

Chinese Bulletin of Botany ›› 2019, Vol. 54 ›› Issue (2): 227-236.DOI: 10.11983/CBB18142 cstr: 32102.14.CBB18142
Special Issue: 逆境生物学专辑 (2019年54卷2期)
• EXPERIMENTAL COMMUNICATIONS • Previous Articles Next Articles
Huaifeng Gao,Yafei Zhang,Guodong Wang,Xiwu Sun,Yue He,Futian Peng( ),Yuansong Xiao(
),Yuansong Xiao( )
)
Received:2018-06-19
Accepted:2018-11-16
Online:2019-03-01
Published:2019-09-01
Contact:
Futian Peng,Yuansong Xiao
Huaifeng Gao,Yafei Zhang,Guodong Wang,Xiwu Sun,Yue He,Futian Peng,Yuansong Xiao. The Effect of Molybdenum on Drought Stress Response in Peach[J]. Chinese Bulletin of Botany, 2019, 54(2): 227-236.
| Primer name | Primer (5′→3′) |
|---|---|
| PpABA3-F | CAATGGTACTGCAGCATCTATGA |
| PpABA3-R | TAAGTTATATGCCTCTCCTGTTGG |
| PpACTIN-F | TGCATTGTGTATGTGTTCATCTACA |
| PpACTIN-R | CTTCACCATTCCAGTTCCATTGTC |
Table 1 Primers used in this study
| Primer name | Primer (5′→3′) |
|---|---|
| PpABA3-F | CAATGGTACTGCAGCATCTATGA |
| PpABA3-R | TAAGTTATATGCCTCTCCTGTTGG |
| PpACTIN-F | TGCATTGTGTATGTGTTCATCTACA |
| PpACTIN-R | CTTCACCATTCCAGTTCCATTGTC |
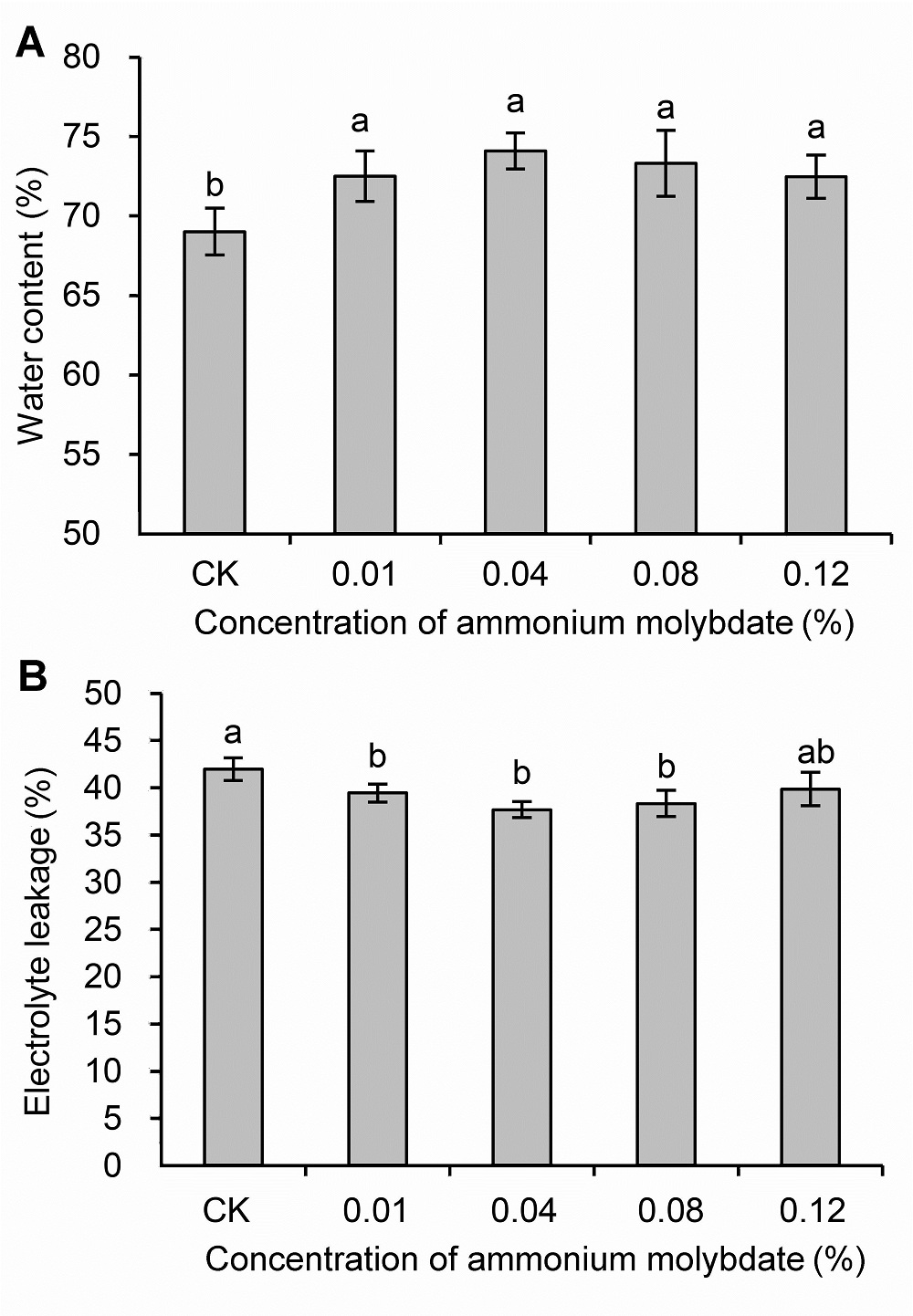
Figure 1 Water content (A) and electrolyte leakage (B) of peach seedlings treated with different concentrations of ammonium molybdate under drought stress Different lowercase letters above the bars indicate significant differences at 0.05 level.
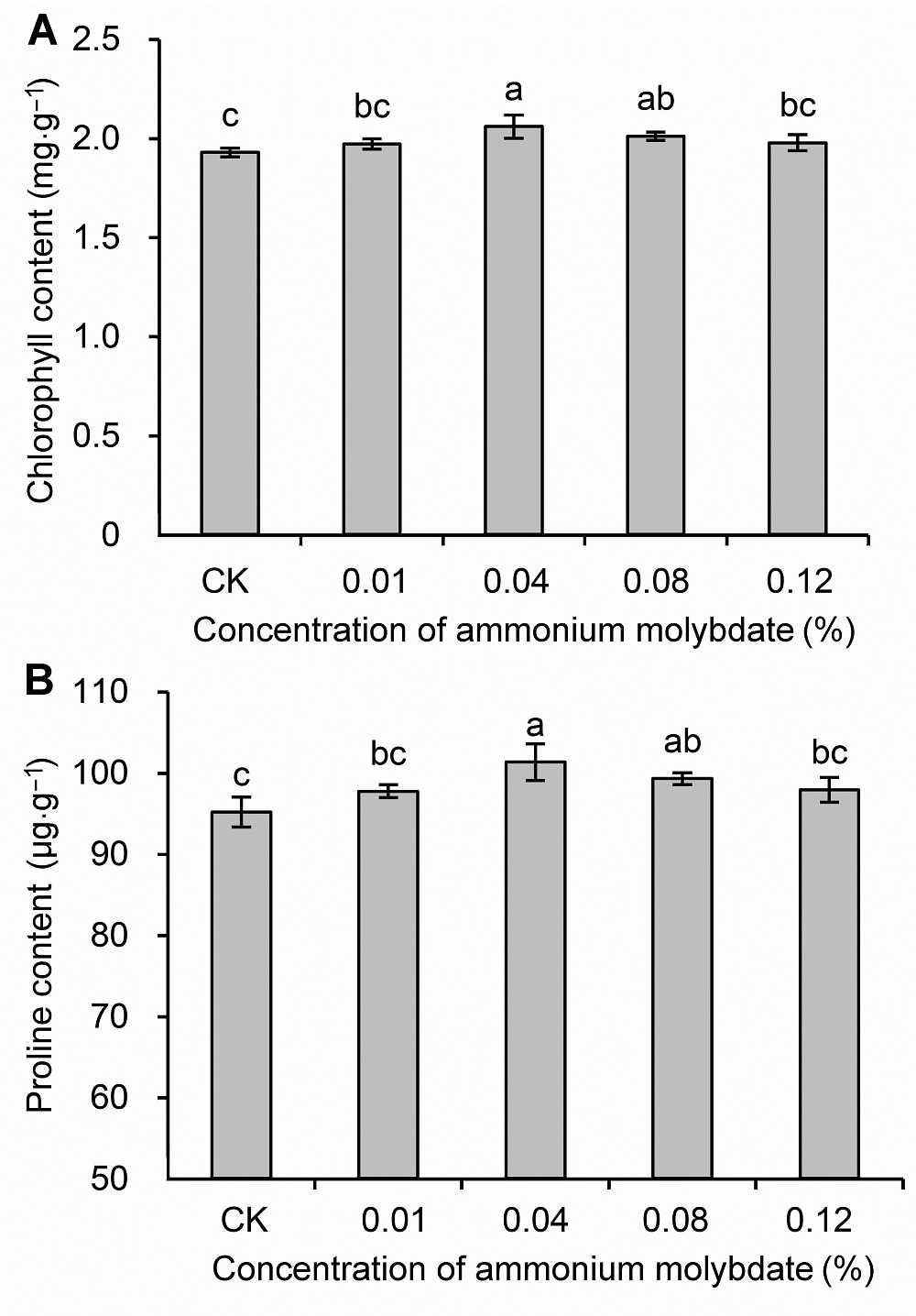
Figure 2 Chlorophyll (A) and proline (B) content of peach seedlings treated with different concentrations of ammonium molybdate under drought stressDifferent lowercase letters above the bars indicate significant differences at 0.05 level.
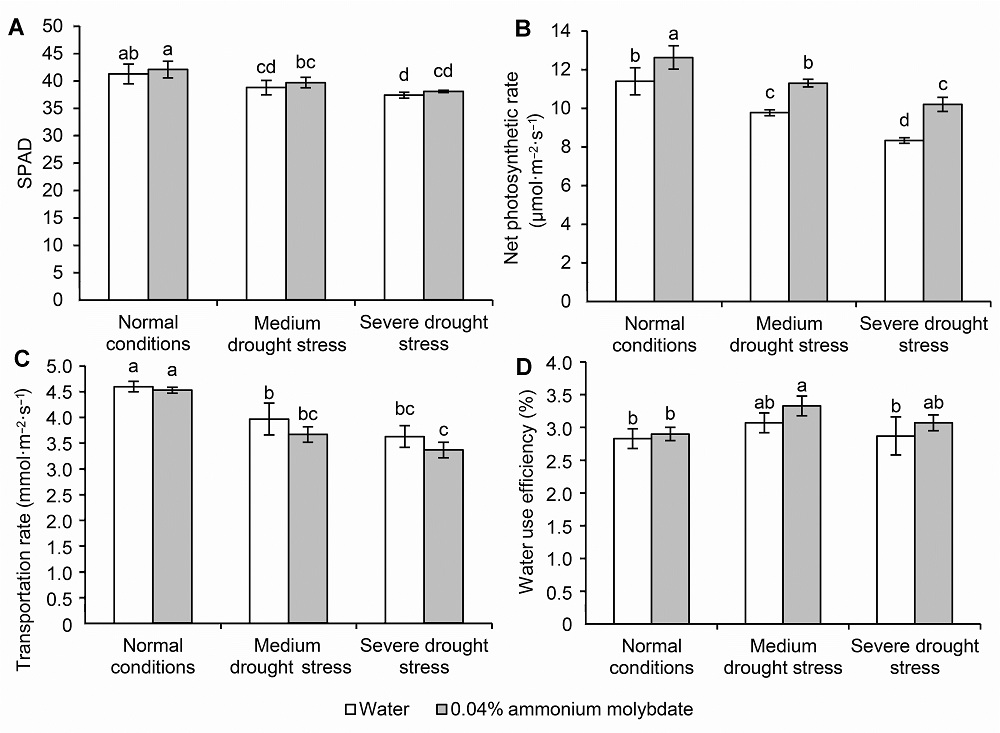
Figure 3 The SPAD (A), net photosynthetic rate (B), transportation rate (C) and water use efficiency (D) of peach seedlings treated with ammonium molybdate under drought stress Different lowercase letters above the bars indicate significant differences at 0.05 level.
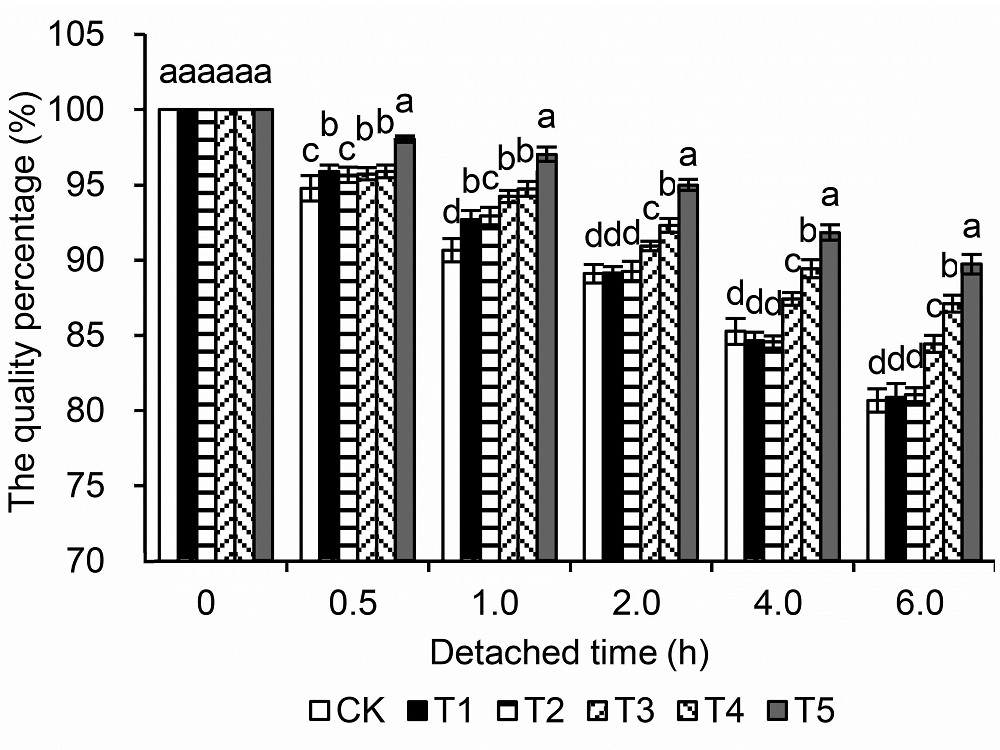
Figure 4 The changes of the quality percentage of detached leaves of peach CK: Normal conditions+water; T1: Normal conditions+0.04% ammonium molybdate; T2: Medium drought stress+water; T3: Medium drought stress+0.04% ammonium molybdate; T4: Severe drought stress+water; T5: Severe drought stress+ 0.04% ammonium molybdate. Different lowercase letters above the bars indicate significant differences at 0.05 level.
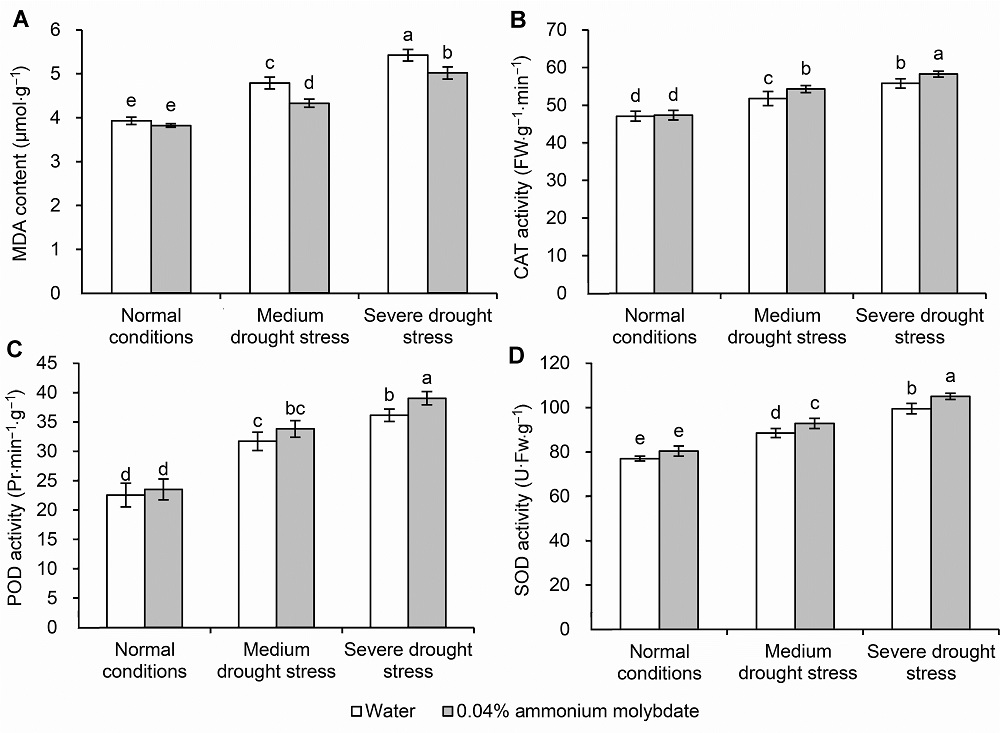
Figure 5 The effect of ammonium molybdate on the MDA content (A) and the activity of CAT (B), POD (C) and SOD (D) of peach seedlingsMDA: Malondialdehyde; CAT: Catalase; POD: Peroxidase; SOD: Superoxide dismutase. Different lowercase letters above the bars indicate significant differences at 0.05 level.
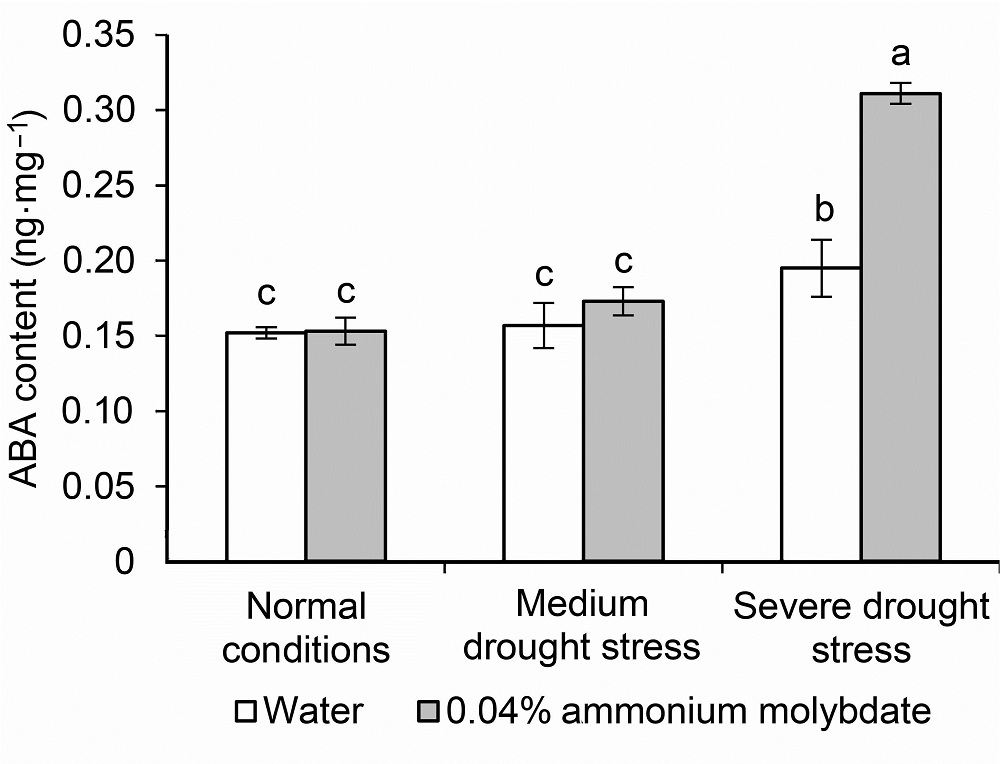
Figure 6 ABA content of peach seedlings treated with ammonium molybdate under drought stress Different lowercase letters above the bars indicate significant differences at 0.05 level.
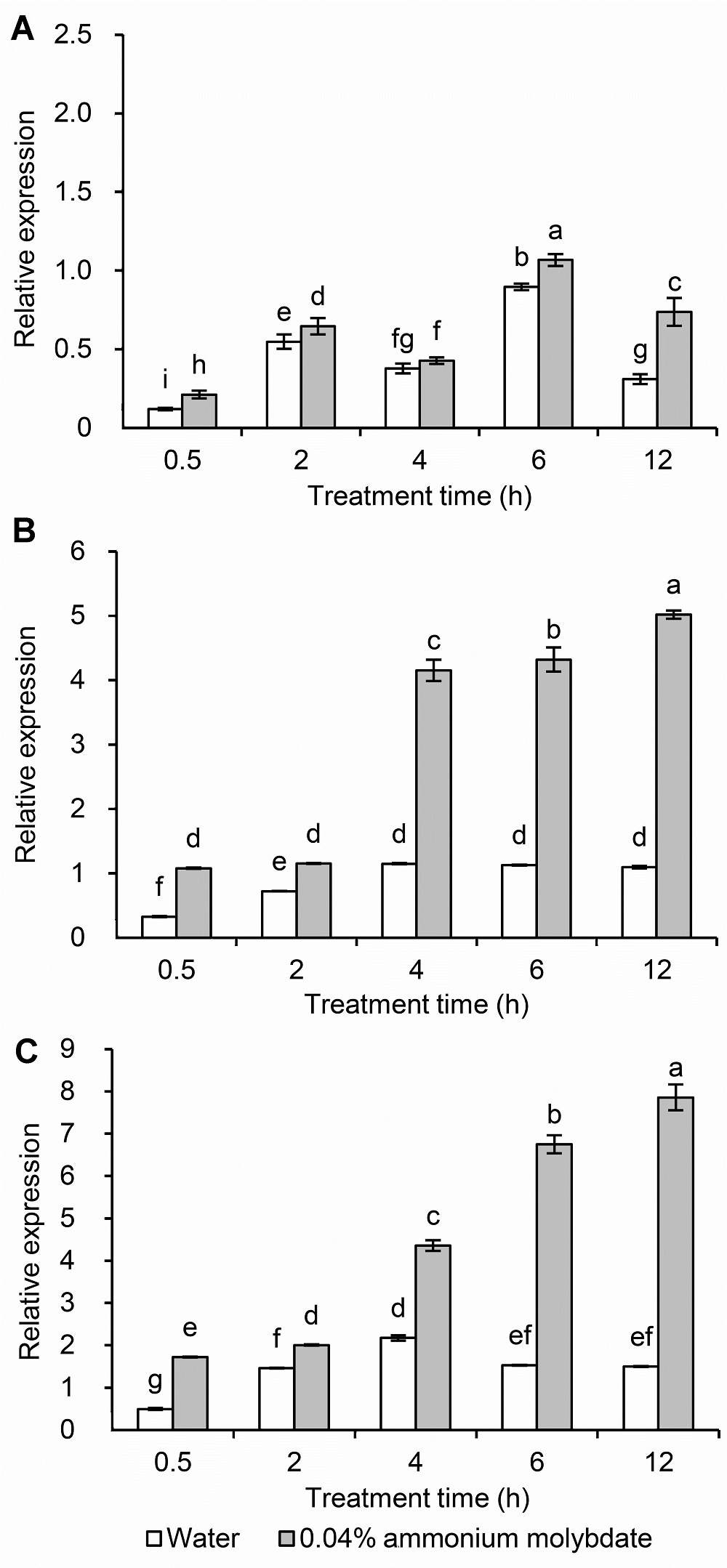
Figure 7 The expression of LOS5/ABA3 in leaves of peach seedlings under different drought stresses(A) Normal conditions; (B) Medium drought stress; (C) Severe drought stress. Different lowercase letters above the bars indicate significant differences at 0.05 level.
| [1] | 李洁, 列志旸, 薛立, 黄威龙, 许建新 ( 2016). 干旱胁迫对3种园林绿化树种幼苗生理指标的影响. 西南林业大学学报 36(2), 56-61. |
| [2] | 邵惠芳, 陈征, 许嘉阳, 范艺宽, 黄五星, 张海枞, 许自成 ( 2016). 两种烟草幼苗叶片对不同强度干旱胁迫的生理响应比较. 植物生理学报 52, 1861-1871. |
| [3] | 宋哲, 章家长, 楮庆全, 李召虎 ( 2008). 转LOS5/ABA3基因早熟禾(P. pratensis L.)的获得及抗旱和抗寒性分析. 中国科技论文在线. . |
| [4] | 苏金, 朱汝财 ( 2001). 渗透胁迫调节的转基因表达对植物抗旱耐盐性的影响. 植物学通报 18, 129-136. |
| [5] | 孙学成 ( 2006). 钼提高冬小麦抗寒力的生理基础及分子机制. 博士论文. 武汉: 华中农业大学. |
| [6] | 王华, 王飞, 陈登文, 丁勤 ( 2000). 低温胁迫对杏花SOD活性和膜脂过氧化的影响. 果树科学 17, 197-201. |
| [7] | 王尉, 乐胜峰, 贺天雨, 杜宁, 林楠 ( 2018). 高效液相色谱法测定苜蓿中脱落酸的含量. 分析仪器 53(2), 53-57. |
| [8] | 吴明才, 肖昌珍 ( 1994). 大豆钼素研究. 大豆科学 13, 245-251. |
| [9] | 武松伟, 胡承孝, 谭启玲, 孙学成 ( 2016). 钼与植物抗寒性研究进展. 湖北农业科学 55, 13-16, 42. |
| [10] | 张士功, 刘国栋, 刘更另 ( 2001). 植物营养与作物抗旱性. 植物学通报 18, 64-69, 63. |
| [11] | 张志良, 瞿伟菁, 李小方 ( 2009). 植物生理学实验指导(第4版). 北京: 高等教育出版社. pp. 4-5. |
| [12] | 赵世杰, 史国安, 董新纯 ( 2002). 植物生理学实验指导. 北京: 中国农业科学技术出版社. pp. 164-165. |
| [13] |
Atwal PS, Scaglia F ( 2016). Molybdenum cofactor deficiency. Mol Genet Metab 117, 1-4.
DOI URL |
| [14] | Barker R, Dawe D, Tuong TP, Bhuiyan SI, Guerra LC ( 1998). The outlook for water resources in the Year 2020: challenges for research on water management in rice production. International Rice Commission Newsletter 49, 7-21. |
| [15] | Garbero M, Pedranzani H, Zirulnik F, Molina A, Pérez- Chaca MV, Vigliocco A, Abdala G ( 2011). Short-term cold stress in two cultivars of Digitaria eriantha : effects on stress-related hormones and antioxidant defense system. Acta Physiol Plant 33, 497-507. |
| [16] | Hanks RJ ( 1983). Yield and water-use relationships: an overview. In: Taylor HM, Jordan WR, Sinclair TR, eds. Limitations to Efficient Water Use in Crop Production. Ma- dison: American Society of Agronomy. pp. 393-411. |
| [17] |
Hille R, Hall J, Basu P ( 2014). The mononuclear molybdenum enzymes. Chem Rev 114, 3963-4038.
DOI URL |
| [18] |
Li YJ, Zhang JC, Zhang J, Hao L, Hua JP, Duan LS, Zhang MC, Li ZH ( 2013). Expression of an Arabidopsis molybdenum cofactor sulphurase gene in soybean enhances drought tolerance and increases yield under field conditions. Plant Biotechnol J 11, 747-758.
DOI URL |
| [19] | Lu Y, Li YJ, Zhang JC, Xiao YT, Yue YS, Duan LS, Zhang MC, Li ZH ( 2013). Overexpression of Arabidopsis molybdenum cofactor sulfurase gene confers drought tolerance in maize ( Zea mays L.). PLoS One 8, e52126. |
| [20] |
Ma FF, Lu R, Liu HY, Shi B, Zhang JH, Tan MP, Zhang AY, Jiang MY ( 2012). Nitric oxide-activated calcium/calmo- dulin-dependent protein kinase regulates the abscisic acid-induced antioxidant defence in maize. J Exp Bot 63, 4835-4847.
DOI URL |
| [21] |
Mahajan S, Tuteja N ( 2005). Cold, salinity and drought stres- ses: an overview. Arch Biochem Biophys 444, 139-158.
DOI URL |
| [22] | Patnaitk D, Khurana P ( 2002). Wheat biotechnology: a minireview. Electron J Biotechnol 4, 74-102. |
| [23] |
Sun XC, Hu CX, Tan QL, Liu JS, Liu HE ( 2009). Effects of molybdenum on expression of cold-responsive genes in abscisic acid (ABA)-dependent and ABA-independent pathways in winter wheat under low-temperature stress. Ann Bot 104, 345-356.
DOI URL |
| [24] |
Wilkinson S, Davies WJ ( 2002). ABA-based chemical signaling: the co-ordination of responses to stress in plants. Plant Cell Environ 25, 195-210.
DOI URL |
| [25] | Wu SW, Hu CX, Tan QL, Nie ZJ, Sun XC ( 2014). Effects of molybdenum on water utilization, antioxidative defense system and osmotic-adjustment ability in winter wheat (Triticum aestivum) under drought stress. Plant Physiol Biochem 83, 365-374. |
| [26] | Xiong LM, Ishitani M, Lee H, Zhu JK ( 2001). The Arabidopsis LOS5/ABA3 locus encodes a molybdenum cof- actor sulfurase and modulates cold stress- and osmotic stress-responsive gene expression. Plant Cell 13, 2063-2084. |
| [27] |
Xiong LM, Zhu JK ( 2001). Abiotic stress signal transduction in plants: molecular and genetic perspectives. Physiol Plant 112, 152-166.
DOI URL |
| [28] | Yue SY, Ming CZ, Zhang JC, Tian XL, Duan LS, Li ZH ( 2012). Overexpression of the AtLOS5 gene increased abscisic acid level and drought tolerance in transgenic cotton. J Exp Bot 63, 3741-3748. |
| [29] | Yue YS, Zhang MC, Zhang JC, Duan LS, Li ZH ( 2011). Arabidopsis LOS5/ABA3 overexpression in transgenic tobacco( Nicotiana tabacum cv. ‘Xanthi-nc’) results in enhanced drought tolerance. Plant Sci 181, 405-411. |
| [30] | Zhang M, Hu CX, Zhao XH, Tan QL, Sun XC, Cao AY, Cui M, Zhang Y ( 2012). Molybdenum improves antioxidant and osmotic-adjustment ability against salt stress in Chinese cabbage ( Brassica campestris L. ssp. pekinensis). Plant Soil 355, 375-383. |
| [31] |
Zhu JK ( 2002). Salt and drought stress signal transduction in plants. Annu Rev Plant Biol 53, 247-273.
DOI URL |
| [1] | Bei Fan, Min Ren, Yanfeng Wang, Fengfeng Dang, Guoliang Chen, Guoting Cheng, Jinyu Yang, Huiru Sun. Functions of SlWRKY45 in Response to Low-temperature and Drought Stress in Tomato [J]. Chinese Bulletin of Botany, 2025, 60(2): 186-203. |
| [2] | LONG Ji-Lan, JIANG Zheng, LIU Ding-Qin, MIAO Yu-Xuan, ZHOU Ling-Yan, FENG Ying, PEI Jia-Ning, LIU Rui-Qiang, ZHOU Xu-Hui, FU Yu-Ling. Effects of drought on plant root exudates and associated rhizosphere priming effect: review and prospect [J]. Chin J Plant Ecol, 2024, 48(7): 817-827. |
| [3] | Ziyang Wang, Shengxue Liu, Zhirui Yang, Feng Qin. Genetic Dissection of Drought Resistance in Maize [J]. Chinese Bulletin of Botany, 2024, 59(6): 883-902. |
| [4] | Xingxin Liao, Yi Niu, Xingwu Duo, Akeyedeli Jumahazi, Marhaba Abdukuyum, Rizwangul Hufur, Haiyan Lan, Jing Cao. Heterologous Expression of Suaeda aralocaspica SaPEPC2 Gene Improves Drought Resistance and Photosynthesis in Transgenic Tobacco [J]. Chinese Bulletin of Botany, 2024, 59(4): 585-599. |
| [5] | Laipeng Zhao, Baike Wang, Tao Yang, Ning Li, Haitao Yang, Juan Wang, Huizhuan Yan. Investigation of the Regulation of Drought Tolerance by the SlHVA22l Gene in Tomato [J]. Chinese Bulletin of Botany, 2024, 59(4): 558-573. |
| [6] | Heping Wang, Zhen Sun, Yuchen Liu, Yanlong Su, Jinyu Du, Yan Zhao, Hongbo Zhao, Zhaoming Wang, Feng Yuan, Yaling Liu, Zhenying Wu, Feng He, Chunxiang Fu. Sequence Identification and Functional Analysis of Cinnamyl Alcohol Dehydrogenase Gene from Agropyron mongolicum [J]. Chinese Bulletin of Botany, 2024, 59(2): 204-216. |
| [7] | Zhang Yingchuan, Wu Xiaomingyu, Tao Baolong, Chen Li, Lu Haiqin, Zhao Lun, Wen Jing, Yi Bin, Tu Jinxing, Fu Tingdong, Shen Jinxiong. Bna-miR43 Mediates the Response of Drought Tolerance in Brassica napus [J]. Chinese Bulletin of Botany, 2023, 58(5): 701-711. |
| [8] | Lei Zhang, Pengfei Jiang, Yiming Wang, Ting Lan, Yanjing Liu, Qingyin Zeng. Comparative Study on the Drought Resistance of Young Seedling from Populus laurifolia × P. simonii F1 Progeny [J]. Chinese Bulletin of Botany, 2023, 58(4): 519-534. |
| [9] | CHEN Tu-Qiang, XU Gui-Qing, LIU Shen-Si, LI Yan. Hydraulic traits adjustments and nonstructural carbohydrate dynamics of Haloxylon ammodendron under drought stress [J]. Chin J Plant Ecol, 2023, 47(10): 1407-1421. |
| [10] | ZHOU Jie, YANG Xiao-Dong, WANG Ya-Yun, LONG Yan-Xin, WANG Yan, LI Bo-Rui, SUN Qi-Xing, SUN Nan. Difference in adaptation strategy between Haloxylon ammodendron and Alhagi sparsifolia to drought [J]. Chin J Plant Ecol, 2022, 46(9): 1064-1076. |
| [11] | LUO Dan-Dan, WANG Chuan-Kuan, JIN Ying. Response mechanisms of hydraulic systems of woody plants to drought stress [J]. Chin J Plant Ecol, 2021, 45(9): 925-941. |
| [12] | Yongmei Che, Yanjun Sun, Songchong Lu, Lixia Hou, Xinxin Fan, Xin Liu. AtMYB77 Involves in Lateral Root Development via Regulating Nitric Oxide Biosynthesis under Drought Stress in Arabidopsis thaliana [J]. Chinese Bulletin of Botany, 2021, 56(4): 404-413. |
| [13] | Yigong Zhang, Yi Zhang, Ayibaiheremu Mutailifu, Daoyuan Zhang. Heterologous Overexpression of Desiccation-tolerance Moss ScABI3 Gene Changes Stomatal Phenotype and Improves Drought Resistance in Transgenic Arabidopsis [J]. Chinese Bulletin of Botany, 2021, 56(4): 414-421. |
| [14] | Jiaxin Li, Xia Li, Yinfeng Xie. Mechanism on Drought Tolerance Enhanced by Exogenous Trehalose in C4-PEPC Rice [J]. Chinese Bulletin of Botany, 2021, 56(3): 296-314. |
| [15] | Chenyang Pan, Yue Zhang, Han Lin, Qianyu Chen, Kairu Yang, Jiaji Jiang, Mengjia Li, Tao Lu, Kexin Wang, Mei Lu, Sheng Wang, Hanfei Ye, Yuchun Rao, Haitao Hu. QTL Mapping and Candidate Gene Analysis on Rice Leaf Water Potential [J]. Chinese Bulletin of Botany, 2021, 56(3): 275-283. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||