

Chinese Bulletin of Botany ›› 2018, Vol. 53 ›› Issue (6): 840-847.DOI: 10.11983/CBB17208 cstr: 32102.14.CBB17208
• TECHNIQUES AND METHODS • Previous Articles Next Articles
Zhang Xuhong1,2, Wang Di2, Liang Zhenxu1,2, Sun Meiyu2, Zhang Jinzheng2, Shi Lei1,2,*( )
)
Received:2017-11-07
Accepted:2018-02-05
Online:2018-11-01
Published:2018-12-05
Contact:
Shi Lei
Zhang Xuhong, Wang Di, Liang Zhenxu, Sun Meiyu, Zhang Jinzheng, Shi Lei. Callus Induction and Establishment of a Plant Regeneration System with Lilium martagon[J]. Chinese Bulletin of Botany, 2018, 53(6): 840-847.
| No. | Concentrations of plant growth regulator (mg·L-1) | |||
|---|---|---|---|---|
| TDZ | PIC | NAA | 6-BA | |
| CK | 0.00 | 0.00 | 0.00 | 0.00 |
| 1 | 0.20 | 0.01 | 0.00 | 0.00 |
| 2 | 0.60 | 0.01 | 0.00 | 0.00 |
| 3 | 0.20 | 0.10 | 0.00 | 0.00 |
| 4 | 0.60 | 0.10 | 0.00 | 0.00 |
| 5 | 0.20 | 0.00 | 0.50 | 0.00 |
| 6 | 0.60 | 0.00 | 0.50 | 0.00 |
| 7 | 0.20 | 0.00 | 1.00 | 0.00 |
| 8 | 0.60 | 0.00 | 1.00 | 0.00 |
| 9 | 0.00 | 0.00 | 0.10 | 0.10 |
| 10 | 0.00 | 0.00 | 0.10 | 0.50 |
| 11 | 0.00 | 0.00 | 0.10 | 1.00 |
Table 1 Media design for callus induction and proliferation of Lilium martagon
| No. | Concentrations of plant growth regulator (mg·L-1) | |||
|---|---|---|---|---|
| TDZ | PIC | NAA | 6-BA | |
| CK | 0.00 | 0.00 | 0.00 | 0.00 |
| 1 | 0.20 | 0.01 | 0.00 | 0.00 |
| 2 | 0.60 | 0.01 | 0.00 | 0.00 |
| 3 | 0.20 | 0.10 | 0.00 | 0.00 |
| 4 | 0.60 | 0.10 | 0.00 | 0.00 |
| 5 | 0.20 | 0.00 | 0.50 | 0.00 |
| 6 | 0.60 | 0.00 | 0.50 | 0.00 |
| 7 | 0.20 | 0.00 | 1.00 | 0.00 |
| 8 | 0.60 | 0.00 | 1.00 | 0.00 |
| 9 | 0.00 | 0.00 | 0.10 | 0.10 |
| 10 | 0.00 | 0.00 | 0.10 | 0.50 |
| 11 | 0.00 | 0.00 | 0.10 | 1.00 |
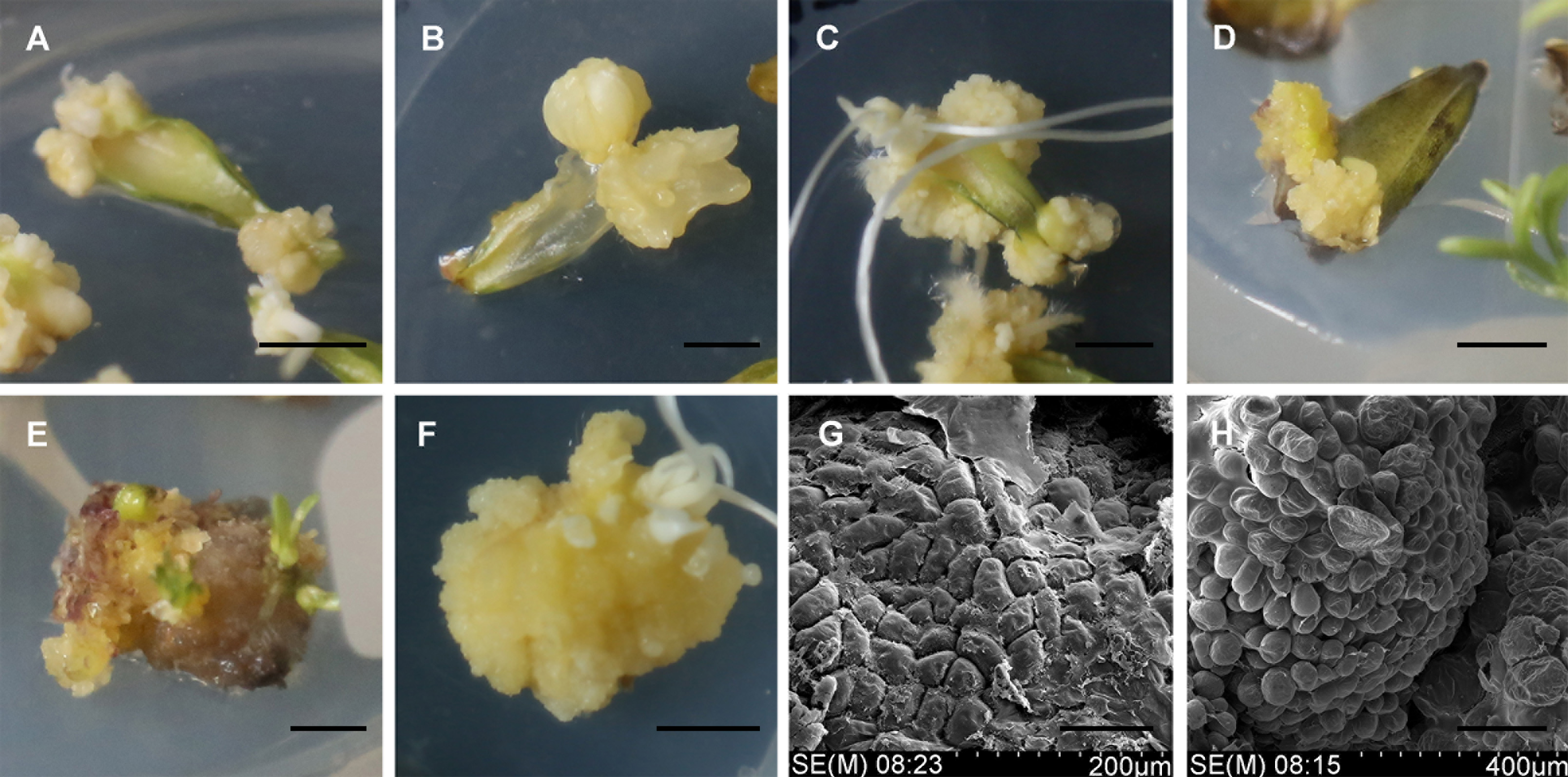
Figure 1 The callus induction and proliferation of Lilium martagon(A), (B) Callus induction in MS medium containing TDZ and PIC in the dark (Bar=5 mm); (C) Callus induction in MS medium containing TDZ and NAA in the dark (Bar=5 mm); (D) Callus induction in MS medium containing TDZ and NAA under 16/8 h light/dark photoperiod (Bar=5 mm); (E), (G) Callus proliferation under 16/8 h light/dark photoperiod ((E) Bar=5 mm; (G) Bar=100 μm); (F), (H) Callus proliferation in the dark ((F) Bar=5 mm; (H) Bar=200 μm)
| No. | Rate of callus induced (%) | Number of buds/explant |
|---|---|---|
| CK | 0.00 Dc | 1.09±0.25 Bc |
| 1 | 63.34±7.46 AB | 1.13±0.33 AB |
| 2 | 66.67±7.86 A | 0.69±0.24 C |
| 3 | 34.44±12.67 C | 1.49±0.29 A |
| 4 | 54.45±8.24 B | 1.16±0.23 AB |
| 5 | 77.14±7.82 a | 1.46±0.28 bc |
| 6 | 48.57±7.82 b | 1.57±0.29 b |
| 7 | 45.72±6.39 b | 1.74±0.26 ab |
| 8 | 68.57±15.65 a | 2.00±0.35 a |
Table 2 Effects of different plant growth regulators combi- nation on the callus induction of Lilium martagon (in the dark)
| No. | Rate of callus induced (%) | Number of buds/explant |
|---|---|---|
| CK | 0.00 Dc | 1.09±0.25 Bc |
| 1 | 63.34±7.46 AB | 1.13±0.33 AB |
| 2 | 66.67±7.86 A | 0.69±0.24 C |
| 3 | 34.44±12.67 C | 1.49±0.29 A |
| 4 | 54.45±8.24 B | 1.16±0.23 AB |
| 5 | 77.14±7.82 a | 1.46±0.28 bc |
| 6 | 48.57±7.82 b | 1.57±0.29 b |
| 7 | 45.72±6.39 b | 1.74±0.26 ab |
| 8 | 68.57±15.65 a | 2.00±0.35 a |
| No. | Rate of callus induced (%) | Number of buds/explant |
|---|---|---|
| CK | 0.00 c | 0.90±0.15 b |
| 5 | 32.86±12.64 a | 1.17±0.37 ab |
| 6 | 16.67±11.78 b | 1.37±0.48 a |
| 7 | 13.34±7.46 bc | 1.00±0.26 ab |
| 8 | 20.00±13.94 ab | 0.46±0.22 c |
Table 3 Effects of TDZ and NAA on the callus induction of Lilium martagon (under 16/8 h light/dark photoperiod)
| No. | Rate of callus induced (%) | Number of buds/explant |
|---|---|---|
| CK | 0.00 c | 0.90±0.15 b |
| 5 | 32.86±12.64 a | 1.17±0.37 ab |
| 6 | 16.67±11.78 b | 1.37±0.48 a |
| 7 | 13.34±7.46 bc | 1.00±0.26 ab |
| 8 | 20.00±13.94 ab | 0.46±0.22 c |
| Light/dark condition | Proliferation coefficient |
|---|---|
| Photoperiod | 1.39±0.21 b |
| Dark condition | 1.86±0.30 a |
Table 4 Effects of light/dark condition on callus proliferation of Lilium martagon
| Light/dark condition | Proliferation coefficient |
|---|---|
| Photoperiod | 1.39±0.21 b |
| Dark condition | 1.86±0.30 a |
| No. | Proliferation coefficient | Number of buds/callus |
|---|---|---|
| 9 | 2.24±0.17 b | 2.37±0.39 a |
| 10 | 2.93±0.51 a | 1.65±0.38 b |
| 11 | 1.86±0.30 b | 0.88±0.41 c |
Table 5 Effects of 6-BA and NAA on callus proliferation of Lilium martagon (in the dark)
| No. | Proliferation coefficient | Number of buds/callus |
|---|---|---|
| 9 | 2.24±0.17 b | 2.37±0.39 a |
| 10 | 2.93±0.51 a | 1.65±0.38 b |
| 11 | 1.86±0.30 b | 0.88±0.41 c |
| Light/dark condition | Differentiation rate of callus (%) | Number of buds/callus |
|---|---|---|
| Photoperiod | 74.00±13.42 a | 2.86±0.88 b |
| Dark condition | 81.11±10.68 a | 5.43±1.60 a |
Table 6 Effects of light/dark condition on the callus differen- tiation of Lilium martagon
| Light/dark condition | Differentiation rate of callus (%) | Number of buds/callus |
|---|---|---|
| Photoperiod | 74.00±13.42 a | 2.86±0.88 b |
| Dark condition | 81.11±10.68 a | 5.43±1.60 a |
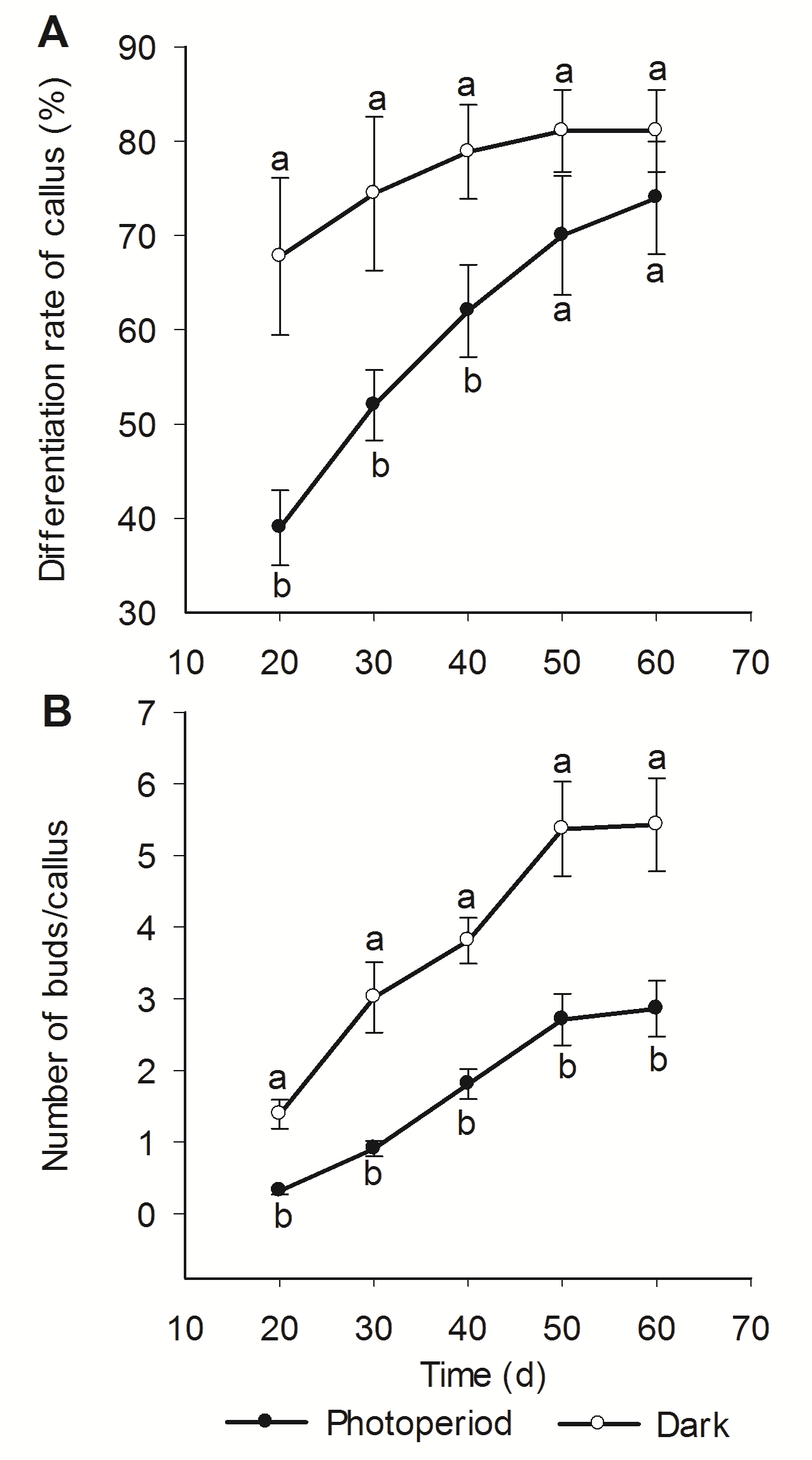
Figure 2 Dynamic change of callus differentiation of Lilium martagon cultured under different light/dark condition(A) Differentiation rate of callus; (B) Number of buds/callus

Figure 3 Somatic embryogenesis and rooting of Lilium martagon(A)-(E) Developmental process of somatic embryos generated from callus cultured in the dark (Bar=0.5 mm); (F) Embryo-like structures obtained from callus under 16/8 h light/dark photoperiod (Bar=1 mm); (G)-(I) Day 1, 5 and 15 of rooting buds differentiated under 16/8 h light/dark photoperiod (Bar=2 mm); (J)-(L) Day 1, 5 and 15 of rooting buds differentiated in the dark (Bar=2 mm); (M) Rooting buds differentiated under 16/8 h light/dark photoperiod (Bar=5 mm); (N) Rooting buds differentiated in the dark (Bar=5 mm); (O) Plantlets (Bar=1 cm); (P) Transplantation (Bar=2 cm)
| [1] | 陈云凤, 张春荣, 黄霞, 黄学林 (2006). TDZ对植物体细胞胚胎发生的作用. 植物生理学通讯 42, 127-133. |
| [2] |
李晓艳, 陈莉, 辛海波, 义鸣放 (2009). 百合鳞片薄层细胞培养高效再生体系的建立. 华中农业大学学报 28, 351-355.
DOI URL |
| [3] |
李雪艳, 严瑞, 张静, 付麟岚, 段鑫, 王春夏, 孙红梅 (2016). 东方百合Tiger Woods离体快繁技术体系的建立. 沈阳农业大学学报 47, 654-660.
DOI URL |
| [4] |
李莺, 李星, 李生玲, 徐薇 (2013). ‘黄天霸’百合花器官愈伤组织诱导及植株再生. 热带作物学报 34, 1507-1512.
DOI URL |
| [5] | 马怡迪 (2015). 野生百合高效再生体系建立及愈伤组织增殖调控研究. 硕士论文. 杭州: 浙江大学. pp. 28-39. |
| [6] |
孙安妮, 张延龙, 牛立新, 王仙芝, 崔雅静, 王润丰 (2011). 宜昌百合体细胞胚诱导及植株再生. 西北农业学报 20, 142-146.
DOI URL |
| [7] | 孙红梅, 王锦霞, 段鑫, 张静, 李雪艳, 高鹤 (2015). 重瓣东方百合Double surprise离体快繁技术体系的建立. 沈阳农业大学学报 46, 391-397. |
| [8] | 王杰, 刘国锋, 包满珠, 黄莉 (2008). 麝香百合胚性愈伤组织状态的调整与植株再生. 园艺学报 35, 1795-1802. |
| [9] |
吴耿, 付春华, 黄永伟, 李为, 余龙江, 栗茂腾 (2011). 岩溶环境下华南忍冬气孔泌钙及其生物矿化. 植物学报 46, 658-664.
DOI URL |
| [10] |
徐晓峰, 黄学林 (2003). TDZ: 一种有效的植物生长调节剂. 植物学通报 20, 227-237.
DOI URL |
| [11] | 袁素霞, 李佳, 明军, 刘春, 徐雷锋, 袁迎迎 (2015). 百合未授粉子房离体培养胚胎形成及植株再生. 植物学报 50, 378-387. |
| [12] | 翟彦, 张宗勤, 贾敏, 王岩, 宋西德, 周雷 (2011). 百合体细胞胚胎发生和植株再生. 西北植物学报 31, 834-841. |
| [13] |
张翔宇, 陈杰, 吉云, 严显进, 王彩云, 阮培均, 王永 (2016). 淡黄花百合珠芽诱导脱分化的研究. 江苏农业科学 44, 58-61.
DOI URL |
| [14] |
张艺萍, 吴丽芳, 吴学尉, 崔光芬, 丁丁, 王继华 (2008). 东方百合胚性愈伤组织诱导和植株再生研究. 江西农业学报 20, 33-36, 39.
DOI URL |
| [15] |
Bakhshaie M, Babalar M, Mirmasoumi M, Khalighi A (2010). Somatic embryogenesis and plant regeneration ofLilium ledebourii (Baker) Boiss, an endangered species. Plant Cell Tiss Org 102, 229-235.
DOI URL |
| [16] | Feldmaier C, McRae J (1982). Lilien. Stuttgart: Verlag Eugen Ulmer. |
| [17] | Kedra M, Bach A (2005). Morphogenesis of Lilium martagon L. explants in callus culture. Acta Biol Cracov Bot 47, 65-73. |
| [18] | Khosravi S, Azghandi AV, Mojtahedi N, Haddad R (2007). In vitro propagation of Lilium longiflorum var. Ceb-Dazzle through direct somatic embryogenesis. Pak J Biol Sci 10, 2517-2521. |
| [19] |
Mori S, Adachi Y, Horimoto S, Suzuki S, Nakano M (2005). Callus formation and plant regeneration in various Lilium species and cultivars. In Vitro Cell Dev-Pl 41, 783-788.
DOI URL |
| [20] | Qi YY, Du LJ, Quan YH, Tian FF, Liu YL, Wang YJ (2014). Agrobacterium-mediated transformation of embryogenic cell suspension cultures and plant regeneration in Lilium tenuifolium Oriental × trumpet ‘Robina’. Acta Physiol Plant 36, 2047-2057. |
| [21] | Synge PM (1980). Lilies. London: B. T. Batsford. pp. 34-38. |
| [22] | Tang YP, Liu XQ, Gituru RW, Chen LQ (2010). Callus induction and plant regeneration from in vitro cultured lea- ves, petioles and scales of Lilium leucanthum(Baker) Bak- er. Biotechnol Biotec Eq 24, 2071-2076. |
| [1] | Jingjing Li, Yanfei Li, Anqi Wang, Jiaying Wang, Chengyan Deng, Min Lu, Jianying Ma, Silan Dai. Establishment of Regeneration and Genetic Transformation System for Chrysanthemum Cultivar ‘Wandai Fengguang’ [J]. Chinese Bulletin of Botany, 2025, 60(4): 1-0. |
| [2] | Yuze Liu, Yifei Wang, Weizhen Ren, Hao Li, Bin Lu, Bingshe Lu, Xiaoyue Yu. Establishment of Immature Embryo Rescue and Regeneration System for Pyrus calleryana cv. ‘Cleveland’ [J]. Chinese Bulletin of Botany, 2024, 59(5): 800-809. |
| [3] | Xuping Tian, Kangjie Yue, Jiali Wang, Huixin Liu, Ziyin Shi, Hongwei Kang. Callus Induction and Plant Regeneration of Dracocephalum rupestre [J]. Chinese Bulletin of Botany, 2024, 59(4): 613-625. |
| [4] | Hao Zeng, Peifang Li, Zhihui Guo, Chunlin Liu, Ying Ruan. Establishment of a Regeneration System for Lunaria annua [J]. Chinese Bulletin of Botany, 2024, 59(3): 433-440. |
| [5] | Xiaoyun Wu, Minling Liao, Xueru Li, Zichun Shu, Jiatong Xin, Bohan Zhang, Silan Dai. Establishment of Regeneration System of Chrysanthemum vestitum with Three Floret Forms [J]. Chinese Bulletin of Botany, 2024, 59(2): 245-256. |
| [6] | Minling Liao, Ya Pu, Xiaoyun Wu, Chaofeng Ma, Wenkui Wang, Silan Dai. Establishment of Regeneration System of Chrysanthemum indicum in Pingtan with Various Ligulate Floret Form [J]. Chinese Bulletin of Botany, 2023, 58(3): 449-460. |
| [7] | Dongrui Zhang, Zhigang Bu, Lingling Chen, Ying Chang. Establishment of a Tissue Culture and Rapid Propagation System of Dryopteris fragrans [J]. Chinese Bulletin of Botany, 2020, 55(6): 760-767. |
| [8] | Hong Luo, Xiaohui Wen, Yuanyuan Zhou, Silan Dai. Establishment of In Vitro Regeneration System of Helenium aromaticum [J]. Chinese Bulletin of Botany, 2020, 55(3): 318-328. |
| [9] | Yue Xu,Yingping Cao,Yu Wang,Chunxiang Fu,Shaojun Dai. Agrobacterium rhizogenes-mediated Transformation System of Spinacia oleracea [J]. Chinese Bulletin of Botany, 2019, 54(4): 515-521. |
| [10] | Xiting Zhao, Liwei Jiang, Miao Wang, Yuting Zhu, Wenfang Zhang, Mingjun Li. Establishment of Transgenic Acceptor by Indirect Somatic Embryogenesis Regeneration and Transformation of CmTGA1 Gene in Chrysanthemum morifolium cv. ‘Huaihuang’ [J]. Chinese Bulletin of Botany, 2016, 51(4): 525-532. |
| [11] | Fang Liu, Yinghong Tang, Youmei Yuan, Qingquan Guo, Fan Shen, Jianrong Chen. Tissue Culture of the Succulent Plant Sedum clavatum [J]. Chinese Bulletin of Botany, 2016, 51(2): 251-256. |
| [12] | Feng Hu, Qiong Shi, Liejian Huang. Acacia melanoxylon Callus Induction and Shoot Regeneration System [J]. Chinese Bulletin of Botany, 2014, 49(5): 603-610. |
| [13] | Huan Feng, Shuli Yi, Jiaheng Xie, Mengqi Lei, Xuan Huang. Callus Induction and Plant Regeneration of Rosa hybrida [J]. Chinese Bulletin of Botany, 2014, 49(5): 595-602. |
| [14] | Xue Chen, Jinzhu Zhang, Bingbing Pan, Chengjin Sang, Xue Ma, Tao Yang, Daidi Che. Callus Induction and Plant Regeneration of Rose [J]. Chinese Bulletin of Botany, 2011, 46(5): 569-574. |
| [15] | Minghao Li;Wei Chen;Liping Xing;Jin Xiao;Haiyan Wang;Aizhong Cao;Xiue Wang. Establishment of High-efficiency Genetic Transformation System for the Wheat Variety Alondra's [J]. Chinese Bulletin of Botany, 2010, 45(04): 466-471. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||