Du JB, Liu J, Yang F, Wang Q, Liu WG, Yong TW, Wang XC, Feng YQ, Yang WU (2017). Salt stress represses soybean seed germination by negatively regulating GA biosynthesis while positively mediating ABA biosynthesis. Front Plant Sci 8, 1372. |
| [26] | Singh DP, Kumar J, Singh M, Singh S, Prasad SM, Dwivedi R, Singh MPVVB (2016). Role of salicylic acid-seed priming in the regulation of chromium (VI) and UV-B toxicity in maize seedlings. Plant Growth Regul 78, 79-91. |
| [27] | Solecka D, Kacperska A (2013). Phenylpropanoid deficiency affects the course of plant acclimation to cold. Physiol Plant 119, 253-262. |
| [28] | Torun H (2019). Time-course analysis of salicylic acid effects on ROS regulation and antioxidant defense in roots of hulled and hulless barley under combined stress of drought, heat and salinity. Physiol Plant 165, 169-182. |
| [29] | Wang JZ, Tao ST, Qi KJ, Wu J, Wu HQ, Zhang SL (2011). Changes in photosynthetic properties and antioxidative system of pear leaves to boron toxicity. Afr J Biotechnol 10, 19693-19700. |
| [30] | Yalpani N, León J, Lawton MA, Raskin I (1993). Pathway of salicylic acid biosynthesis in healthy and virus-inoculated tobacco. Plant Physiol 103, 315-321. |
| [31] | Zhang JL, Flowers TJ, Wang SM (2010). Mechanisms of sodium uptake by roots of higher plants. Plant Soil 326, 45-60. |
| [32] | Zhu JK (2001). Plant salt tolerance. Trends Plant Sci 6, 66-71. |
| [1] | Zhou xin-yu, huiliang liu, GAO Bei, LU Yuting, TAO Lingqing, WEN Xiaohu, ZHANG Lan, ZHANG Yuan-Ming. Reproductive Biology of the Endangered and Endemic Species Nymphaea candida C. Presl in Xinjiang [J]. , 2025, 49(濒危植物的保护与恢复): 0-. |
| [2] | Can Ye, Linbo Yao, Ying Jin, Rong Gao, Qi Tan, Xuying Li, Yanjun Zhang, Xifeng Chen, Bojun Ma, Wei Zhang, Kewei Zhang. Establishment and Application of a High-throughput Screening Method for Salicylic Acid Metabolic Mutants in Rice [J]. Chinese Bulletin of Botany, 2025, 60(4): 1-0. |
| [3] | Yang Li, Qu Xitong, Chen Zihang, Zou Tingting, Wang Quanhua, Wang Xiaoli. Identification of the Spinach AT-hook Gene Family and Analysis of Expression Profiles [J]. Chinese Bulletin of Botany, 2025, 60(3): 377-392. |
| [4] | Xu Tiantian, Yang Peijian, Zhou Xiaoxi, Cao Yi, Chen Yanhong, Liu Guoyuan, Zhang Jian, Wei Hui. Analysis of Physicochemical Characteristics and Expression Characteristics of Lagerstroemia indica GolS Family Genes [J]. Chinese Bulletin of Botany, 2025, 60(3): 393-406. |
| [5] | SUN Long, LI Wen-Bo, LOU Hu, YU Cheng, HAN Yu, HU Tong-Xin. Effects of fire disturbance on seed germination of Larix gmelinii [J]. Chin J Plant Ecol, 2024, 48(6): 770-779. |
| [6] | Yan Luo, Qiyuan Liu, Yuanbing Lü, Yue Wu, Yaoyu Tian, Tian An, Zhenhua Li. Photothermal Sensitivity of Phytochrome Mutants During Seed Germination in Arabidopsis thaliana [J]. Chinese Bulletin of Botany, 2024, 59(5): 752-762. |
| [7] | YUAN Han, ZHONG Ai-Wen, LIU Song-Ping, PENG Yan-Song, XU Lei. Differences in the germination characteristics of Schoenoplectiella triangulata seeds and methods for breaking seed dormancy [J]. Chin J Plant Ecol, 2024, 48(5): 638-650. |
| [8] | Jinyu Du, Zhen Sun, Yanlong Su, Heping Wang, Yaling Liu, Zhenying Wu, Feng He, Yan Zhao, Chunxiang Fu. Identification and Functional Analysis of an Agropyron mongolicum Caffeic Acid 3-O-methyltransferase Gene AmCOMT1 [J]. Chinese Bulletin of Botany, 2024, 59(3): 383-396. |
| [9] | Xiaobo Zhu, Zhang Dong, Mengjin Zhu, Jin Hu, Cheng Lin, Min Chen, Yajing Guan. Indispensable Material for Germination: Long-lived mRNAs of Plant Seed [J]. Chinese Bulletin of Botany, 2024, 59(3): 355-372. |
| [10] | Yuan Yuan, Enhebayaer, Qi Yanhua. Research Advances in Biological Functions of GH3 Gene Family in Plants [J]. Chinese Bulletin of Botany, 2023, 58(5): 770-782. |
| [11] | Cai Shuyu, Liu Jianxin, Wang Guofu, Wu Liyuan, Song Jiangping. Regulatory Mechanism of Melatonin on Tomato Seed Germination Under Cd2+ Stress [J]. Chinese Bulletin of Botany, 2023, 58(5): 720-732. |
| [12] | Qi Zhang, Wenjing Zhang, Xiankai Yuan, Ming Li, Qiang Zhao, Yanli Du, Jidao Du. The Regulatory Mechanism of Melatonin on Nucleic Acid Repairing of Common Bean (Phaseolus vulgaris) at the Sprout Stage Under Salt Stress [J]. Chinese Bulletin of Botany, 2023, 58(1): 108-121. |
| [13] | Feifei Wang, Zhenxiang Zhou, Yi Hong, Yangyang Gu, Chao Lü, Baojian Guo, Juan Zhu, Rugen Xu. Identification of the NF-YC Genes in Hordeum vulgare and Expression Analysis Under Salt Stress [J]. Chinese Bulletin of Botany, 2023, 58(1): 140-149. |
| [14] | LI Shao-Yang, MA Hong-Yuan, ZHAO Dan-Dan, MA Meng-Yao, QI Wen-Wen. Research progress on the effects of fire-related cues on seed germination [J]. Chin J Plant Ecol, 2021, 45(11): 1177-1190. |
| [15] | Zeyi Wang, Hengjia Zhang, Yucai Wang, Xietian Chen, Yuchun Ba. Effects of Deficit Irrigation on the Photosynthetic and Physiological Characteristics of Leaves and Yield of Isatis tinctoria [J]. Chinese Bulletin of Botany, 2020, 55(6): 705-714. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||