

Chinese Bulletin of Botany ›› 2023, Vol. 58 ›› Issue (5): 687-700.DOI: 10.11983/CBB22204 cstr: 32102.14.CBB22204
• EXPERIMENTAL COMMUNICATIONS • Previous Articles Next Articles
Qiu Tianhang, Wang An’an, Li Li, Wang Yingchun, Cui Jipeng, Wang Ziyao, Wang Rui, Cui Suxia( )
)
Received:2022-08-31
Accepted:2022-10-24
Online:2023-09-01
Published:2023-09-21
Contact:
*E-mail: sxcui@cnu.edu.cn
Qiu Tianhang, Wang An’an, Li Li, Wang Yingchun, Cui Jipeng, Wang Ziyao, Wang Rui, Cui Suxia. Characteristics and Expression Specificity of RCA Genes in Two Ecotypes of Phragmites australis[J]. Chinese Bulletin of Botany, 2023, 58(5): 687-700.
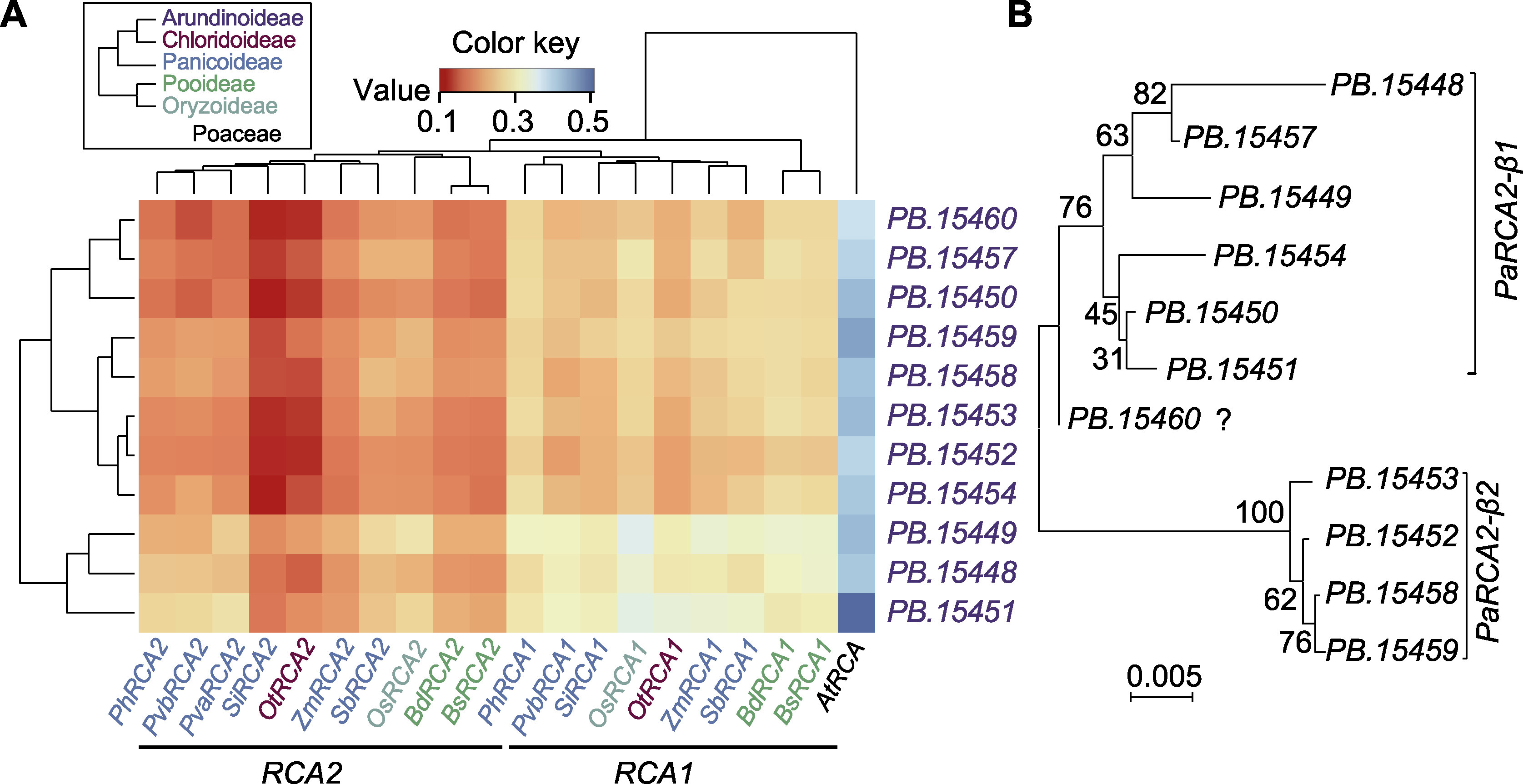
Figure 1 Classification of predicted RCA genes in Phragmites australis (A) The pairwise genetic distance matrix was generated by using 11 predicted RCA genes of P. australis and 19 known RCA gene sequences of Poaceae (the color key shows genetic distance from close (red) to far (blue); the phylogeny tree in the legend shows the taxonomic relationships of Poaceae subfamilies used in this study; the different subfamilies are highlighted by different colors, while out-group Arabidopsis thaliana shown in black; the data based Poisson clustering tree is on the left, and the phylogeny tree is on the top); (B) Evolutionary analysis of 11 predicted RCA genes of P. australis (all phylogeny trees in this figure were constructed using RCA genomic sequences (including introns and UTR) by MUSCLE alignment of MEGA7 with Neighbor-joining method)
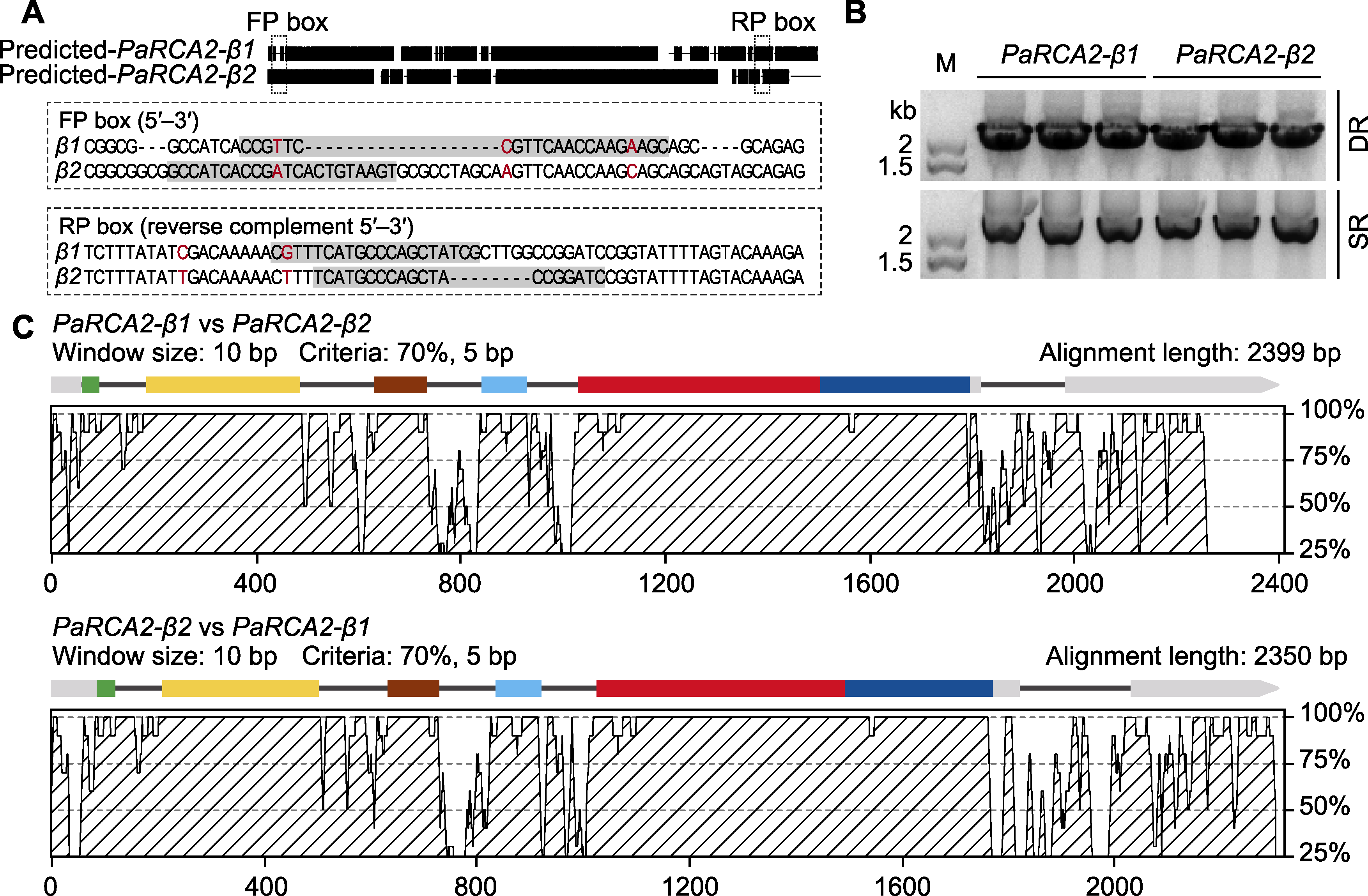
Figure 2 Identification and differential analysis of two RCA genes in Phragmites australis (A) The design of specific primers for the RCA2 genes (the boxes show the primer positions and sequences, the differential nucleotide residues are highlighted in red); (B) The RCA bands from swamp reed (SR) and desert-dune reed (DR) obtained by PCR amplification with genomic DNA as template; (C) Analysis of PaRCA2-β1 and PaRCA2-β2 in sequence and gene structure (the AVID program of mVISTA was used for comparative analysis; the color-coded exon segments were homologous to exons of Arabidopsis thaliana RCA gene)
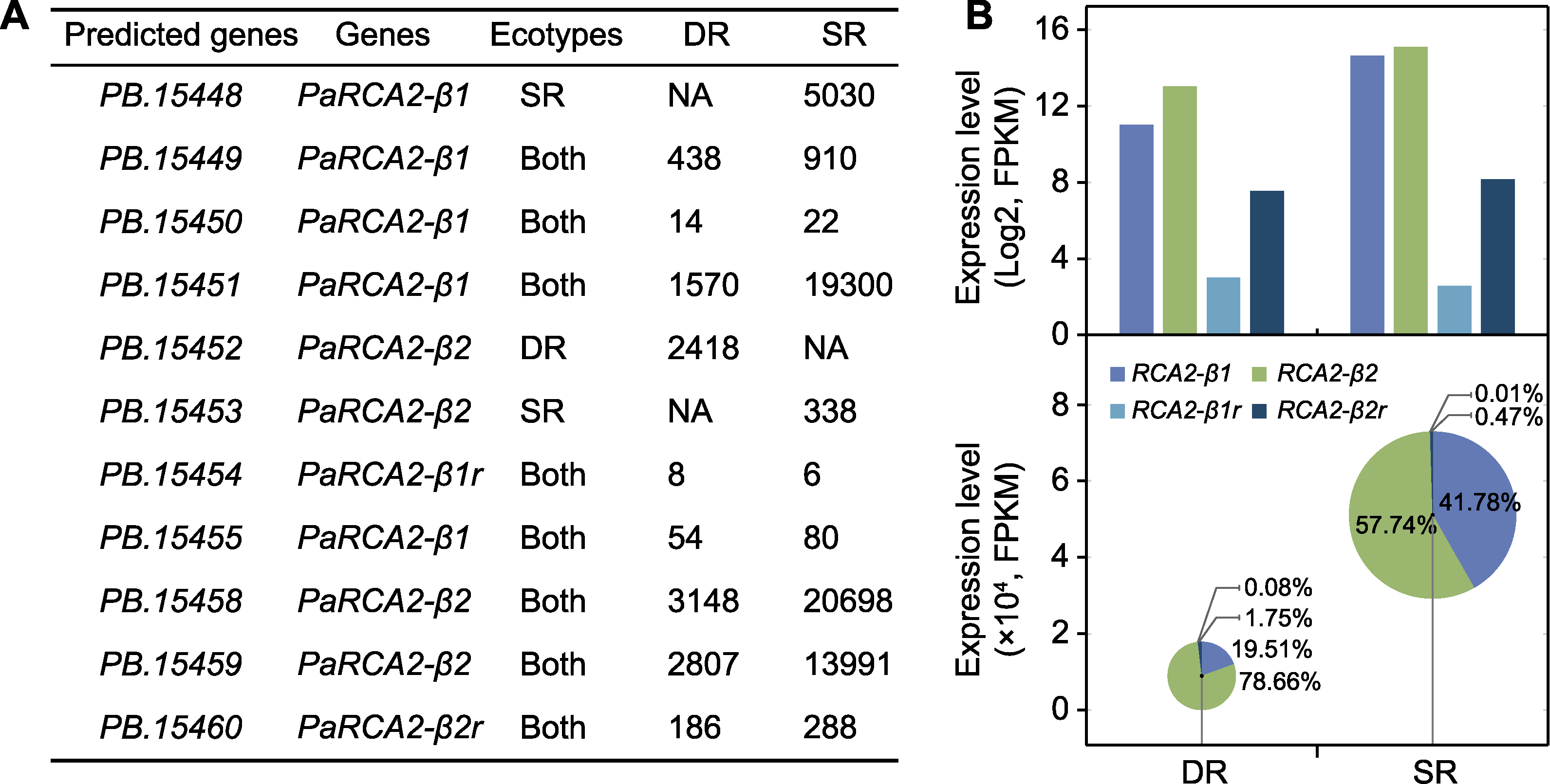
Figure 3 Expression pattern of RCA in two ecotypes of Phragmites australis (A) Expression level of PaRCA2 genes; (B) Logarithmic histogram (top) and bubble-pie graph (bottom) of transcriptional differences of RCA genes in swamp reed (SR) and desert-dune reed (DR). The proportion of each transcript is annotated with percentage.
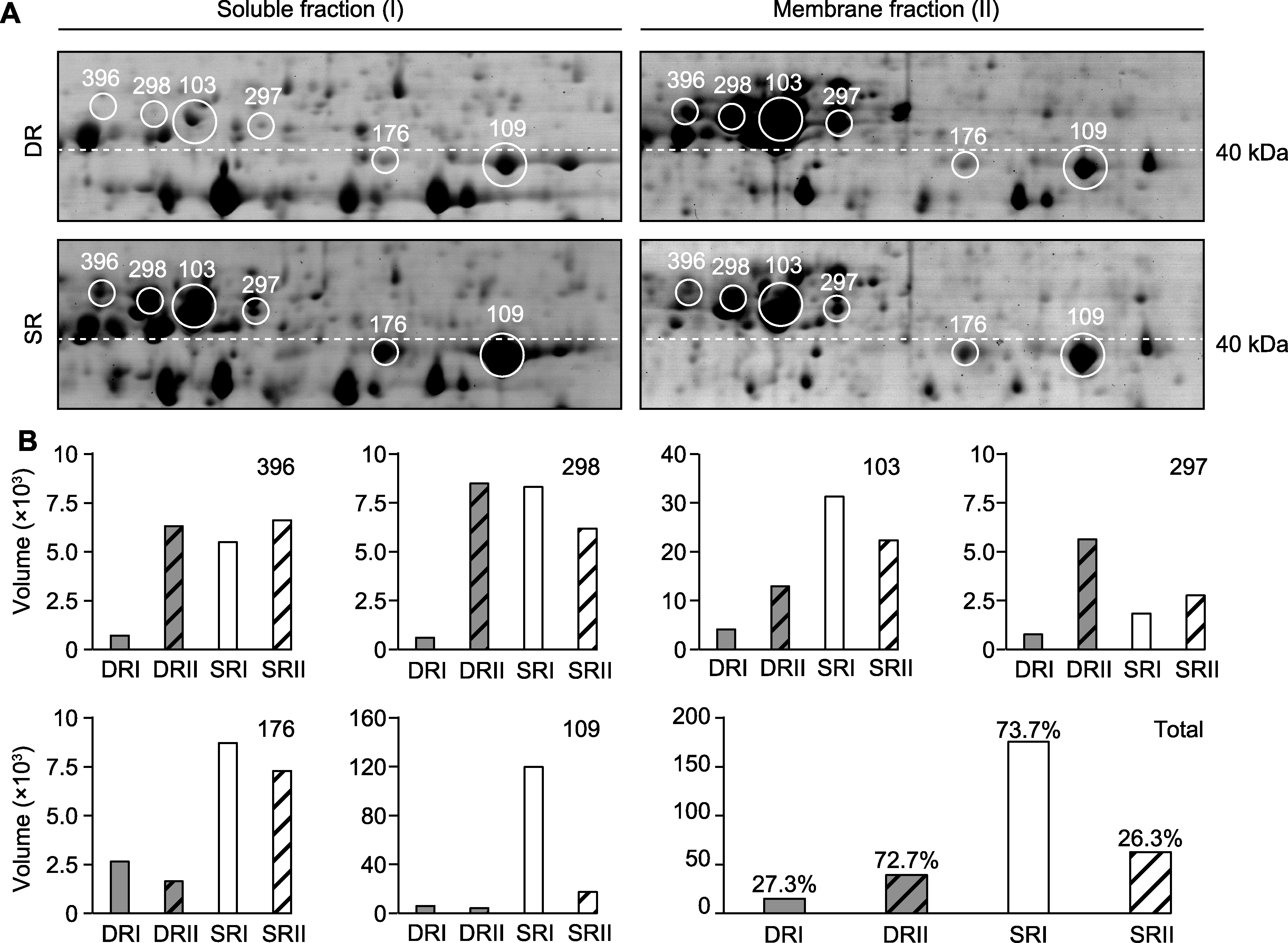
Figure 4 Two-dimensional electrophoresis analysis of RCA proteins in two ecotypes of Phragmites australis (A) The protein profiles of the soluble fraction (I) and membrane fraction (II) (six isoforms of PaRCA are marked with white circles and numbers; the white dashed lines indicate the standard molecular weight position of 40 kDa protein); (B) Quantitative analysis of PaRCA, showing 6 RCA isoforms and total RCA content. SR: Swamp reed; DR: Desert-dune reed
| Ecotypes | Soluble fraction (FI) (%) | Membrane fraction (FII) (%) | FII/FI | |||
|---|---|---|---|---|---|---|
| L-RCA | S-RCA | L-RCA | S-RCA | L-RCA | S-RCA | |
| Swamp reed (SR) | 19.7 | 54.0 | 15.9 | 10.4 | 0.81 | 0.19 |
| Desert-dune reed (DR) | 11.4 | 15.9 | 61.7 | 11.0 | 5.41 | 0.69 |
Table 1 Localization difference of RCA isoforms in two ecotypes of Phragmites australis
| Ecotypes | Soluble fraction (FI) (%) | Membrane fraction (FII) (%) | FII/FI | |||
|---|---|---|---|---|---|---|
| L-RCA | S-RCA | L-RCA | S-RCA | L-RCA | S-RCA | |
| Swamp reed (SR) | 19.7 | 54.0 | 15.9 | 10.4 | 0.81 | 0.19 |
| Desert-dune reed (DR) | 11.4 | 15.9 | 61.7 | 11.0 | 5.41 | 0.69 |

Figure 5 Subcellular distribution of RCA immune gold particles in two ecotypes of Phragmites australis SR: Swamp reed; DR: Desert-dune reed; Mt: Mitochondria; V: Vacuoles; Chl: Chloroplast; CW: Cell wall; S: Cytoplasmic stroma; IS: Intercellular stroma; CS: Chloroplast stroma; Gr: Granum. Bars=1 μm
| Ecotypes | Membrane fraction | Soluble fraction | |||
|---|---|---|---|---|---|
| Chl envelope | Thy membrane | Chl stroma | Thy lumen | Others | |
| Swamp reed (SR) | 17.5±7.0 | 29.1±7.5 | 19.1±6.3 | 15.7±5.2 | 18.7±7.3 |
| Desert-dune reed (DR) | 26.8±4.2 | 32.4±5.7 | 17.1±5.1 | 11.6±3.3 | 12.1±4.6 |
| P-value (t-test) | 9.95E-08 | 0.056 | 0.173 | 6.85E-04 | 1.26E-04 |
| DR/SR | 1.27 | 0.76 | |||
Table 2 The distribution of RCA immune gold particles in the chloroplasts of two Phragmites australis ecotypes (values were means±SD, n=30)
| Ecotypes | Membrane fraction | Soluble fraction | |||
|---|---|---|---|---|---|
| Chl envelope | Thy membrane | Chl stroma | Thy lumen | Others | |
| Swamp reed (SR) | 17.5±7.0 | 29.1±7.5 | 19.1±6.3 | 15.7±5.2 | 18.7±7.3 |
| Desert-dune reed (DR) | 26.8±4.2 | 32.4±5.7 | 17.1±5.1 | 11.6±3.3 | 12.1±4.6 |
| P-value (t-test) | 9.95E-08 | 0.056 | 0.173 | 6.85E-04 | 1.26E-04 |
| DR/SR | 1.27 | 0.76 | |||
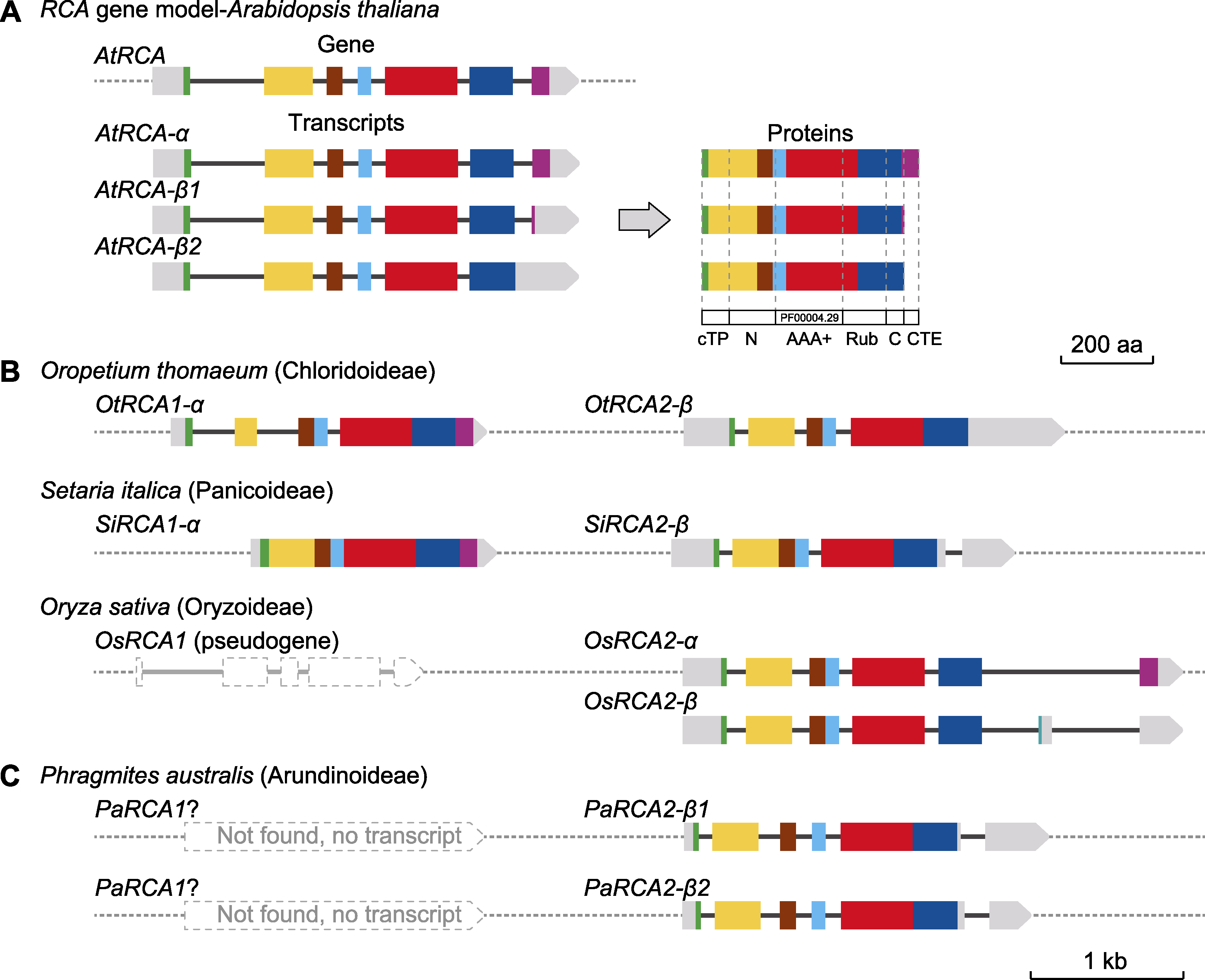
Figure 6 RCA gene/transcript models of Phragmites australis and Poaceae model plants (A) Gene structure and expression products of AtRCA (the seven exons were shown in different colors, gray represented untranslated region, thin dark gray lines represented introns, and dotted lines represented intergenic sequences); (B) RCA gene model in three Poaceae species (the gene characters were similar between Oropetium thomaeum and Setaria italica; pseudo OsRCA1 had lost its function, while OsRCA2 had two expression products simultaneously); (C) PaRCA gene model. Color labeling and definition in (B) and (C) were aligned with AtRCA by sequence similarity. cTP: Chloroplast transport peptide; N: N-terminal domain; AAA+: ATPase domain; Rub: Rubisco large subunit binding domain; C: C-terminal domain; CTE: Redox related C-terminal extension
| [1] | 张承烈, 陈国仓 (1991). 河西走廊不同生态类型芦苇的气体交换特点的研究. 生态学报 11, 250-255. |
| [2] | 张茜, 裘天航, 王安安, 周华健, 袁敏, 李利, 白素兰, 崔素霞 (2020). 北京地区芦苇资源状态及其多样性. 植物学报 55, 693-704. |
| [3] |
Bayramov S, Guliyev N (2014). Changes in Rubisco activase gene expression and polypeptide content in Brachypodium distachyon. Plant Physiol Biochem 81, 61-66.
DOI URL |
| [4] |
Boex-Fontvieille E, Daventure M, Jossier M, Hodges M, Zivy M, Tcherkez G (2014). Phosphorylation pattern of Rubisco activase in Arabidopsis leaves. Plant Biol 16, 550-557.
DOI URL |
| [5] |
Carmo-Silva E, Scales JC, Madgwick PJ, Martin AJP (2015). Optimizing Rubisco and its regulation for greater resource use efficiency. Plant Cell Environ 38, 1817-1832.
DOI URL |
| [6] |
Chen J, Wang P, Mi HL, Chen GY, Xu DQ (2010). Reversible association of Ribulose-1,5-bisphosphate carboxylase/oxygenase activase with the thylakoid membrane depends upon the ATP level and pH in rice without heat stress. J Exp Bot 61, 2939-2950.
DOI URL |
| [7] | Chen YX, Chen YS, Shi CM, Huang ZB, Zhang Y, Li SK, Li Y, Ye J, Yu C, Li Z, Zhang XQ, Wang J, Yang HM, Lin F, Chen Q (2018). SOAPnuke: a MapReduce acceleration- supported software for integrated quality control and preprocessing of high-throughput sequencing data. GigaScience 7, gix120. |
| [8] | Clevering OA, Lissner J (1999). Taxonomy, chromosome numbers, clonal diversity and population dynamics of Phragmites australis. Aquat Bot 66, 185-208. |
| [9] |
Cui S, Wang W, Zhang C (2002). Plant regeneration from callus cultures in two ecotypes of reed (Phragmites communis Trinius). In Vitro Cell Dev Biol Plant 38, 325-329.
DOI URL |
| [10] |
Cui SX, Hu J, Yang B, Shi L, Huang F, Tsai SN, Ngai SM, He YK, Zhang JH (2009). Proteomic characterization of Phragmites communis in ecotypes of swamp and desert dune. Proteomics 9, 3950-3967.
DOI URL |
| [11] |
Demirevska-kepova K, Hölzer R, Simova-stoilova L, Feller U (2005). Heat stress effects on Ribulose-1,5-bisphosphate carboxylase/oxygenase, Rubisco binding protein and Rubisco activase in wheat leaves. Biol Plant 49, 521-525.
DOI URL |
| [12] |
Deridder BP, Shybut ME, Dyle MC, Kremling KAG, Shapiro MB (2012). Changes at the 3′-untranslated region stabilize Rubisco activase transcript levels during heat stress in Arabidopsis. Planta 236, 463-476.
DOI URL |
| [13] |
Flecken M, Wang H, Popilka L, Hartl FU, Bracher A, Hayer-Hartl M (2020). Dual functions of a Rubisco activase in metabolic repair and recruitment to carboxysomes. Cell 183, 457-473.
DOI PMID |
| [14] |
Frazer KA, Pachter L, Poliakov A, Rubin EM, Dubchak I (2004). VISTA: computational tools for comparative genomics. Nucleic Acids Res 32 (Web Server issue),W273-9.
DOI PMID |
| [15] | Hall T (2011). BioEdit: an important software for molecular biology. GERF Bull Biosci 2, 60-61. |
| [16] | Kim D, Langmead B, Salzberg SL (2015). HISAT: a fast spliced aligner with low memory requirements. Nature Me- thods 12, 357-360. |
| [17] |
Kim SY, Stessman DJ, Wright DA, Spalding MH, Huber SC, Ort DR (2020). Arabidopsis plants expressing only the redox-regulated RCA-α isoform have constrained photosynthesis and plant growth. Plant J 103, 2250-2262.
DOI URL |
| [18] | Kumar RR, Goswami S, Singh K, Dubey K, Singh S, Sharma R, Verma N, Kala YK, Rai GK, Grover M, Mishra DC, Singh B, Pathak H, Chinnusamy V, Rai A, Praveen S (2016). Identification of putative Rubisco activase (TaRca1)—the catalytic chaperone regulating carbon assimilatory pathway in wheat (Triticum aestivum) un- der the heat stress. Front Plant Sci 7, 986. |
| [19] |
Kurek I, Chang TK, Bertain SM, Madrigal A, Liu L, Lassner MW, Zhu GH (2007). Enhanced thermostability of Arabidopsis Rubisco activase improves photosynthesis and growth rates under moderate heat stress. Plant Cell 19, 3230-3241.
DOI URL |
| [20] |
Li B, Dewey CN (2011). RSEM: accurate transcript quantification from RNA-seq data with or without a reference genome. BMC Bioinformatics 12, 323.
DOI PMID |
| [21] |
Li F, Fan G, Lu C, Xiao G, Zou C, Kohel RJ, Ma Z, Shang H, Ma X, Wu J, Liang X, Huang G, Percy RG, Liu K, Yang W, Chen W, Du X, Shi C, Yuan Y, Ye W, Liu X, Zhang X, Liu W, Wei H, Wei S, Huang G, Zhang X, Zhu S, Zhang H, Sun F, Wang X, Liang J, Wang J, He Q, Huang L, Wang J, Cui J, Song G, Wang K, Xu X, Yu JZ, Zhu Y, Yu S (2015). Genome sequence of cultivated upland cotton (Gossypium hirsutum TM-1) provides insights into genome evolution. Nat Biotechnol 33, 524-530.
DOI |
| [22] | Li H, Lin WF, Shen ZJ, Peng H, Zhou JJ, Zhu XY (2021). Physiological and proteomic analyses of different ecotypes of reed (Phragmites communis) in adaption to natural drought and salinity. Front Plant Sci 12, 1-15. |
| [23] |
Li L, Chen X, Shi L, Wang CJ, Fu B, Qiu TH, Cui SX (2017). A proteome translocation response to complex desert stress environments in perennial Phragmites sympatric ecotypes with contrasting water availability. Front Plant Sci 8, 511.
DOI PMID |
| [24] |
Love MI, Huber W, Anders S (2014). Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15, 550.
DOI URL |
| [25] | Merchant SS, Prochnik SE, Vallon O, Harris EH, Karpowicz SJ, Witman GB, Terry A, Salamov A, Fritz- Laylin LK, Maréchal-Drouard L, Marshall WF, Qu LH, Nelson DR, Sanderfoot AA, Spalding MH, Kapitonov VV, Ren QH, Ferris P, Lindquist E, Shapiro H, Lucas SM, Grimwood J, Schmutz J, Cardol P, Cerutti H, Chanfreau G, Chen CL, Cognat V, Croft MT, Dent R, Dutcher S, Fernández E, Fukuzawa H, González- Ballester D, González-Halphen D, Hallmann A, Hanikenne M, Hippler M, Inwood W, Jabbari K, Kalanon M, Kuras R, Lefebvre PA, Lemaire SD, Lobanov AV, Lohr M, Manuell A, Meier I, Mets L, Mittag M, Mittelmeier T, Moroney JV, Moseley J, Napoli C, Nedelcu AM, Niyogi K, Novoselov SV, Paulsen IT, Pazour G, Purton S, Ral JP, Riaño-Pachón DM, Riekhof W, Rymarquis L, Schroda M, Stern D, Umen J, Willows R, Wilson N, Zimmer SL, Allmer J, Balk J, Bisova K, Chen CJ, Elias M, Gendler K, Hauser C, Lamb MR, Ledford H, Long JC, Minagawa J, Page MD, Pan JM, Pootakham W, Roje S, Rose A, Stahlberg E, Terauchi AM, Yang PF, Ball S, Bowler C, Dieckmann CL, Gladyshev VN, Green P, Jorgensen R, Mayfield S, Mueller-Roeber B, Rajamani S, Sayre RT, Brokstein P, Dubchak I, Goodstein D, Hornick L, Huang YW, Jhaveri J, Luo YG, Martínez D, Ngau WCA, Otillar B, Poliakov A, Porter A, Szajkowski L, Werner G, Zhou KM, Grigoriev IV, Rokhsar DS, Grossman AR (2007). The Chlamydomonas genome reveals the evolution of key animal and plant functions. Science 318, 245-250. |
| [26] |
Nagarajan R, Gill KS (2018). Evolution of Rubisco activase gene in plants. Plant Mol Biol 96, 69-87.
DOI PMID |
| [27] |
Oh DH, Kowalski KP, Quach QN, Wijesinghege C, Tanford P, Dassanayake M, Clay K (2022). Novel genome characteristics contribute to the invasiveness of Phragmites australis (common reed). Mol Ecol 31, 1142-1159.
DOI URL |
| [28] |
Porebski S, Bailey LG, Baum BR (1997). Modification of a CTAB DNA extraction protocol for plants containing high polysaccharide and polyphenol components. Plant Mol Biol Rep 15, 8-15.
DOI URL |
| [29] |
Portis AR Jr, Li C, Wang D, Salvucci ME (2008). Regulation of Rubisco activase and its interaction with Rubisco. J Exp Bot 59, 1597-1604.
DOI PMID |
| [30] |
Qian J, Rodermel SR (1993). Ribulose-1,5-bisphosphate carboxylase oxygenase activase cDNAs from Nicotiana tabacum. Plant Physiol 102, 683-684.
PMID |
| [31] |
Qiu TH, Cui SX (2021). Evolutionary analysis for Phragmites ecotypes based on full-length plastomes. Aquat Bot 170, 103349.
DOI URL |
| [32] |
Rozas J, Ferrer-Mata A, Sánchez-DelBarrio JC, Guirao- Rico S, Librado P, Ramos-Onsins SE, Sánchez-Gracia A (2017). DnaSP 6: DNA sequence polymorphism analysis of large datasets. Mol Biol Evol 34, 3299-3302.
DOI URL |
| [33] |
Salse J, Bolot S, Throude M, Jouffffe V, Piegu B, Quraishi UM, Calcagno T, Cooke R, Delseny M, Feuillet C (2008). Identification and characterization of shared duplications between rice and wheat provide new insight into grass genome evolution. Plant Cell 20, 11-24.
DOI PMID |
| [34] |
Salvucci ME, Crafts-Brandner SJ (2004). Relationship between the heat tolerance of photosynthesis and the thermal stability of Rubisco activase in plants from contrasting thermal environments. Plant Physiol 134, 1460-1470.
DOI PMID |
| [35] |
Salvucci ME, van de Loo FJ, Stecher D (2003). Two isoforms of Rubisco activase in cotton, the products of separate genes not alternative splicing. Planta 216, 736-744.
DOI PMID |
| [36] |
Salvucci ME, Werneke JM, Ogren WL, Portis AR Jr (1987). Purification and species distribution of Rubisco activase. Plant Physiol 84, 930-936.
DOI PMID |
| [37] |
Shen JB, Orozco EM, Ogren WL (1991). Expression of the two isoforms of spinach Ribulose-1,5-bisphosphate carboxylase activase and essentiality of the conserved lysine in the consensus nucleotide-binding domain. J Biol Chem 266, 8963-8968.
PMID |
| [38] |
Sheng X, Dong X, Zhang S, Jiang LP, Tan LL, Li X (2011). Unequal distribution of ubiquitinated proteins during Pinus bungeana pollen development. Trees 25, 407-414.
DOI URL |
| [39] |
To KY, Suen DF, Chen SCG (1999). Molecular characterization of Ribulose-1,5-bisphosphate carboxylase/oxygenase activase in rice leaves. Planta 209, 66-76.
DOI PMID |
| [40] | Tseng HHE (2016). Cogent: reconstructing the coding genome using full-length transcriptome sequences without a reference genome. Plant & Animal Genome Conference XXIV. San Diego, CA. |
| [41] |
Wang D, Li XF, Zhou ZJ, Feng XP, Yang WJ, Jiang DA (2010). Two Rubisco activase isoforms may play different roles in photosynthetic heat acclimation in the rice plant. Physiol Plant 139, 55-67.
DOI PMID |
| [42] |
Werneke JM, Zielinski RE, Ogren WL (1988). Structure and expression of spinach leaf cDNA encoding Ribulosebisphosphate carboxylase/oxygenase activase. Proc Natl Acad Sci USA 85, 787-791.
PMID |
| [43] | Wickham H (2016). Ggplot2: Elegant Graphics for Data Analysis (2nd edition). New York: Springer-Verlag. |
| [44] |
Yin Z, Meng F, Song H, Wang X, Xu XM, Yu DY (2010). Expression quantitative trait loci analysis of two genes encoding Rubisco activase in soybean. Plant Physiol 152, 1625-1637.
DOI PMID |
| [45] |
Yin Z, Zhang Z, Deng D, Chao MN, Gao QS, Wang YJ, Yang ZF, Bian YL, Hao DR, Xu CW (2014). Characterization of Rubisco activase genes in maize: an α-isoform gene functions alongside a β-isoform gene. Plant Physiol 164, 2096-2106.
DOI PMID |
| [46] |
Zhang JH, Wang LJ, Pan QH, Wang YZ, Zhan JC, Huang WD (2008). Accumulation and subcellular localization of heat shock proteins in young grape leaves during cross- adaptation to temperature stresses. Sci Hortic 117, 231-240.
DOI URL |
| [47] |
Zhang N, Kallis RP, Ewy RG, Portis AR (2002). Light modulation of Rubisco in Arabidopsis requires a capacity for redox regulation of the larger Rubisco activase isoform. Proc Natl Acad Sci USA 99, 3330-3334.
DOI PMID |
| [48] |
Zhang N, Portis AR (1999). Mechanism of light regulation of Rubisco: a specific role for the larger Rubisco activase isoform involving reductive activation by thioredoxin-f. Proc Natl Acad Sci USA 96, 9438-9443.
DOI PMID |
| [1] | Su Chen, Niu Yufan, Xu Hang, Wang Xiling, Yu Yingjun, He Yuqing, Wang Lei. Advances of Plant Circadian Clock Response to Light and Temperature Signals [J]. Chinese Bulletin of Botany, 2025, 60(3): 315-341. |
| [2] | Jiayu Lu, Xiaoyi Shi, Li’an Duo, Tianming Wang, Zhilin Li. Circadian rhythms of urban terrestrial mammals in Tianjin based on camera trapping method [J]. Biodiv Sci, 2024, 32(8): 23369-. |
| [3] | Qiguang Xie, Xiaodong Xu. Plant Circadian Clock in Agricultural Production in Response to Global Warming [J]. Chinese Bulletin of Botany, 2024, 59(4): 635-650. |
| [4] | Lei Gu, Qi Zhang, Xia Zhang, Bingbing Yang, Fanglan Wang, Wen Liu, Faju Chen. Cloning and Functional Analysis of APETALA3/DEFICIENS Homologous Gene from Rhus chinensis [J]. Chinese Bulletin of Botany, 2024, 59(4): 533-543. |
| [5] | Jingci Meng, Guodong Wang, Guanglan Cao, Nanlin Hu, Meiling Zhao, Yantong Zhao, Zhenshan Xue, Bo Liu, Wenhua Piao, Ming Jiang. Patterns and drivers of plant species richness in Phragmites australis marshes in China [J]. Biodiv Sci, 2024, 32(2): 23194-. |
| [6] | JIANG Hai-Gang, ZENG Yun-Hong, TANG Hua-Xin, LIU Wei, LI Jie-Lin, HE Guo-Hua, QIN Hai-Yan, WANG Li-Chao, Victor RESCO de DIOS, YAO Yin-An. Rhythmic regulation of carbon fixation and water dissipation in three mosses [J]. Chin J Plant Ecol, 2023, 47(7): 988-997. |
| [7] | Dandan Wu, Yongkun Chen, Yu Yang, Chunyan Kong, Ming Gong. Identification of the Cysteine Protease Family and Corresponding miRNAs in Jatropha curcas and Their Response to Chill-hardening [J]. Chinese Bulletin of Botany, 2021, 56(5): 544-558. |
| [8] | Xi Zhang, Tianhang Qiu, Anan Wang, Huajian Zhou, Min Yuan, Li Li, Sulan Bai, Suxia Cui. Morphology and Genetic Diversity of Phragmites australis in Beijing [J]. Chinese Bulletin of Botany, 2020, 55(6): 693-704. |
| [9] | Yingjun Yu,Hang Xu,Lei Wang. A Non-invasive Method for Measuring and Analyzing Circadian Phenotype in Living Plants [J]. Chinese Bulletin of Botany, 2020, 55(2): 177-181. |
| [10] | Chi Xu, Haijun Wang, Quanxing Liu, Bo Wang. Alternative stable states and tipping points of ecosystems [J]. Biodiv Sci, 2020, 28(11): 1417-1430. |
| [11] | Yegeng Fan,Lihang Qiu,Xing Huang,Huiwen Zhou,Chongkun Gan,Yangrui Li,Rongzhong Yang,Jianming Wu,Rongfa Chen. Expression Analysis of Key Genes in Gibberellin Biosynthesis and Related Phytohormonal Dynamics During Sugarcane Internode Elongation [J]. Chinese Bulletin of Botany, 2019, 54(4): 486-496. |
| [12] | GUO Rui, ZHOU Ji, LIU Qi, GU Feng-Xue. Characterization of nutrient elements at different leaf positions in Phragmites australis in Songnen degraded grassland [J]. Chin J Plant Ecol, 2018, 42(7): 734-740. |
| [13] | Wei Hua, Wang Yan, Liu Baohui, Wang Lei. Deciphering the Underlying Mechanism of the Plant Circadian System and Its Regulation on Plant Growth and Development [J]. Chinese Bulletin of Botany, 2018, 53(4): 456-467. |
| [14] | Qun LI, Cheng-Zhang ZHAO, Lian-Chun ZHAO, Jian-Liang WANG, Wei-Tao ZHANG, Wen-Xiu YAO. Empirical relationship between specific leaf area and thermal dissipation of Phragmites australis in salt marshes of Qinwangchuan [J]. Chin J Plant Ecol, 2017, 41(9): 985-994. |
| [15] | Jiangmin Xu, Hongzhen Jiang, Han Lin, Miaomiao Huang, Qiaoli Fu, Dali Zeng, Yuchun Rao. EARLY SENESCENCE 1 Participates in the Expression Regulation of Circadian Clock Genes and Response to Stress in Rice [J]. Chinese Bulletin of Botany, 2016, 51(6): 743-756. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||