

Chinese Bulletin of Botany ›› 2025, Vol. 60 ›› Issue (6): 914-930.DOI: 10.11983/CBB24191 cstr: 32102.14.CBB24191
• RESEARCH ARTICLES • Previous Articles Next Articles
Yunxiang Xu1,2, Liwen Zhang1,2, Peng Wang1, Yingchen Gu1,2, Madan Lal Kolhi1,2, Biao Zhang1,2, Yingying Zhu1, Haiwei Liu1,*( )
)
Received:2024-12-09
Accepted:2024-03-18
Online:2025-11-10
Published:2025-03-18
Contact:
Haiwei Liu
Yunxiang Xu, Liwen Zhang, Peng Wang, Yingchen Gu, Madan Lal Kolhi, Biao Zhang, Yingying Zhu, Haiwei Liu. Differences in the Adaptive Development of Suberin in the Tobacco Root Endothelial Layer under Different Potassium Levels[J]. Chinese Bulletin of Botany, 2025, 60(6): 914-930.
| Reagent | Configuration method |
|---|---|
| 0.01% (w/v) FY 088 | Dissolve 0.01 g of FY 088 powder in 100 mL of distilled water, mix well, and heat to 70°C before using |
| 0.05% (w/v) toluidine Blue O | Dissolve 0.05 g of toluidine blue O powder in 100 mL of distilled water, mix well and store away from light |
| 4% (w/v) agar | Dissolve 4 g of agar powder in 100 mL of distilled water, mix well and heat in a microwave oven over medium heat for 3 min |
| 50% (v/v) glycerol-ethanol | Mix absolute ethanol 1:1 with glycerol well |
Table 1 Preparation methods for the reagents required for suberization fluorescence staining of tobacco root
| Reagent | Configuration method |
|---|---|
| 0.01% (w/v) FY 088 | Dissolve 0.01 g of FY 088 powder in 100 mL of distilled water, mix well, and heat to 70°C before using |
| 0.05% (w/v) toluidine Blue O | Dissolve 0.05 g of toluidine blue O powder in 100 mL of distilled water, mix well and store away from light |
| 4% (w/v) agar | Dissolve 4 g of agar powder in 100 mL of distilled water, mix well and heat in a microwave oven over medium heat for 3 min |
| 50% (v/v) glycerol-ethanol | Mix absolute ethanol 1:1 with glycerol well |
| Treatments (mmol∙L-1 K+) | Shoot fresh weight (g) | Root fresh weight (g) | Shoot dry weight (g) | Root dry weight (g) | Root-shoot ratio |
|---|---|---|---|---|---|
| 0.1 | 7.32±0.96 c | 0.70±0.08 b | 0.30±0.04 b | 0.06±0 b | 0.19±0.02 a |
| 0.5 | 10.51±2.25 bc | 0.84±0.08 ab | 0.47±0.09 a | 0.06±0 b | 0.14±0.03 bc |
| 1.0 | 12.33±2.43 b | 0.93±0.07 ab | 0.53±0.10 a | 0.06±0.01 ab | 0.12±0.03 c |
| 2.0 | 13.24±2.51 ab | 1.09±0.41 ab | 0.54±0.13 a | 0.08±0.02 a | 0.16±0.01 b |
| 4.0 | 16.03±2.76 a | 1.03±0.26 a | 0.60±0.10 a | 0.08±0.02 a | 0.14±0.01 bc |
Table 2 Differences in tobacco biomass and the root/shoot ratio under different concentrations of potassium
| Treatments (mmol∙L-1 K+) | Shoot fresh weight (g) | Root fresh weight (g) | Shoot dry weight (g) | Root dry weight (g) | Root-shoot ratio |
|---|---|---|---|---|---|
| 0.1 | 7.32±0.96 c | 0.70±0.08 b | 0.30±0.04 b | 0.06±0 b | 0.19±0.02 a |
| 0.5 | 10.51±2.25 bc | 0.84±0.08 ab | 0.47±0.09 a | 0.06±0 b | 0.14±0.03 bc |
| 1.0 | 12.33±2.43 b | 0.93±0.07 ab | 0.53±0.10 a | 0.06±0.01 ab | 0.12±0.03 c |
| 2.0 | 13.24±2.51 ab | 1.09±0.41 ab | 0.54±0.13 a | 0.08±0.02 a | 0.16±0.01 b |
| 4.0 | 16.03±2.76 a | 1.03±0.26 a | 0.60±0.10 a | 0.08±0.02 a | 0.14±0.01 bc |
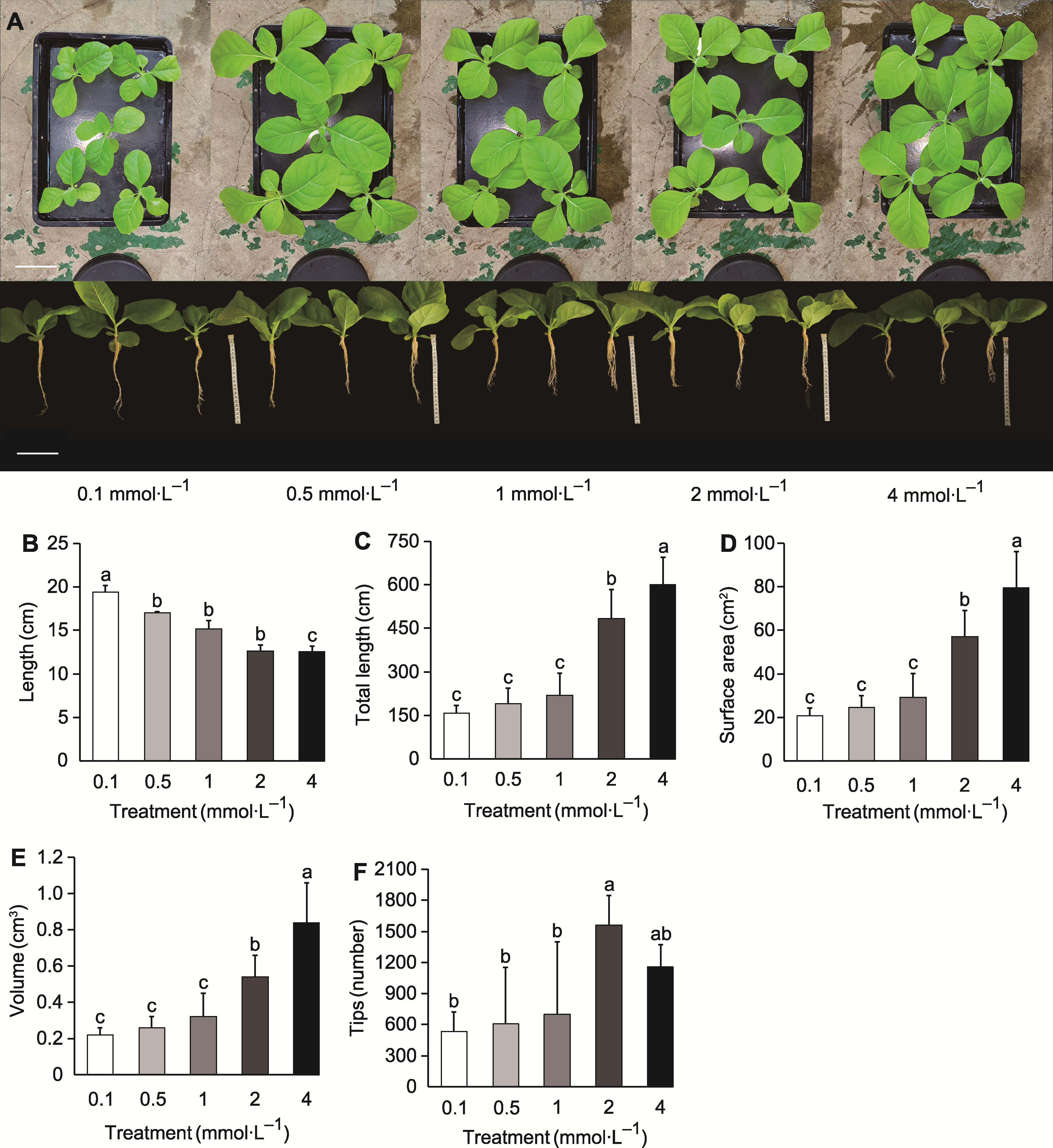
Figure 1 Growth and root morphological development of tobacco plants under different potassium concentrations (A) Root and leaf conditions of tobacco seedlings after 15 days of treatment (bars=10 cm); (B) Length of the longest root; (C) Total root length; (D) Root surface area; (E) Root volume; (F) Number of root tips. Different lowercase letters indicate significant differences among different treatments (P<0.05).
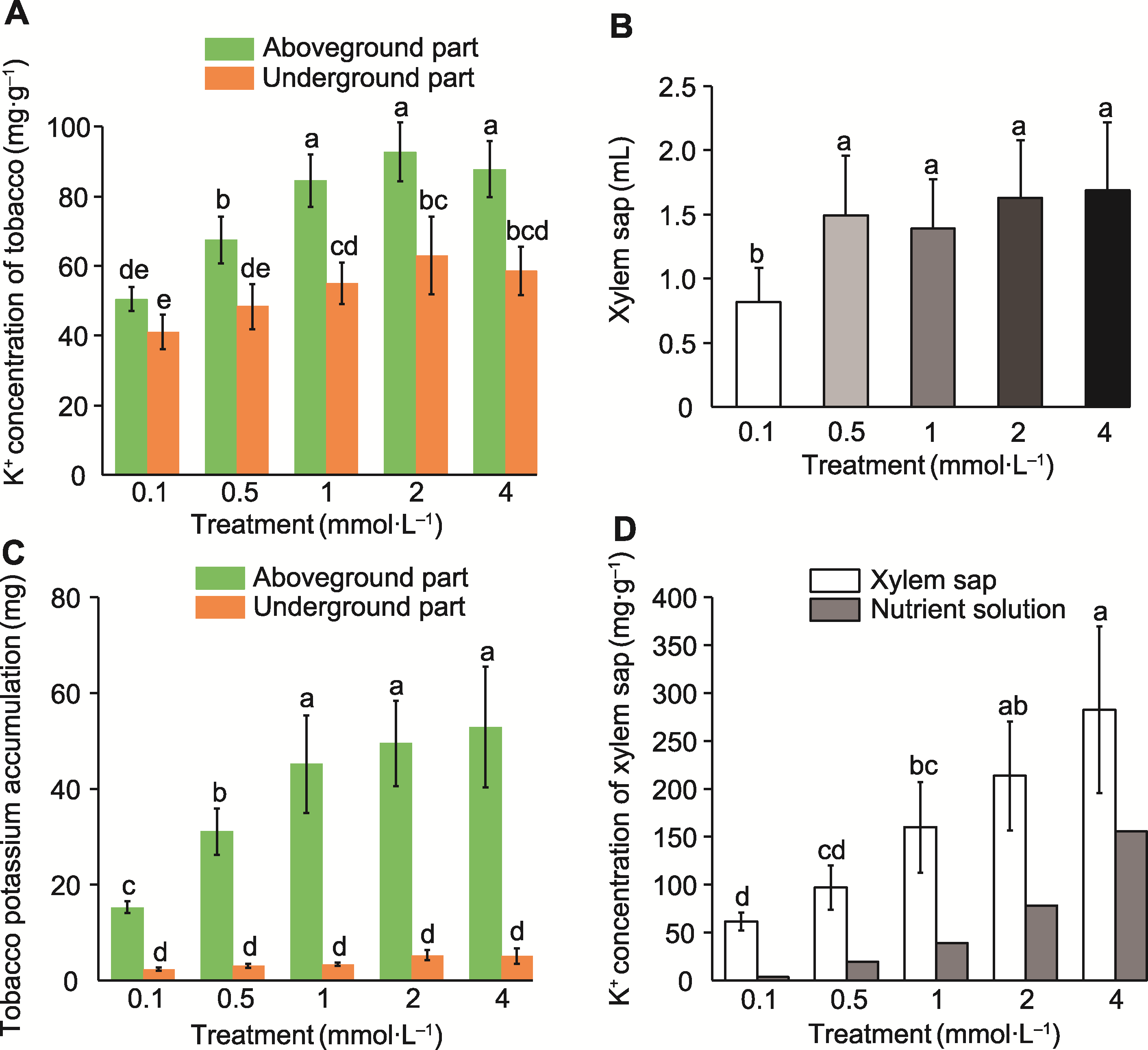
Figure 2 Potassium concentration in tobacco plants and xylem sap under different potassium concentration treatments (A) Potassium ion concentrations of the aboveground and underground parts; (B) Xylem sap collection volume; (C) Potassium accumulation in the aboveground and underground parts; (D) Potassium ion concentration in the xylem sap. Different lowercase letters indicate significant differences among different treatments (P<0.05).
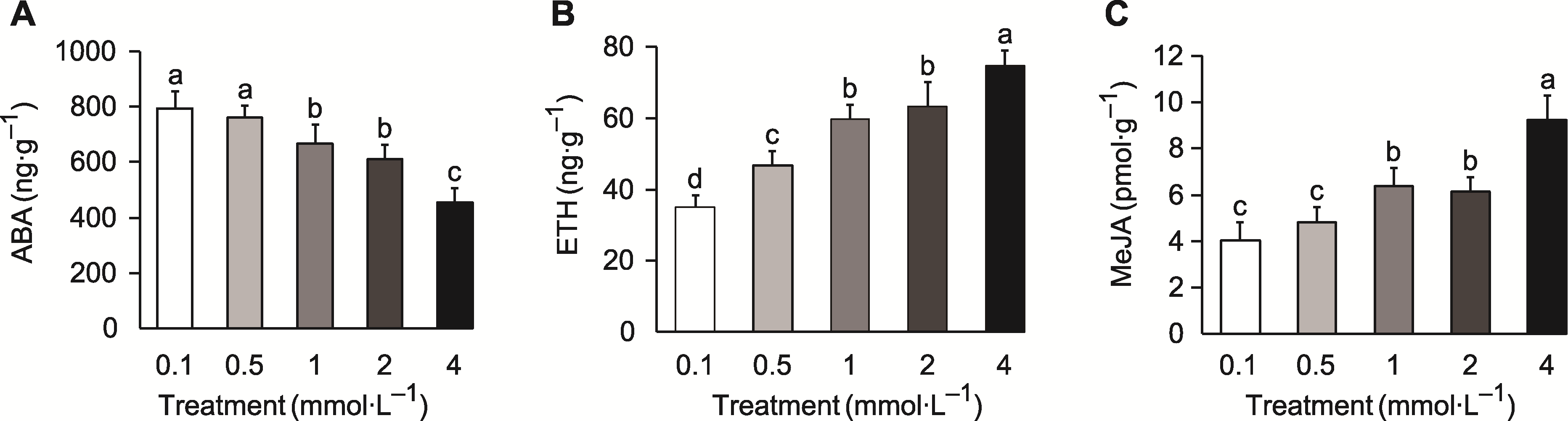
Figure 3 Differences in the levels of endogenous hormones in tobacco roots under different potassium concentrations (A) Root endogenous abscisic acid (ABA) concentration; (B) Root endogenous ethylene (ETH) concentration; (C) Root endogenous methyl jasmonate (MeJA) concentration. Different lowercase letters indicate significant differences among different treatments (P<0.05).
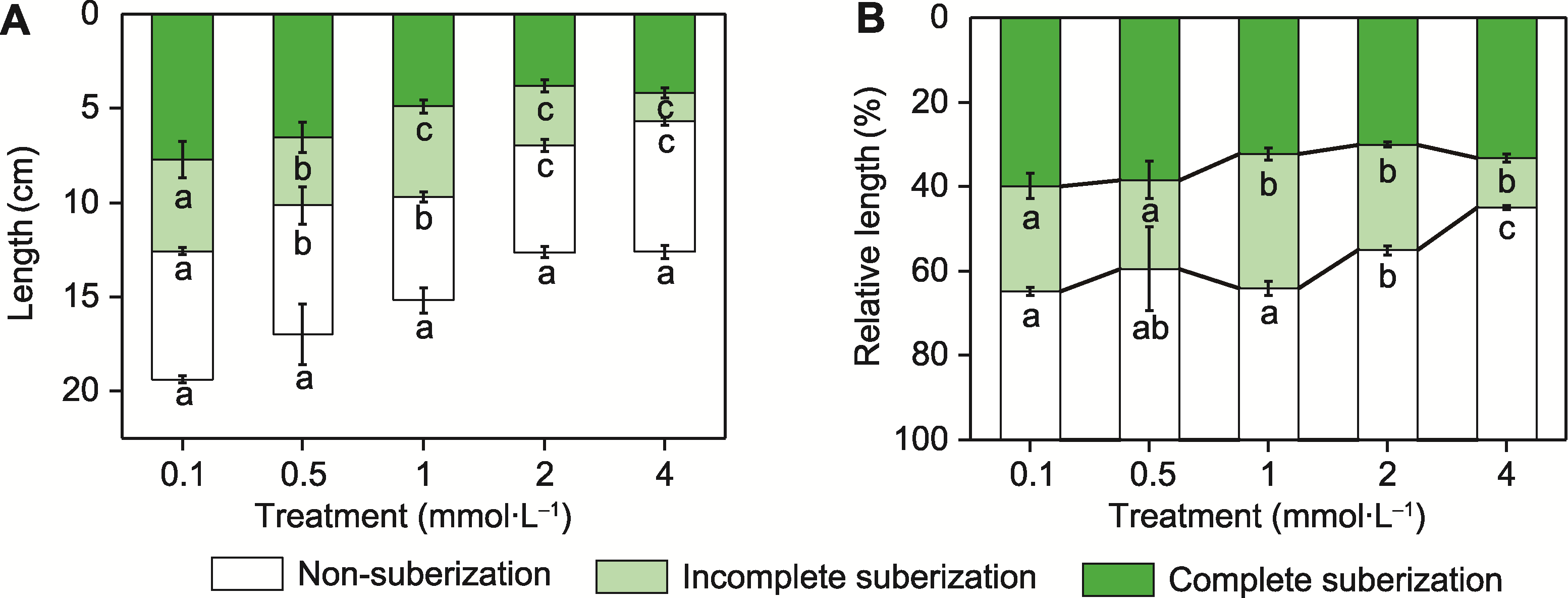
Figure 5 Developmental status of three suberized regions in the endodermis of tobacco roots under different potassium concentration treatments (A) Absolute lengths; (B) Relative lengths. Different lowercase letters indicate significant differences among different treatments (P<0.05).
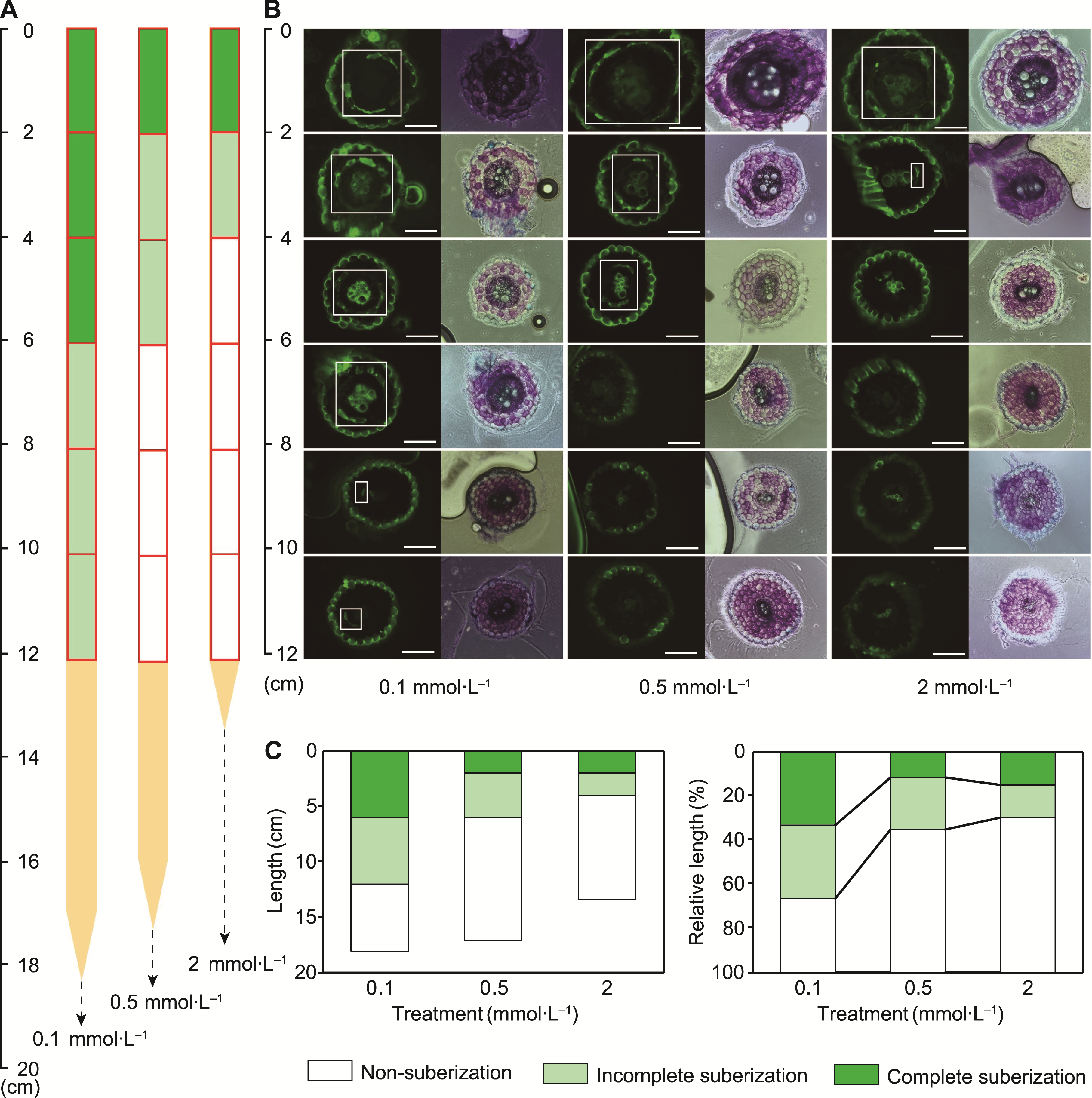
Figure 6 Fluorescence images of transverse sections of endodermis development in tobacco roots at three potassium concentrations (A) Schematic diagram of the suberization development in the endodermis of tobacco roots at three potassium concentrations; (B) FY 088 fluorescence imaging of different root segment cross-sections of tobacco at three potassium concentrations (bars=130 μm); (C) Absolute length and relative length of suberization development in the endodermis of tobacco roots at three potassium concentrations
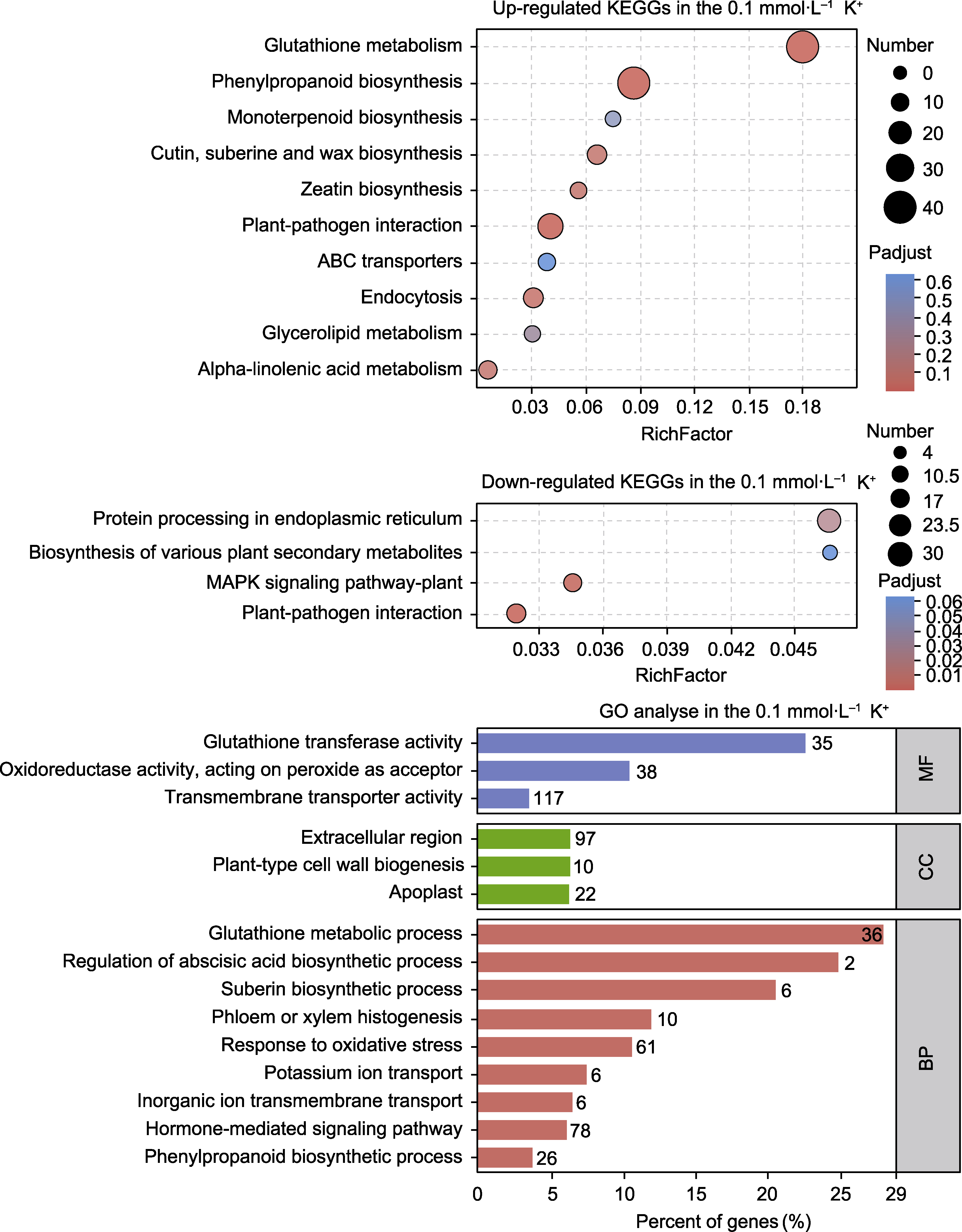
Figure 8 KEGG and GO enrichment analyses of differentially expressed genes (DEGs) under 0.1 mmol∙L-1 K+ treatment MF: Molecular function; CC: Cellular component; BP: Biological process
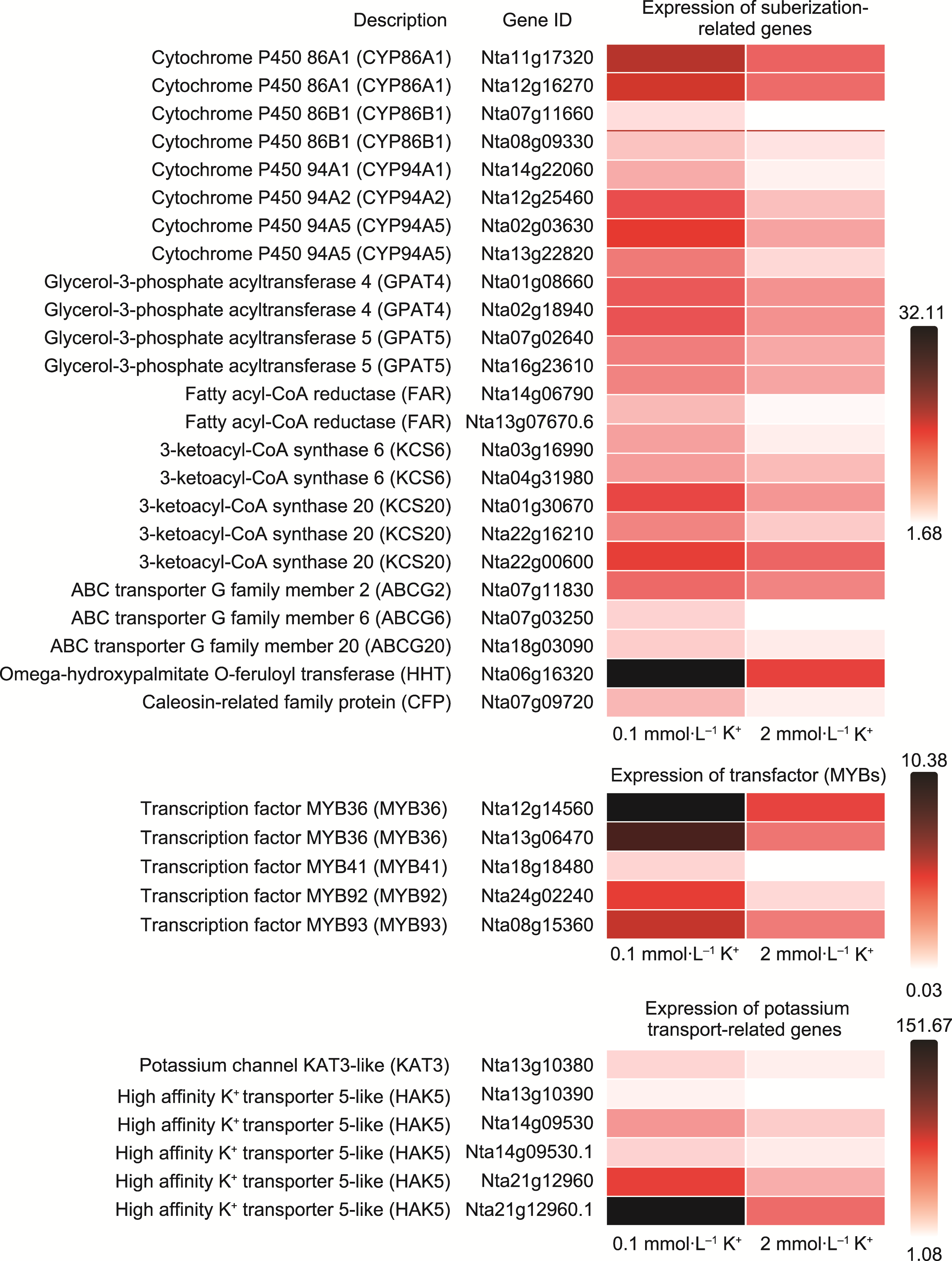
Figure 9 Expression levels of endodermal suberization-related genes, MYB transcription factors, and potassium transporter-related genes among the differentially expressed genes under 0.1 mmol∙L-1 K+ treatment
| [1] |
Andersen TG, Barberon M, Geldner N (2015). Suberization—the second life of an endodermal cell. Curr Opin Plant Biol 28, 9-15.
DOI PMID |
| [2] |
Barberon M (2017). The endodermis as a checkpoint for nutrients. New Phytol 213, 1604-1610.
DOI PMID |
| [3] |
Barberon M, Vermeer JEM, De Bellis D, Wang P, Naseer S, Andersen TG, Humbel BM, Nawrath C, Takano J, Salt DE, Geldner N (2016). Adaptation of root function by nutrient-induced plasticity of endodermal differentiation. Cell 164, 447-459.
DOI PMID |
| [4] | Baxter I, Hosmani PS, Rus A, Lahner B, Borevitz JO, Muthukumar B, Mickelbart MV, Schreiber L, Franke RB, Salt DE (2009). Root suberin forms an extracellular barrier that affects water relations and mineral nutrition in Arabidopsis. PLoS Genet 5, e1000492. |
| [5] | Chen AL, Liu T, Deng Y, Xiao R, Zhang T, Wang Y, Yang YH, Lakshmanan P, Shi XJ, Zhang FS, Chen XP (2023). Nitrate-dependent suberization regulates cadmium uptake and accumulation in maize. Sci Total Environ 878, 162-848. |
| [6] |
Doblas VG, Geldner N, Barberon M (2017). The endodermis, a tightly controlled barrier for nutrients. Curr Opin Plant Biol 39, 136-143.
DOI PMID |
| [7] | Guo Z, Li ZS, Dai XY, Wang YF (2019). Effects of auxin on tobacco root growth and potassium uptake under low potassium stress. Plant Nutr Fert Sci 25, 1173-1184. (in Chinese) |
| 郭泽, 李子绅, 代晓燕, 王英锋 (2019). 低钾胁迫下外源生长素对烟草根系生长及钾吸收的影响. 植物营养与肥料学报 25, 1173-1184. | |
| [8] |
Leide J, Hildebrandt U, Hartung W, Riederer M, Vogg G (2012). Abscisic acid mediates the formation of a suberized stem scar tissue in tomato fruits. New Phytol 194, 402-415.
DOI PMID |
| [9] |
Liu HW, Zhang Y, Wang HY, Zhang B, He Y, Wang HH, Zhu YY, Holm PE, Shi Y (2023). Comparing cadmium uptake kinetics, xylem translocation, chemical forms, and subcellular distribution of two tobacco (Nicotiana tabacum L.) cultivars. Ecotoxicol Environ Saf 254, 114738.
DOI URL |
| [10] | Liu YK, Lu M, Persson DP, Luo JP, Liang YC, Li TQ (2022). The involvement of nitric oxide and ethylene on the formation of endodermal barriers in response to Cd in hyperaccumulator Sedum alfredii. Environ Pollut 307, 11-9530. |
| [11] |
Liu YK, Tao Q, Li JX, Guo XY, Luo JP, Jupa R, Liang YC, Li TQ (2021). Ethylene-mediated apoplastic barriers development involved in cadmium accumulation in root of hyperaccumulator Sedum alfredii. J Hazard Mater 403, 123729.
DOI URL |
| [12] |
Lulai EC, Suttle JC, Pederson SM (2008). Regulatory involvement of abscisic acid in potato tuber wound-healing. J Exp Bot 59, 1175-1186.
DOI PMID |
| [13] | Luo HB, Huang CM, Cao HQ, Jiang SL, Wu XJ, Ye LP, Wei YW (2022). Effects of different potassium levels on root development and endogenous hormone content for passion fruit tissue cultured seedling. China Fruits (4), 53-58. (in Chinese) |
| 罗海斌, 黄诚梅, 曹辉庆, 蒋胜理, 吴兴剑, 叶丽萍, 魏源文 (2022). 不同浓度钾元素对西番莲组培苗根系生长和内源激素含量的影响. 中国果树 (4), 53-58. | |
| [14] |
Lux A, Morita S, Abe J, Ito K (2005). An improved method for clearing and staining free-hand sections and whole- mount samples. Ann Bot 96, 989-996.
DOI URL |
| [15] |
Melino VJ, Plett DC, Bendre P, Thomsen HC, Zeisler- Diehl VV, Schreiber L, Kronzucker HJ (2021). Nitrogen depletion enhances endodermal suberization without restricting transporter-mediated root NO3- influx. J Plant Physiol 257, 153334.
DOI URL |
| [16] |
Naseer S, Lee Y, Lapierre C, Franke R, Nawrath C, Geldner N (2012). Casparian strip diffusion barrier in Arabidopsis is made of a lignin polymer without suberin. Proc Natl Acad Sci USA 109, 10101-10106.
DOI PMID |
| [17] | Pfister A, Barberon M, Alassimone J, Kalmbach L, Lee Y, Vermeer JE, Yamazaki M, Li GW, Maurel C, Takano J, Kamiya T, Salt DE, Roppolo D, Geldner N (2014). A receptor-like kinase mutant with absent endodermal diffusion barrier displays selective nutrient homeostasis defects. eLife 3, e03115. |
| [18] |
Tao Q, Jupa R, Liu YK, Luo JP, Li JX, Kováč J, Li B, Li QQ, Wu KR, Liang YC, Lux A, Wang CQ, Li TQ (2019). Abscisic acid-mediated modifications of radial apoplastic transport pathway play a key role in cadmium uptake in hyperaccumulator Sedum alfredii. Plant Cell Environ 42, 1425-1440.
DOI |
| [19] |
Tao Q, Li M, Xu Q, Kováč J, Yuan S, Li B, Li QQ, Huang R, Gao XS, Wang CQ (2022). Radial transport difference mediated by root endodermal barriers contributes to differential cadmium accumulation between japonica and indica subspecies of rice (Oryza sativa L.). J Hazard Mater 425, 128008.
DOI URL |
| [20] | Vestenaa MW, Husted S, Minutello F, Persson DP (2024). Endodermal suberin restricts root leakage of cesium: a suitable tracer for potassium. Physiol Plant 176, e14393. |
| [21] |
Wang JB, Zhang QL, Tung J, Zhang X, Liu D, Deng YT, Tian ZD, Chen HL, Wang TT, Yin WX, Li B, Lai ZB, Dinesh-Kumar SP, Baker B, Li F (2024). High-quality assembled and annotated genomes of Nicotiana tabacum and Nicotiana benthamiana reveal chromosome evolution and changes in defense arsenals. Mol Plant 17, 423-437.
DOI URL |
| [22] | Wang LM, Liu YQ, Ruan YJ (2015). The research progress of potassium to plant. Chin Hortic Abstr 31(5), 71, 148. (in Chinese) |
| 王立梅, 刘奕清, 阮玉娟 (2015). 植物钾素研究进展. 中国园艺文摘 31(5), 71, 148. | |
| [23] |
Wang P, Wang CM, Gao L, Cui YN, Yang HL, de Silva NDG, Ma Q, Bao AK, Flowers TJ, Rowland O, Wang SM (2020). Aliphatic suberin confers salt tolerance to Arabidopsis by limiting Na+ influx, K+ efflux and water backflow. Plant Soil 448, 603-620.
DOI |
| [24] |
Wei XP, Liu LY, Jin XY, Xue J, Geng P, Xu ZH, Zhang LH, Wang XY, Zong W, Zhang L, Mao LC (2024a). Exogenous methyl jasmonate promotes wound healing of Chinese yam tubers (Dioscorea opposita) through the deposition of suberin polyaliphatics at the wound sites. Postharvest Biol Technol 207, 112586.
DOI URL |
| [25] |
Wei XP, Liu LY, Liu G, Geng P, Wei XB, Yao X, Chen JY, Gong WJ, Ge ZZ, Liu MP, Mao LC (2024b). Methyl jasmonate promotes suberin biosynthesis by stimulating transcriptional activation of AchMYC2 on AchFHT in wound healing of kiwifruit. Postharvest Biol Technol 210, 112741.
DOI URL |
| [26] |
Wei XP, Liu LY, Xu ZH, Xue J, Geng P, Ge ZZ, Wang XY, Zhang L, Zong W, Mao LC (2023). Methyl jasmonate facilitates wound healing of Chinese yam tubers via positively regulating the biosynthesis and polymerization of suberin polyphenolics. Sci Hortic 312, 111840.
DOI URL |
| [27] |
Xu HM, Liu P, Wang CH, Wu SS, Dong CQ, Lin QY, Sun WR, Huang BB, Xu MZ, Tauqeer A, Wu S (2019). Transcriptional networks regulating suberin and lignin in endodermis link development and ABA response. Plant Physiol 190, 1165-1181.
DOI URL |
| [28] | Yan HF, Shi Y, Li NH, Zhang YC (2013). Progress in tobacco potassium nutrition. J Agric Sci Technol 15, 123-129. (in Chinese) |
| 闫慧峰, 石屹, 李乃会, 张永春 (2013). 烟草钾素营养研究进展. 中国农业科技导报 15, 123-129. | |
| [29] | Yang TZ, Shu HY, Zhao XZ (2002). Recent advances in tobacco potassium nutrition in China. Tob Sci Technol (7), 39-43. (in Chinese) |
| 杨铁钊, 舒海燕, 赵献章 (2002). 我国烟草钾素营养研究现状与进展. 烟草科技 (7), 39-43. | |
| [30] | Zhang B, Wu J, Zhang Y, Dong XW, Han S, Gao X, Du CW, Li HY, Chong XF, Zhu YY, Liu HW (2023). Research progress on physiological functions of suberin lamellae in water and solutes transport. Chin Bull Bot 58, 1008-1018. (in Chinese) |
|
张标, 吴健, 张杨, 董小卫, 韩硕, 高昕, 杜从伍, 李慧英, 种学法, 朱莹莹, 刘海伟 (2023). 木栓层在水和溶质运输中的生理功能研究进展. 植物学报 58, 1008-1018.
DOI |
|
| [31] |
Zhang B, Xu YX, Zhang LW, Yu SY, Zhu YY, Liu CJ, Wang P, Shi Y, Li LZ, Liu HW (2024). Root endodermal suberization induced by nitrate stress regulate apoplastic pathway rather than nitrate uptake in tobacco (Nicotiana tabacum L.). Plant Physiol Biochem 216, 109166.
DOI URL |
| [32] | Zhang B, Xu YX, Zhang LW, Zhu YY, Liu HW (2024). Mechanism of low NO3- stress inhibiting apoplastic transport in tobacco roots. Chin Tob Sci 45(2), 25-34. (in Chinese) |
| 张标, 许耘祥, 张莉汶, 朱莹莹, 刘海伟 (2024). 低NO3-胁迫抑制烟草根系质外体运输的机制研究. 中国烟草科学 45(2), 25-34. | |
| [33] | Zhou Y, An YP, Ma R, Wang P (2024). Transcriptional regulation of suberin and its response to the environment. Acta Bot Boreali-Occident Sin 44, 1993-2006. (in Chinese) |
| 周月, 安永平, 马蓉, 王沛(2024). 木栓质的转录调控及其对环境的响应. 西北植物学报 44, 1993-2006. |
| [1] | Miao Lin. The Hormonal 'Code' for the Number of Maize Tassel Branches [J]. Chinese Bulletin of Botany, 2026, 61(1): 1-0. |
| [2] | Haodong Luo, Yongbo Liu. Research Progress in the Development and Regulatory Mechanisms of Plant Tendrils [J]. Chinese Bulletin of Botany, 2025, 60(6): 993-1004. |
| [3] | Rui Wang, Weijun Zhao, Yang Bai, Qingjun Cheng, Haiyan Zhang, Fengxia Yan, Liang Ling. Effects of Endogenous Hormones on Height Difference Between Main Stem and Tiller of Sorghum bicolor [J]. Chinese Bulletin of Botany, 2025, 60(6): 901-913. |
| [4] | Jie Zhao, Jing Li, Yuxin Li, Yi Huang, Jie Yang, Xia Li. Research Progress of the Function of Reactive Oxygen Species in Plant Seed Dormancy Release and Germination [J]. Chinese Bulletin of Botany, 2025, 60(6): 978-992. |
| [5] | Liu Xupeng, Wang Min, Han Shou'an, Zhu Xuehui, Wang Yanmeng, Pan Mingqi, Zhang Wen. Research Progress on Factors and Molecular Mechanisms Regulating Plant Organ Abscission [J]. Chinese Bulletin of Botany, 2025, 60(3): 472-482. |
| [6] | Tingxin Chen, Min Fu, Na Li, Leilei Yang, Lingfei Li, Chunmei Zhong. Identification and Expression Analysis of DNA Methyltransferase in Begonia masoniana [J]. Chinese Bulletin of Botany, 2024, 59(5): 726-737. |
| [7] | Yuejing Zhang, Hetian Sang, Hanqi Wang, Zhenzhen Shi, Li Li, Xin Wang, Kun Sun, Ji Zhang, Hanqing Feng. Research Progress of Plant Signaling in Systemic Responses to Abiotic Stresses [J]. Chinese Bulletin of Botany, 2024, 59(1): 122-133. |
| [8] | Yindu Liu, Junkang Tuo, Chengju Li, Feng Zhang, Chunli Zhang, Ying Zhang, Yunjiao Wang, Youfang Fan, Panfeng Yao, Chao Sun, Yuhui Liu, Zhen Liu, Zhenzhen Bi, Jiangping Bai. Screening and Evaluation of Low-potassium Tolerance Potato Varieties [J]. Chinese Bulletin of Botany, 2024, 59(1): 75-88. |
| [9] | Ziwen Tang, Dongping Zhang. Research Progress on the Molecular Mechanism of Starch Accumulation in Rice Endosperm [J]. Chinese Bulletin of Botany, 2023, 58(4): 612-621. |
| [10] | Dai Chen, Wang Jin, Lu Yaping. Determination of Acidic Plant Hormones by Derivative UPLC-MS [J]. Chinese Bulletin of Botany, 2022, 57(4): 500-507. |
| [11] | Li Yue, Hu Desheng, Tan Jinfang, Mei Hao, Wang Yi, Li Hui, Li Fang, Han Yanlai. Chaetomium uniseriatum Promotes Maize Growth by Accelerating Straw Degradation and Regulating the Expression of Hormone Responsive Genes [J]. Chinese Bulletin of Botany, 2022, 57(4): 422-433. |
| [12] | Yanyan Meng, Nan Zhang, Yan Xiong. Novel Links in the Plant Target of Rapamycin Signaling Networks [J]. Chinese Bulletin of Botany, 2022, 57(1): 1-11. |
| [13] | Xiaoting Zhao, Kaitao Mao, Jiahui Xu, Chuan Zheng, Xiaofeng Luo, Kai Shu. Protein Phosphorylation and Its Regulatory Roles in Seed Dormancy and Germination [J]. Chinese Bulletin of Botany, 2021, 56(4): 488-499. |
| [14] | Qilu Yu, Jiangzhe Zhao, Xiaoxian Zhu, Kewei Zhang. Regulation of Rice Growth by Root-secreted Phytohormones [J]. Chinese Bulletin of Botany, 2021, 56(2): 175-182. |
| [15] | Lulu Xie, Qingqing Cui, Chunjuan Dong, Qingmao Shang. Recent Advances in Molecular Mechanisms of Plant Graft Healing Process [J]. Chinese Bulletin of Botany, 2020, 55(5): 634-643. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||